Abstract
Testing platforms that leverage automation, require minimal sample volume, and enable various tests to be performed simultaneously on a single sample have the potential to improve workflow and efficiency in veterinary diagnostic laboratories. We evaluated a barcoded magnetic bead (BMB) technology using established immunoassays for detection of feline leukemia virus (FeLV) p27 antigen and antibody against feline immunodeficiency virus (FIV). Analytical sensitivity, limit of blank, and limit of detection were used to establish a functional sensitivity of 1.00 ng/mL of inactivated FeLV antigen and 35.7 ng/mL of anti-FIV monoclonal antibody. Common interferents, such as hemoglobin, lipid, and bilirubin, were not found to interfere with the performance of the assay. Intra- and inter-assay CVs were <13% for both assays using manufactured samples. Using a set of 116 feline samples, the diagnostic accuracy of our multiplex assay was 100% compared to reference assays. Performance in a convenience set of 1,000 feline samples submitted to a commercial diagnostic laboratory revealed a proportion of positive results of 1.3% for FeLV and 3.7% for FIV. BMB technology should enable rapid screening of samples for various markers in a single immunoassay well.
Keywords: antibody, antigen, feline immunodeficiency virus, feline leukemia virus, immunoassay, multiplex
Immunoassay technology has evolved in veterinary diagnostic laboratories to include particle-based flow cytometric assays and peptide arrays. These technologies not only enable various immunoassays to be performed simultaneously on an individual sample but are also compatible with high-throughput laboratory testing.4,13 More recently, a barcoded, magnetic bead (BMB) technology (Applied BioCode) developed for detecting multiple PCR amplicons was proposed for use with immunoassays. 8 The BMBs are 40 × 65 × 5 µm wafer-like particles with functionalized surfaces for either passive or covalent attachment of nucleic acids or proteins. Each bead has a digital barcode bonded to the surface using a semiconductor lithography process that allows identification by brightfield microscopy. The magnetic property of the bead permits routine processing in a 96-well microtiter plate, as in an ELISA, and can be adapted for automated, high-throughput screening. Immunoreactivity on the bead surface is detected using a fluorescent dye coupled to the specific detection reagent, and median fluorescence is quantified across each set of unique BMBs. Unique BMBs, each representing a different assay, can be added to a single well of the microtiter plate thereby reducing the amount of patient sample needed per test.
We evaluated the BMB technology for immunoassay feasibility using existing assays for feline leukemia virus (FeLV) p27 antigen and antibody against feline immunodeficiency virus (FIV) performed simultaneously on individual samples.2,12,14 Mouse anti-FeLV monoclonal antibody (mAb) was covalently coupled to p-carboxyl functionalized BMBs for the FeLV p27 antigen immunoassay. Detection was performed with a second anti-FeLV mAb covalently attached to biotin. A peptide from the immunodominant region (IDR) of the envelope protein of FIV was covalently linked to amine-functionalized BMBs for the FIV antibody immunoassay. 9 Detection was performed with the same IDR peptide covalently coupled to biotin. Assay fluorescence was generated using an 8 μg/mL solution of streptavidin, R-phycoerythrin (SA-PE) conjugate (Moss). Negative control BMBs, used to detect reagent- or sample-specific background when performing the assay, were prepared by covalently coating amine-functionalized BMBs with L-cysteine (MilliporeSigma) to block the functionalized amine groups. Each type of coated BMB was stored at a concentration of 50,000 BMB/mL in a PBS-Tween 20 (PBST; 0.1%) buffer (pH 7.4) containing 1% BSA and 0.05% ProClin 950 (MilliporeSigma). The multiplexed BMB mixture was prepared by diluting each of the coated BMBs in the same PBST-BSA buffer at a final concentration of 500 BMBs/mL. A multiplexed detection mix was prepared by combining biotinylated FeLV antibody and FIV peptide in the same PBST-BSA buffer at concentrations of 0.5 µg/mL and 2 µg/mL, respectively.
For the assay, 100 µL of the multiplexed BMB mixture was added to uncoated clear polystyrene microwells (Greiner Bio-One) for a targeted concentration of 50 BMBs per barcode per well. Plates were then washed 5 times with 300 µL/well of a 0.5% PBST buffer (pH 7.4) using a magnetic plate washer (405LS; BioTek Instruments). After washing, 50 µL of sample was added and incubated, with mixing, for 30 min at room temperature. Plates were washed as above, and 50 µL of the multiplexed detection mixture was added and allowed to incubate for 15 min with mixing. Plates were washed, and 50 µL of the SA-PE reagent was added and allowed to incubate with mixing for 10 min. After a final wash, 200 µL of detection buffer (Applied BioCode) was added to each microwell and the plate was read (BioCode 2500 detection system; Applied BioCode) by measuring the fluorescence and decoding the barcode of each BMB. A median fluorescence intensity (MFI) was calculated for each BMB in the microwell. The mean MFI of the negative control beads was subtracted as background from all other beads in the same well. The final assay result represents the background-corrected MFI of all beads of a particular barcode within a single well.
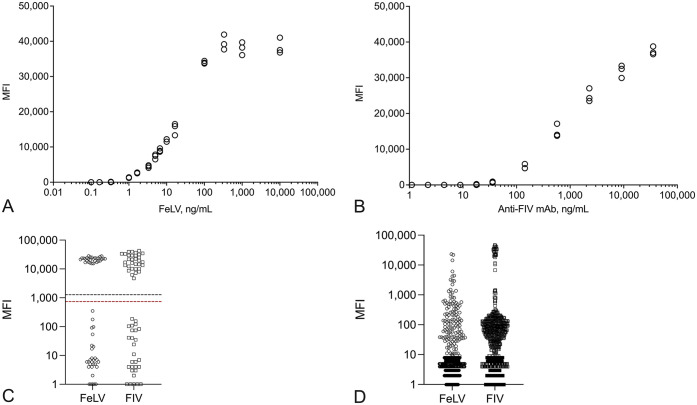
We evaluated the analytical sensitivity of our FeLV/FIV BMB assay using a dilution series of inactivated FeLV (Advanced Biotechnologies) and a dilution series of mouse mAb against the FIV envelope protein (clone HB12529; ATCC), both prepared in fetal bovine serum (FBS; MilliporeSigma). Dilutions were designed to span a broad range of concentrations: 0.1–10,000 ng/mL of inactivated FeLV, and 1.1–35,400 ng/mL of FIV mAb. These dilutions were tested in triplicate on our FeLV/FIV BMB assay. For FeLV, a concentration of 1.00 ng/mL of virus produced an MFI of 1,270; the subsequent dilution at 0.33 ng/mL was <10 MFI (Fig. 1A). A concentration of 1.00 ng/mL was consistent with the previously determined limit of quantification for the microtiter plate ELISA for FeLV p27 antigen. 2 For the FIV BMB assay, mAb concentrations of 17.8 ng/mL and 8.9 ng/mL resulted in MFIs of 168 and <10, respectively (Fig. 1B).
Figure 1.
Performance of the feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) barcoded magnetic bead (BMB) assay with manufactured and native feline samples. A. Detection of a serial dilution of inactivated FeLV in the FeLV BMB assay. B. Detection of a serial dilution of anti-FIV monoclonal antibody in the FIV BMB assay. C. FeLV/FIV BMB assay results for a characterized sample set of 116 feline sera. Median fluorescence intensity (MFI) cutoffs were 1.00 ng/mL (1,270 MFI) for FeLV (black dashed line) and 35.7 ng/mL (738 MFI) for FIV (red dashed line). D. Distribution of the FeLV/FIV BMB assay MFI results from a set of 1,000 feline samples obtained from a commercial diagnostic laboratory.
The limit of blank (LOB) and limit of detection (LOD) for our FeLV/FIV BMB assay were determined according to practices recommended by the Clinical Laboratory Standards Institute. 5 A negative sample consisting of FBS and a low-concentration positive manufactured sample for each assay were utilized. 1 Inactivated FeLV was diluted in FBS to a concentration of 6.65 ng/mL; FIV mAb was diluted in FBS to a concentration of 143 ng/mL. The negative and low-positive manufactured samples were tested in replicates of 60 to determine the x̄ and SD. For the FeLV assay, the LOB was 10 MFI and the LOD was 492 MFI; for the FIV assay, the LOB was 36 MFI and the LOD was 738 MFI. Extrapolating from the results of the analytical sensitivity study, the LODs equate to a concentration of 0.42 ng/mL of inactivated FeLV and 35.7 ng/mL of FIV mAb. The LOD for the FeLV assay was less than the analytical sensitivity of the assay, indicating that the functional sensitivity should be equivalent to the analytical sensitivity. However, the LOD for the FIV assay exceeded the analytical sensitivity study, indicating that functional sensitivity for the FIV assay should be no lower than the determined LOD, which incorporates estimates of bias and imprecision in the assay.
The precision of our FeLV/FIV BMB assay was evaluated using a panel of samples that included the negative and low-positive manufactured samples (as described above) along with high-positive manufactured samples containing 100 ng/mL of inactivated FeLV and 2,280 ng/mL of FIV mAb. The low- and high-positive manufactured samples produced comparable signal at ~5,000 MFI and >25,000 MFI, respectively (Table 1). The panels were tested on 20 consecutive FeLV/FIV BMB assays over 5 d for a total of 40 replicates. Each replicate of the negative sample fell below the LOD for both assays (FeLV median = 4.5, range: 1–71 MFI; FIV median = 4.5, range: 1–447 MFI). A nested ANOVA of the MFI results was performed to determine intra- and inter-assay variance components for the low- and high-positive manufactured samples in each assay (JMP 15; SAS Institute). The intra- and inter-assay SDs were calculated by taking the square root of the intra- and inter-assay variances, respectively. The intra- and inter-assay CVs were determined by dividing the intra- and inter-assay SDs by the grand mean MFI for each assay-panel combination and converted to a percentage via multiplying by 100%. The resulting intra-assay CV was <10% and the inter-assay CV was <13% for both assays when using the low- and high-positive manufactured samples (Table 1).
Table 1.
Intra- and inter-assay precision of the feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) barcoded magnetic bead (BMB) assay with low- and high-positive manufactured samples as indicated by concentration and median fluorescence intensity (MFI).
| Assay/Panel, ng/mL | MFI | Intra-assay CV, % | Inter-assay CV, % |
|---|---|---|---|
| FeLV BMB assay | |||
| FeLV low, 6.65 | 5,130 | 7.2 | 12.6 |
| FeLV high, 100 | 24,400 | 8.1 | 8.0 |
| FIV BMB assay | |||
| FIV low, 143 | 4,750 | 7.0 | 12.3 |
| FIV high, 2,280 | 25,100 | 5.0 | 3.3 |
The effect of elevated hemoglobin, lipids, and bilirubin on our FeLV/FIV BMB assay was evaluated using the negative and low-positive manufactured samples. Each interfering substance was spiked into the manufactured samples at increasing concentrations; bovine hemoglobin (MilliporeSigma) was tested at concentrations of 0.30–5.5 g/L, bilirubin (synthetic ditaurobilirubin, disodium salt; Scripps) was tested at concentrations of 0.001–0.144 g/L, and lipid (Intralipid, 20% emulsion; MilliporeSigma) was tested across optical density at 660 nm (OD660) values of 0.20–6.40. Each condition was tested in duplicate, and results were evaluated for evidence of trends across the range of interfering substances. A one-way ANOVA with an alpha risk of 0.05 was performed on each condition. No significant differences in MFI measurements were obtained when the negative and low-positive manufactured samples without interfering substances were compared to those manufactured samples containing hemoglobin up to a concentration of 5.5 g/L, bilirubin up to a concentration of 0.144 g/L, or lipids up to an OD660 of 6.40.
To assess the accuracy of our FeLV/FIV BMB assay and to evaluate its performance in a population of cats receiving veterinary care, we obtained 2 convenience sample sets of feline sera from a commercial laboratory (Idexx). The samples had been submitted by practicing veterinarians and, per terms of the service contract, were made available once requested testing was complete. The first sample set consisted of 116 samples that were screened for Dirofilaria immitis and FeLV p27 antigen and antibodies to FIV (SNAP feline triple test; Idexx).3,10 Fifty-six samples were selected for the FeLV population (27 FeLV p27 antigen-positive, 29 FeLV p27 antigen-negative), randomized, and blinded before testing on a commercial FeLV p27 antigen microtiter plate ELISA (Idexx). 2 Fifty-eight samples were selected for the FIV population (30 FIV antibody-positive, 28 FIV antibody-negative), randomized, and blinded before testing on a commercial FIV western blot (Idexx). The remaining 2 samples were found to be positive for both FeLV p27 antigen and FIV antibodies when screened, and these 2 samples were tested on both FeLV and FIV reference methods. When the SNAP feline triple test results for FeLV and FIV were compared to the respective reference methods, all results were found to be concordant.
All 116 samples were randomized and blinded before testing on our FeLV/FIV BMB assay. Diagnostic accuracy was assessed by comparing the results of our FeLV/FIV BMB assay to the results obtained from the reference methods. The MFI cutoffs for our FeLV/FIV BMB assay were aligned to 1.00 ng/mL (1,270 MFI) for FeLV and 35.7 ng/mL (738 MFI) for FIV. All samples tested from each population agreed with the reference methods, resulting in a diagnostic accuracy of 100% (Fig. 1C). Because all samples were tested simultaneously in both assays, it was possible to evaluate the FIV results for the FeLV-characterized population and the FeLV results for the FIV-characterized population. Test results from these additional negative samples did not alter the diagnostic accuracy or MFI differences between positive and negative populations (Table 2).
Table 2.
Feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) barcoded magnetic bead (BMB) assay results represented as the median and range of median fluorescence intensity (MFI) for each assay. The MFI cutoffs for the FeLV and FIV BMB assays were 1,270 and 738, respectively.
| MFI | ||
|---|---|---|
| Median | Range | |
| FeLV BMB assay | ||
| Positive (n = 29) | 22,500 | 15,400–28,000 |
| Negative (n = 87) | 4 | 1–533 |
| FIV BMB assay | ||
| Positive (n = 32) | 18,400 | 4,700–42,600 |
| Negative (n = 84) | 5 | 0–281 |
The second convenience sample set consisted of 1,000 feline sera of unknown retroviral status that were obtained from U.S. laboratories between June 2019 and June 2020 following routine blood chemistry testing. Retroviral status was determined by the results of our FeLV/FIV BMB assay, and the proportion of positive samples, along with the exact binomial 95% CIs, was calculated using the MFI cutoffs applied previously. The FeLV BMB assay generated results for all samples, with 1.3% (13 of 1,000; CI: 0.69–2.2%) determined to be positive. Reports of FeLV seroprevalence range from 1.99% in a 2020 dataset to 3.1% in a field study from 2010.3,6 Although the FIV BMB assay failed to generate a result for 23 samples because of insufficient BMBs per well, 3.7% (36 of 977; CI: 2.6–5.1%) of the samples were determined to be positive (Fig. 1D). This result aligns with reported seroprevalence of FIV infections within the United States of 2.5% in a 2006 serosurvey to 4.21% in 2020 field data.7,11 Although the measured seroprevalence for both FeLV and FIV aligned with prior studies, one limitation of our study was the lack of a direct comparison to established reference methods.
Our results demonstrate the feasibility of the BMB technology to perform 2 immunoassays per sample with the intention of improving laboratory testing efficiency and reducing required sample volume. Further evaluation of this new technology will likely reveal additional areas for improvement as we observed with the FIV BMB assay when testing the large convenience sample set. Well-designed experiments are needed to further explore the effects of different lots of BMBs under various immunoassay conditions so that standardized manufacturing and assay conditions may be adopted for broader application of this technology.
Acknowledgments
We acknowledge the support of Melissa Nielsen for technical support with western blot testing, and Jancy Hanscom, Molly Donovan, Rebecca Foley, Ashley Reynolds, Jessica Donahue, and Danica Douglas for assistance with sample acquisition.
Footnotes
All authors are employees of Idexx Laboratories.
Funding: This study was funded by Idexx Laboratories.
ORCID iD: Melissa Beall  https://orcid.org/0000-0002-8165-5361
https://orcid.org/0000-0002-8165-5361
References
- 1. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 2008;29(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 2. Buch JS, et al. Analytical validation of a reference laboratory ELISA for the detection of feline leukemia virus p27 antigen. J Vet Diagn Invest 2017;29:654–659. [DOI] [PubMed] [Google Scholar]
- 3. Burling AN, et al. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. J Am Vet Med Assoc 2017;251:187–194. [DOI] [PubMed] [Google Scholar]
- 4. Christopher-Hennings J, et al. Opportunities for bead-based multiplex assays in veterinary diagnostic laboratories. J Vet Diagn Invest 2013;25:671–691. [DOI] [PubMed] [Google Scholar]
- 5. Clinical Laboratory Standards Institute (CLSI). Protocols for determination of limits of detection and limits of quantitation; approved guideline. CLSI, 2004. CLSI document EP17-A. [Google Scholar]
- 6. Companion Animal Parasite Council. CAPC parasite map—FeLV. [Cited 2022 Mar 13]. https://capcvet.org/maps/#/2020/all-year/felv/cat/united-states
- 7. Companion Animal Parasite Council. CAPC parasite map—FIV. [Cited 2022 Mar 13]. https://capcvet.org/maps/#/2020/all-year/fiv/cat/united-states
- 8. Duncan R, et al. Advances in multiplex nucleic acid diagnostics for blood-borne pathogens: promises and pitfalls. Expert Rev Mol Diagn 2016;16:83–95. [DOI] [PubMed] [Google Scholar]
- 9. Fontenot JD, et al. Evaluation of feline immunodeficiency virus and feline leukemia virus transmembrane peptides for serological diagnosis. J Clin Microbiol 1992;30:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levy JK, et al. Seroprevalence of heartworm infection, risk factors for seropositivity, and frequency of prescribing heartworm preventives for cats in the United States and Canada. J Am Vet Med Assoc 2017;250:873–880. [DOI] [PubMed] [Google Scholar]
- 11. Levy JK, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 2006;228:371–376. [DOI] [PubMed] [Google Scholar]
- 12. O’Connor TP, et al. Immunoassay applications in veterinary diagnostics. In: Wild D, ed. The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques. 4th ed. Elsevier, 2013:623–645. [Google Scholar]
- 13. Pang S, et al. A comparability study of the emerging protein array platforms with established ELISA procedures. J Immunol Methods 2005;302:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Tonelli QJ. Enzyme-linked immunosorbent assay methods for detection of feline leukemia virus and feline immunodeficiency virus. J Am Vet Med Assoc 1991;199:1336–1339. [PubMed] [Google Scholar]