Abstract
Objective
To investigate safety, feasibility, and effectiveness of platelet-rich plasma (PRP) injection into the olfactory clefts of COVID-19 patients with persistent olfactory dysfunction (OD).
Methods
From March 2022 to July 2022, COVID-19 patients with persistent OD were consecutively recruited to benefit from PRP injection into the olfactory clefts. Patient pain, annoyance, time of procedure, and adverse events were evaluated. Olfactory function was evaluated at baseline and 2-month post-injection with the olfactory disorder questionnaire (ODQ) and threshold, discrimination, and identification (TDI) test.
Results
Eighty-seven patients with anosmia (N = 30), hyposmia (N = 40), or parosmia (N = 17) with a mean OD duration of 15.7 months completed the evaluations. The PRP injection was successfully performed in all patients with a mean procedure time of 18.4 ± 3.4 min. The adverse events included transient epistaxis (N = 31), parosmia related to xylocaine spray (N = 10), and vasovagal episode (N = 2). The injection procedure was evaluated as somewhat or moderately painful by 41 (47%) and 22 (25%) patients, respectively. Thirty-seven patients were assessed after 2 months post-injection. The mean ODQ and TDI scores significantly improved from baseline to 2-month post-injection (p < 0.01). The olfactory improvement occurred after a mean of 3.6 ± 1.9 weeks.
Conclusion
The injection of PRP into the olfactory clefts is safe and associated with adequate patient-reported outcomes. The findings of this preliminary study suggest possible efficacy on subjective and psychophysical evaluations, but future randomized controlled studies are needed to determine the superiority of PRP injection over placebo.
Keywords: COVID-19, Otolaryngology, Rhinology, Coronavirus, SARS-CoV-2, Anosmia, Hyposmia, Olfactory, Smell, Recovery, Platelet-rich plasma
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) led to an increase of the prevalence of olfactory dysfunction (OD) in the population [1]. The OD is one of the most common symptoms of the infection, reaching 30% to 86% of patients according to variants [2–4]. Most patients recover smell sense over the post-infection weeks, but some individuals report mid- to long-term OD, including anosmia, hyposmia, phantosmia, or parosmia [5]. Thus, the 12-month persistence of OD according to psychophysical olfactory evaluations may reach 46% of cases [6]. Others reported that the prevalence of patient-reported OD ranged from 15 to 70% 1 year after the infection [7, 8]. To date, there is no treatment for the long-term OD. Patients are recommended to adhere to an olfactory training protocol [8, 9], while some dietary supplements (e.g., omega 3, zinc, and B12 vitamin) may be advised [10]. In 2019, Yan et al. [11] published a preliminary paper describing the injection of platelet-rich plasma (PRP) into the olfactory cleft of seven individuals with post-viral OD as a new potential approach to improve the smell recovery. To date, there is no study assessing feasibility, safety, and tolerance of this procedure in large cohort of patients.
In the present study, we investigated the feasibility, safety, and tolerance of the injection of PRP into the olfactory clefts of patients with COVID-19-related OD.
Methods
Setting and patients
From March 2022 to July 2022, patients with post-COVID-19 persistent OD were consecutively recruited from two medical centers [Ear Nose and Throat Dour Medical Center (Dour) and CHU Saint-Pierre University Hospital (Brussels, Belgium)]. The OD occurred after the COVID-19, which was diagnosed with RT-PCR. The persistent OD was defined as smell sense disorder lasting more than 6 months and consisted of anosmia, hyposmia, phantosmia, or parosmia. Anosmia and hyposmia were defined with the threshold, discrimination, and identification testing (TDI) [12]. Anosmia consisted of a TDI score ≤ 16 points, while hyposmia was established as a TDI score of less than 30.75. TDI > 30.75 was considered as normal [12]. Patients benefited from tomodensitometry or magnetic resonance imaging, which did not report sinus or olfactory region abnormalities (e.g., rhinosinusitis, olfactory, or nasal tumor).
Patients with the following conditions were excluded: OD before the pandemic (e.g., post-viral, post-traumatic, neurological, and idiopathic); chronic rhinosinusitis with or without nasal polyposis; nasal obstruction related to rhinitis; history of nasal radiation or functional endoscopic sinus surgery.
The study protocol was approved by the ethics committee of the University hospital CHU Saint-Pierre (CHUSP2102028). The electronic informed consent was obtained for all patients.
Epidemiological, clinical, and olfactory data
The following epidemiological and clinical outcomes were collected with a standardized online questionnaire at the first evaluation: age; gender; comorbidities; allergy and tobacco consumption. The nasal symptoms were assessed with the French version of the sinonasal outcome tool-22 (SNOT-22) [13].
The olfactory and gustatory questions were based on the smell and taste component of the National Health and Nutrition Examination Survey [14]. The impact of OD on quality of life was assessed with the French version of the Olfactory Disorder Questionnaire, which includes parosmia (/12), quality of life (/57), and sincerity (/18) outcome scores [15]. The ODQ total score ranges from 0 (no OD) to 87 (important impact of OD on quality of life). Patients benefited from psychophysical evaluations with the TDI (Medisense, Groningen, The Netherlands) [12]. The olfactory cleft endoscopy scale [16] was scored at the first consultation and at the time of the PRP injection. This scale is a validated scale reporting the findings of discharge, polyps, edema, scarring, or crusting on a scale of 0, 1, or 2 on each side, giving a total score ranging from 0 to 20. Patients were evaluated for TDI and ODQ at the time of inclusion and 2 months after the PRP injection.
Injection procedure and outcomes
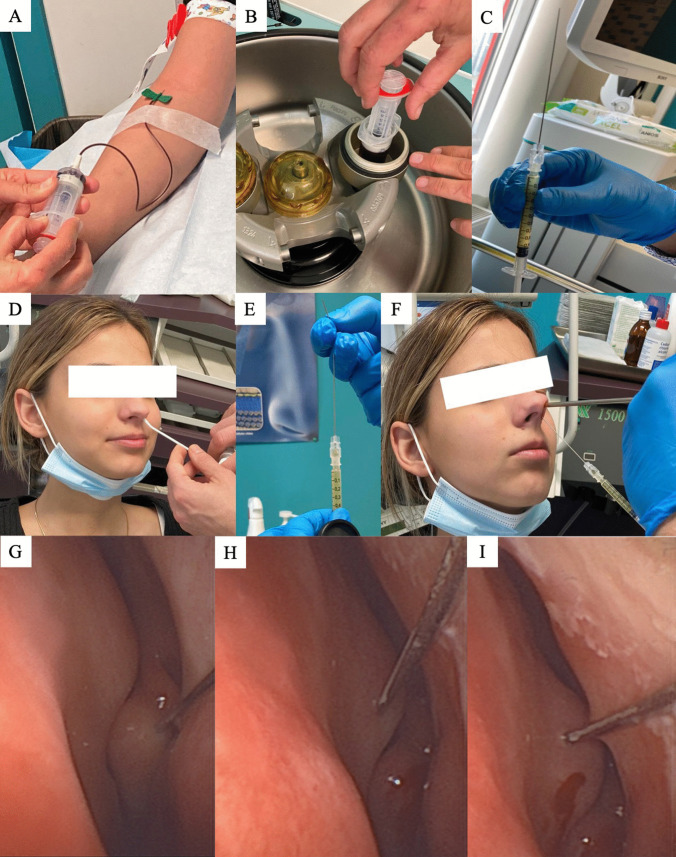
The PRP injections were performed by the same otolaryngologist (J.R.L.). The procedure was briefly described in Fig. 1. The blood extraction was performed into a 20 mL tube with sodium citrate anticoagulant and the isolation of PRP was made through a 10-min centrifugation at 4200 rpm. The supernatant was drawn up into a 10 mL syringe. The PRP was transferred into a 1 mL syringe. The injection was performed with a 27-G needle (10 cm). The local (nasal) anesthesia was performed in patients with Xylocain 10% spray, while otolaryngologist used xylometazoline chlorhydrate drops to have a better access to the nose and the olfactory cleft. The injection was performed through a 0° rigid optic to guide the needle direction. In some cases (septal deviation), the needle was bent to have a better access to the olfactory cleft. Several points of 0.2–0.5 mL were performed in the middle turbinate and in the nasal septum in regard of the head of the middle turbinate. The procedure was similarly performed in the contralateral nasal fossae. Note that common precautions were taken to ensure no injection into intravascularly, while patients were awake during the procedure about any visual changes occurring during the procedure. Patient was observed for 15 min after the procedure for potential adverse events and was discharged.
Fig. 1.
Procedure. The figure shows: blood extraction (A); centrifugation (B); 27-G needle syringe preparation (C, E); local anesthesia (D), injection of PRP through a 0° rigid optic in the middle turbinate/nasal septum in regard of the head of the middle turbine (F–I)
At the end of the procedure, patient was invited to fulfill an online questionnaire assessing pain and annoyance with a 4-point visual analog scale ranging from totally disagree (= 0) to totally agree (= 3) for the following steps: blood extraction, local anesthesia, and procedure.
The physician assessed the following outcomes: time of PRP preparation (e.g., blood extraction and centrifugation); time of local anesthesia; time of injection; quantity of injected PRP and immediate and delayed adverse events.
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS, v23,0; IBM Corp, Armonk, NY, USA). The evolution of subjective and objective olfactory evaluations was studied with the Wilcoxon Rank test. A p value < 0.05 was considered as significant. The relationship between epidemiological, clinical, olfactory, and procedure outcomes was analyzed with Spearman coefficient (rs < 0.30 = low correlation; 0.30–0.60 = moderate correlation; > 0.60 = strong correlation).
Results
Eighty-seven patients benefited from PRP injections (Table 1). There were 62 females and 25 males, respectively. The mean age was 41.6 ± 14.6 years. The most common comorbidities included thyroid disorders (16%), hypertension (9%), arthrosis (9%), and diabetes (9%; Table 1). Sixty patients (69%) received at least one dose of vaccine.
Table 1.
Epidemiological and clinical characteristics of patients
| Outcomes | Patients (N = 87) |
|---|---|
| Age (mean, SD—years) | 41.6 ± 14.6 |
| Sex (N (%)) | |
| Male | 25 (28.7) |
| Female | 62 (71.3) |
| Comorbidities [N (%)] | |
| Thyroid disorder | 14 (16.1) |
| Hypertension | 8 (9.2) |
| Arthrosis | 8 (9.2) |
| Diabetes | 8 (9.2) |
| Hypercholesterolemia | 7 (8.0) |
| Depression | 5 (5.7) |
| Psoriasis | 5 (5.7) |
| Asthma | 4 (4.6) |
| Reflux | 4 (4.6) |
| Renal insufficiency | 2 (2.3) |
| Rheumatoid polyarthritis | 2 (2.3) |
| Cancer history | 1 (1.1) |
| Hepatic insufficiency | 1 (1.1) |
| Cardiologic affections | 1 (1.1) |
| Allergy | 5 (5.7) |
| Tobacco consumption | 4 (4.6) |
| Vaccine [N (%)] | |
| No response | 13 (14.9) |
| No vaccine | 14 (16.1) |
| One-dose vaccine | 5 (5.7) |
| Two-dose vaccine | 20 (23.0) |
| Three-dose vaccine | 35 (40.2) |
N number, SD standard deviation
Olfactory features
The olfactory features are reported in Table 2. The included patients reported a mean duration of OD of 15.7 ± 7.5 months. Fifty-eight patients (67%) recognized to have adhered to a 12-week olfactory training at the onset of the OD, while 39 (45%) and 37 (42%) received nasal or oral corticosteroids in the first days after the onset of the OD. Some dietary supplements were prescribed in some patients in the first weeks of the OD (Table 2). At the time of the inclusion, the mean SNOT-22 and ODQ were 32.5 ± 18.1 and 51.0 ± 18.0, respectively. According to psychophysical evaluations, 30 (34%) and 40 (46%) patients reported anosmia and hyposmia, respectively (Table 2). Seventeen patients (19%) had normal TDI score but severe parosmia.
Table 2.
Olfactory dysfunction features of patients
| Olfactory dysfunction outcomes | |
|---|---|
| Duration of OD (mean ± SD (range); mo) | 15.7 ± 7.5 (14.1–17.3) |
| Intervention pre-injection (N (%)) | |
| Olfactory training (12 weeks) | 58 (66.7) |
| Alpha lipoic acid | 16 (18.4) |
| Nasal corticosteroids | 39 (44.8) |
| Oral corticosteroids | 37 (42.5) |
| Vitamin B | 26 (29.9) |
| Vitamin A | 14 (16.1) |
| Omega 3 | 12 (13.8) |
| Zinc | 37 (42.5) |
| SNOT-22 (mean, SD) | 32.5 ± 18.1 |
| ODQ outcomes (mean, SD) | |
| Parosmia statement | 7.8 ± 3.8 |
| Life quality statement | 34.1 ± 13.8 |
| Sincerity statement | 9.1 ± 4.4 |
| ODQ total score | 51.0 ± 18.0 |
| Psychophysical evaluations (mean, SD) | |
| Threshold | 4.3 ± 3.8 |
| Discrimination | 8.5 ± 4.5 |
| Identification | 8.2 ± 4.6 |
| TDI total score | 20.3 ± 10.5 |
| OD types (TDI; N (%)) | |
| Anosmia | 30 (34.5) |
| Hyposmia | 40 (46.0) |
| Normosmia with parosmia | 17 (19.5) |
The results consisted of mean standard ± deviation or number (%)
mo months, OD olfactory dysfunction, ODQ olfactory disorder questionnaire, SNOT-22 sinonasal outcome 22, TDI threshold discrimination identification
Procedure outcomes
The injection of PRP was successfully performed in all patients. Thirty-five patients reported unilateral nasal deviation, limiting the injection of PRP into the olfactory cleft of the deviation side. In case of deviation, the injection was performed closest to the olfactory cleft region. The mean times of PRP preparation (i.e., blood collection, centrifugation, and syringe preparation), local anesthesia, and PRP injection were reported in Table 3. The mean procedure time was 18.4 ± 3.4 min. Thirty-one patients (36%) had post-injection transient epistaxis, which was the primary acute adverse event. The local anesthesia with the xylocaine spray led to transient parosmia in ten patients (11%). Note that the two coagulations of PRP into the syringe occurred in patients who had vasovagal episode. Postnasal drip sensation (N = 5) and nausea (N = 2) were the only two adverse events occurring in the post-injection days.
Table 3.
Procedure outcomes
| Procedure outcomes | Mean (SD) |
|---|---|
| PRP preparation time (min) | 12.8 ± 2.6 |
| Local anesthesia time (min) | 1.1 ± 0.3 |
| Right olfactory cleft score | 0.6 ± 1.9 |
| Right olfactory cleft injection time (min) | 2.3 ± 1.0 |
| Right olfactory cleft amount (mL) | 1.2 ± 0.4 |
| Left olfactory cleft score | 0.1 ± 0.4 |
| Left olfactory cleft injection time (min) | 2.4 ± 1.0 |
| Left olfactory cleft amount (mL) | 1.2 ± 0.3 |
| Total duration (min) | 18.4 ± 3.4 |
| N (%) | |
|---|---|
| Acute adverse events | |
| Transient epistaxis | 31 (35.6) |
| Parosmia during local anesthesia (spray) | 10 (11.5) |
| Vasovagal episode | 4 (4.6) |
| Panic attack | 2 (2.3) |
| PRP coagulation | 2 (2.3) |
| Delayed adverse events | |
| Postnasal drip sensation | 5 (5.7) |
| Nausea | 2 (2.3) |
The data of this table concerned the entire cohort (n = 87)
SD standard deviation
The patient outcomes are reported in Table 4. Among the procedure steps, the injection was judged as the most painful and annoying step compared with other steps. Seventeen patients (19%) reported severe pain during the injection, while 41 (47%) and 22 (25%) evaluated the pain as moderate or low, respectively. According to the visual analog scale ranging from 0 (ineffective) to 3 (fully effective), the mean score of the local anesthesia effectiveness was 2.1 ± 0.9. The local anesthesia was evaluated as optimal, adequate, moderately adequate, and ineffective in 33 (38%), 33 (38%), 18 (21%), and 3 (3%) patients, respectively.
Table 4.
Patient-reported outcomes about procedure
| Patient-reported outcomes | Mean (SD) | Range | |||
|---|---|---|---|---|---|
| 0 (no p/a) | 1 (mild p/a) | 2 (mod. p/a) | 3 (full p/a) | ||
| Injection pain | 1.8 ± 0.9 | 7 (8.0) | 22 (25.3) | 41 (47.1) | 17 (19.5) |
| Blood collection pain | 0.3 ± 0.6 | 65 (74.7) | 21 (24.1) | 0 (0.0) | 1 (1.1) |
| Blood collection annoyance | 0.3 ± 0.5 | 67 (77.0) | 17 (19.5) | 3 (3.4) | 0 (0.0) |
| Local anesthesia pain | 1.1 ± 1.0 | 30 (34.5) | 26 (29.9) | 25 (28.7) | 6 (6.9) |
| Local anesthesia annoyance | 1.1 ± 0.9 | 26 (29.9) | 29 (33.3) | 29 (33.3) | 3 (3.4) |
| Injection annoyance | 1.5 ± 0.9 | 16 (18.4) | 19 (21.8) | 45 (51.7) | 7 (8.0) |
The data of this table concerned the entire cohort (n = 87)
p/a pain/annoyance, SD standard deviation
Evolution of olfactory outcomes and predictors
Thirty-seven patients were re-evaluated 2 months after the PRP injection. There were no synechia, mucosal disturbances, or inflammation at the nasofibroscopic examination. Among them, 8 patients (22%) did not report subjective improvement of OD, while 20 (54%) and 9 individuals (24%) reported substantial improvement of anosmia/hyposmia or parosmia, respectively. Thirty-three patients (89%) adhered to the olfactory training, which was performed 2.3 ± 1.5 times daily for 8 weeks. According to the patient experience, the significant improvement of olfaction occurred after a mean of 3.6 ± 1.9 weeks. The pre- to post-injection changes in ODQ and TDI scores are reported in Table 5. Both ODQ and TDI scores significantly improved from baseline to 2-month post-injection.
Table 5.
Evolution of olfactory outcomes after PRP injection
| Outcomes | Baseline | 2 mo | p value |
|---|---|---|---|
| Parosmia score | 7.8 ± 3.8 | 7.5 ± 3.1 | 0.047 |
| Life quality statement score | 34.1 ± 13.8 | 24.4 ± 8.0 | 0.001 |
| Sincerity statement score | 9.1 ± 4.4 | 8.9 ± 3.3 | NS |
| Fr-ODQ total score | 51.0 ± 18.0 | 40.7 ± 10.9 | 0.001 |
| Threshold | 3.5 ± 4.0 | 5.8 ± 4.5 | 0.024 |
| Discrimination | 8.5 ± 4.5 | 11.2 ± 3.9 | 0.007 |
| Identification | 8.3 ± 4.6 | 10.4 ± 3.5 | 0.002 |
| TDI total score | 20.3 ± 10.5 | 26.0 ± 11.2 | 0.009 |
The data of this table concerned 37 patients who were assessed at 2-month post-injection
mo month, NS non-significant, ODQ olfactory disorder questionnaire, PRP platelet-rich plasma, TDI threshold discrimination identification
There were negative significant associations between age and the following baseline outcomes: ODQ-Life quality score (rs = − 0.309; p = 0.007), sincerity score (rs = − 0.237; p = 0.041), and ODQ total score (rs = − 0.323; p = 0.005); meaning that young patients reported stronger impact of OD on the quality of life.
Discussion
The injection of platelet-rich plasma into injured tissues is an old approach used in orthopedic, plastic surgery, dermatology, or rehabilitation [17]. In otolaryngology, PRP was used in the management of neck fistula [18], vocal fold scars [19], or tympanic membrane perforation [20], reporting encouraging results.
The primary finding of this pilot study was the demonstration of the safety, feasibility, and tolerance of PRP injection into the olfactory cleft. The injection-related pain was judged as tolerable by 81% of patients, who assessed the local anesthesia as effective. The occurrence of transient epistaxis was related to the realization of several mucosa injection points and was the main adverse event. Both injection pain and risk of transient epistaxis were, however, not reported in the study of Yan et al., which limits the comparison of our data with the current literature [11]. The mean time of the procedure was 18.4 min, which makes the PRP olfactory cleft injection a rapid procedure. The mean time of PRP extraction and injection found in the present study corroborated those of studies in which PRP procedure was performed for other otolaryngological indications [19, 21]. The mean injected PRP amount was 1.2 mL/side, which was consistent with the data of Yan et al. [11].
The main advantage of this approach is the safety and the easiness of the technique. Because PRP is an autologous biological product derived from the patient blood, there is no risk of reject, disease transmission, or blood adverse event. However, from a practical standpoint, the injections need to be performed in the minutes following the end of the centrifugation, because there is a risk of coagulation of the supernatant. In the present study, two patients had vasovagal event, delaying the injection of few minutes, which led to the coagulation of plasma.
A proportion of patients (n = 37) were re-evaluated 2 months after the PRP injection, reporting significant improvements of ODQ and TDI scores. These data supported those of the preliminary study of Yan et al., who reported a substantial improvement of TDI scores in five out of seven patients [11]. The usefulness of PRP injection into the olfactory cleft was recently supported by Steffens et al. [22] who observed that patients treated by PRP injection for a persistent (> 1 year) OD reported higher increase of TDI score improvements 1 month after the PRP injection compared with patients who did not benefit from injection. The study of Steffens et al. [22] may support our observations. In the present study, patients recovered subjectively smell sense 3.6 weeks after the injection, which may corroborate the current knowledge about the physiological effect of PRP [23]. From a physiological standpoint, the PRP pockets in the mucosa will progressively release anti-inflammatory and pro-regenerative factors of the platelets, leading to the upregulation of some factors by the cells of nasal and olfactory tissues, e.g., growth and transforming factors, vascular endothelial growth molecules, epidermal growth factor, and insulin-like growth factor [17, 23]. It was moreover suggested that PRP may promote axon regeneration and neuroregeneration [17]. The anti-inflammatory effects of PRP are particularly relevant in patients with COVID-19 OD, because a recent multicenter study supported that OD patients may have persistent virus in the olfactory region and associated inflammation in the neuroepithelium, which may account for prolonged or relapsing loss of smell [24]. Theoretically, the potential anti-inflammatory effect of PRP may reduce the chronic inflammation and the cell-related injuries, promoting the regeneration of the olfactory tissues.
However, the effectiveness of PRP injection on persistent OD cannot be formally established without the conduction of randomized controlled study. Because the injection of a ‘therapeutic material’ into the olfactory cleft may have a placebo effect [11], the design of future studies may include the injection of saline solution in the olfactory cleft of patients of the control group.
The lack of control group and the low number of patients who completed the 2-month follow-up evaluations are the primary limitations of the present study. However, the main objective of this preliminary study was the evaluation of the safety, feasibility, and tolerance of the technique. The publication of our preliminary results about the potential effectiveness of PRP was motivated by the potential impact of this approach in COVID-19 patients with a persistent OD. The uses of ODQ and TDI scores are the main strengths of the present study, because they are both validated approaches providing different but complementary olfactory findings.
Conclusion
The injection of PRP into the olfactory cleft of patients with OD related to COVID-19 is a safe approach associated with adequate patient-reported outcomes. The findings of this preliminary study suggest possible efficacy on subjective and psychophysical evaluations, but future randomized controlled studies are needed to determine the superiority of PRP injection over placebo.
Acknowledgements
B. Johnson for the proofreading.
Funding
None.
Data availability
Data are available on request to the first author according the rights of University (data protection/copyright).
Declarations
Conflict of interest
Authors have no conflict of interest.
Sponsorships
None.
Research involving human participants and/or animals
IRB was not required for this study.
Informed consent
Experts agreed to participate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karamali K, Elliott M, Hopkins C. COVID-19 related olfactory dysfunction. Curr Opin Otolaryngol Head Neck Surg. 2022;30(1):19–25. doi: 10.1097/MOO.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of smell and taste in 2,013 European Mild-to-moderation COVID-19 patients. Ann Int Med. 2020;173(8):672–675. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 4.Boscolo-Rizzo P, Tirelli G, Meloni P, Hopkins C, Madeddu G, De Vito A, Gardenal N, Valentinotti R, Tofanelli M, Borsetto D, Lechien JR, Polesel J, De Riu G, Vaira LA. Coronavirus disease 2019 (COVID-19)-related smell and taste impairment with widespread diffusion of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) Omicron variant. Int Forum Allergy Rhinol. 2022 doi: 10.1002/alr.22995.10.1002/alr.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Ascanio L, Pandolfini M, Cingolani C, Latini G, Gradoni P, Capalbo M, Frausini G, Maranzano M, Brenner MJ, Di Stadio A. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg. 2021;164(1):82–86. doi: 10.1177/0194599820943530. [DOI] [PubMed] [Google Scholar]
- 6.Boscolo-Rizzo P, Hummel T, Hopkins C, Dibattista M, Menini A, Spinato G, Fabbris C, Emanuelli E, D'Alessandro A, Marzolino R, Zanelli E, Cancellieri E, Cargnelutti K, Fadda S, Borsetto D, Vaira LA, Gardenal N, Polesel J, Tirelli G. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-COVID-19 patients: a matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59(6):517–527. doi: 10.4193/Rhin21.249. [DOI] [PubMed] [Google Scholar]
- 7.Ferreli F, Gaino F, Russo E, et al. Long-term olfactory dysfunction in COVID-19 patients: 18-month follow-up study. Int Forum Allergy Rhinol. 2022 doi: 10.1002/alr.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortunato F, Martinelli D, Iannelli G, Milazzo M, Farina U, Di Matteo G, De Nittis R, Ascatigno L, Cassano M, Lopalco PL, Prato R. Self-reported olfactory and gustatory dysfunctions in COVID-19 patients: a 1-year follow-up study in Foggia district, Italy. BMC Infect Dis. 2022;22(1):77. doi: 10.1186/s12879-022-07052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic reviewand meta-analysis. Otolaryngol Head Neck Surg. 2021;164(2):244–254. doi: 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- 10.Oleszkiewicz A, Bottesi L, Pieniak M, Fujita S, Krasteva N, Nelles G, Hummel T. Olfactory training with Aromastics: olfactory and cognitive effects. Eur Arch Otorhinolaryngol. 2022;279(1):225–232. doi: 10.1007/s00405-021-06810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helman SN, Adler J, Jafari A, Bennett S, Vuncannon JR, Cozart AC, Wise SK, Kuruvilla ME, Levy JM. Treatment strategies for postviral olfactory dysfunction: a systematic review. Allergy Asthma Proc. 2022;43(2):96–105. doi: 10.2500/aap.2022.43.210107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan CH, Mundy DC, Patel ZM. The use of platelet-rich plasma in treatment of olfactory dysfunction: a pilot study. Laryngoscope Investig Otolaryngol. 2020;5(2):187–193. doi: 10.1002/lio2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin' Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276(3):719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Dorlodot C, Horoi M, Lefebvre P, Collet S, Bertrand B, Eloy P, Poirrier AL. French adaptation and validation of the sino-nasal outcome test-22: a prospective cohort study on quality of life among 422 subjects. Clin Otolaryngol. 2015;40(1):29–35. doi: 10.1111/coa.12315. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015;125:1102–1106. doi: 10.1002/lary.24999. [DOI] [PubMed] [Google Scholar]
- 16.Lechien JR, Vaira LA, Le Bon SD, Geerts R, Boscolo-Rizzo P, Saussez S (2022) Validity and reliability of a French version of the olfactory disorders questionnaire. J Otolaryngol Head Neck Surg (Submitted to) [DOI] [PMC free article] [PubMed]
- 17.Soler ZM, Hyer JM, Karnezis TT, Schlosser RJ. The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(3):293–298. doi: 10.1002/alr.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24(3):159–167. doi: 10.1055/s-2008-1076752. [DOI] [PubMed] [Google Scholar]
- 19.Eryılmaz A, Demirci B, Gunel C, Kacar Doger F, Yukselen O, Kurt Omurlu I, Basal Y, Agdas F, Basak S. Can tissue adhesives and platelet-rich plasma prevent pharyngocutaneous fistula formation? Auris Nasus Larynx. 2016;43(1):62–67. doi: 10.1016/j.anl.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Suresh A, Balouch B, Martha VV, Sataloff RT. Laryngeal applications of platelet rich plasma and platelet poor plasma: a systematic review. J Voice. 2021 doi: 10.1016/j.jvoice.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Mandour MF, Elsheikh MN, Khalil MF. Platelet-rich plasma fat graft versus cartilage perichondrium for repair of medium-size tympanic membrane perforations. Otolaryngol Head Neck Surg. 2019;160(1):116–121. doi: 10.1177/0194599818789146. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt NK, Gao WZ, Timmons Sund L, Castro ME, O'Dell K, Johns MM., 3rd Platelet-rich plasma for vocal fold scar: a preliminary report of concept. J Voice. 2021 doi: 10.1016/j.jvoice.2020.12.040. [DOI] [PubMed] [Google Scholar]
- 23.Steffens Y, Lebon SD, Prunier L, Rodíguez A, Lechien JR, Saussez S, Horoi M. Effectiveness and safety of PRP on persistent olfactory dysfunction related to COVID-19: towards a new therapeutic hope. Eur Arch Oto-Rhino-Larynol. 2022 doi: 10.1101/2022.02.14.22270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Fan H, Li Y, Yun Z, Zhang Z, Zhu Q. Effectiveness of platelet-rich plasma injections for the treatment of acute Achilles tendon rupture: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100(41):e27526. doi: 10.1097/MD.0000000000027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo PM. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request to the first author according the rights of University (data protection/copyright).