Abstract
Purpose
Antibiotic-loaded bone cement (ALBC) was usually used to prevent periprosthetic joint infection (PJI) in primary total knee arthroplasty (PTKA), but whether to use ALBC or plain bone cement in PTKA remains unclear. We aimed to compare the occurrence rate of PJI using two different cements, and to investigate the efficacy of different antibiotic types and doses administered in preventing surgical site infection (SSI) with ALBC.
Methods
The availability of ALBC for preventing PJI was evaluated by using a systematic review and meta-analysis referring to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Existing articles until December 2021 involving PTKA patients with both ALBC and plain bone cement cohorts were scanned by searching “total knee arthroplasty”, “antibiotic-loaded cement”, “antibiotic prophylaxis”, “antibiotic-impregnated cement” and “antibiotic-laden cement” in the database of PubMed/MEDLINE, Embase, Web of Science and the Cochrane Library. Subgroup analysis included the effectiveness of different antibiotic types and doses in preventing SSI with ALBC. The modified Jadad scale was employed to score the qualities of included articles.
Results
Eleven quantitative studies were enrolled, including 34,159 knees undergoing PTKA. The meta-analysis results demonstrated that the use of prophylactic ALBC could significantly reduce the prevalence of deep incisional SSI after PTKA, whereas there was no significant reduction in the rate of superficial incisional SSI. Moreover, gentamicin-loaded cement was effective in preventing deep incisional SSI, and the use of high-dose ALBC significantly reduced the rate of deep incisional SSI after PTKA. Besides, no significant adverse reactions and complications were stated during the use of ALBC in PTKA.
Conclusion
The preventive application of ALBC during PTKA could reduce the rates of deep PJI. Furthermore, bone cement containing gentamicin and high-dose ALBC could even better prevent deep infection after PTKA. However, the existing related articles are mostly single-center and retrospective studies, and further high-quality ones are needed for confirmation.
Keywords: Surgical site infection, Antibiotic-loaded cement, Plain bone cement, Periprosthetic joint infection, Primary total knee arthroplasty, Meta
Introduction
As we all know, periprosthetic joint infection (PJI) is one of the serious complications after primary total knee arthroplasty (PTKA),1 which could lead to costly revision surgery, decline in patients’ functional status, and so on. Preoperative and postoperative intravenous antibiotics are usually recommended to preclude PJI during PTKA. However, due to the devastated blood vessel and limited blood supply, PJI may not be sufficiently and effectively excluded, which results in a low concentration of antibiotic around the implant prostheses.2,3 Moreover, increasing the dose or the time period using antibiotics may disequilibrate immune system and cause other adverse effects.
As early as in 1970, Buchholz et al.4 initially utilized antibiotic-loaded bone cement (ALBC) to preclude PJI. The preventive application of ALBC has been reported to decrease the incidence of deep infection in several studies.5,6 In many northern countries, using ALBC was considered a routine procedure in total joint arthroplasty (TJA).7,8 However, currently, it is more commonly used in periprosthetic infection revision rather than in primary TJA. Although some objectors introduce several disadvantages of ALBC, such as antibiotic resistance, allergic reaction, toxicity, compromised mechanical strength of the cement and increased cost, there is insufficient clinical evidence to support these disadvantages.
Using ALBC or plain bone cement (PBC) in PTKA remains unclear in the literature. ALBC has been reported to reduce serious complication, but has the disadvantage of reducing the strength of cement.9 In the year of 2015, a meta-analysis found no significantly difference of the rates of PJI in patients receiving two cement materials during PTKA.10 Nevertheless, this meta-analysis had a small sample size and did not analyze the efficacy of antibiotic types and doses in preventing infection. Currently, it still remains controversial whether ALBC is recommended, as described in the 2018 International Consensus on Orthopaedic Infections.11
Our study represented an update of the previous studies. This meta-analysis was designed (1) to ask whether prophylactic use of ALBC reduces deep and superficial infection rates after PTKA, (2) to determine whether different antibiotic types could affect the outcomes in preventing surgical site infection (SSI), and (3) to investigate the effectiveness of high- and low-dose antibiotics in avoiding SSI.
Methods
Study design and search strategy
This comprehensive meta-analysis was executed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for verifying the efficacy of ALBC for preventing PJI.12 The studies relevant to ALBC and PBC in PTKA, were identified according to the following retrieval words: “total knee arthroplasty”, “antibiotic-loaded cement”, “antibiotic prophylaxis”, “antibiotic-impregnated cement” and “antibiotic-laden cement.” The databases involved PubMed (MEDLINE), Embase, Web of Science and the Cochrane Library. The last date for this search was December 2021. Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist was shown in Appendix A1.
Study selection and eligibility criteria
The inclusion criteria were: (1) studies involving PTKA, (2) studies comparing the ALBC trial group with the PBC control group, and (3) studies in which the patients were without a history of pyogenic arthritis or previous surgeries. The exclusion criteria were: (1) targeting patients with poor health, such as diabetes and malignant tumor, (2) unavailable to obtain or extrapolate the data and outcomes of publication results, (3) animal experimentations, non-controlled studies, revision knee arthroplasty and conference abstracts, and (4) significantly mismatched baseline. After reading the title, abstract, full text and references in turn, relevant studies were screened out by two reviewers. If disagreements were noted, the third author would make the final decision after discussion.
Data extraction and items
The baseline, deep incisional SSI, superficial incisional SSI, type and dosage of antibiotics loaded in the cement were recorded in each study. Postoperative PJI event following the use of ALBC and PBC was the main analytical target. Subgroup analysis included the efficacy of different types and doses of antibiotic in preventing SSI with ALBC. Additionally, other relevant adverse effects of bone cement were also recorded.
Risk of study bias
Two reviewers evaluated the bias of selected articles by utilizing the modified Jadad scale,13 which was executed according to four primary appraisal items: randomization or not and specific random method, concealment or not, blinding or not, and quit or loss. The quality of included study contains two categories: high (score 4–7) and low (score 1–3).
Statistical analyses
Statistical analyses regarding the occurrence rate of PJI after PTKA were performed utilizing Review Manager (RevMan) 5.3 software (Cochrane Informatics & Knowledge Management, Oxford, UK). Binary variables were calculated with the risk ratio (RR) with 95% confidence intervals (CI), while continuous variables were calculated as mean differences with 95% CI. Fixed-effect (Mantel-Haenszel test) models were utilized, if there was statistical homogeneity among the included data (p ≥ 0.10, I2 ≤ 50%). If not, random-effect (DerSimonian-Laird method) models were utilized.
Results
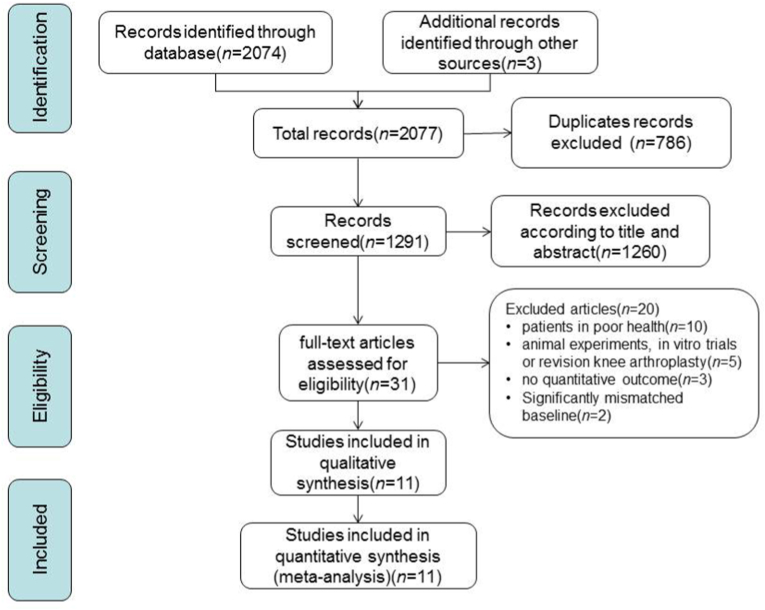
In total, 2074 potentially relevant studies from electronic databases were identified depending on the search strategy, of which 786 duplicates were removed and 1260 studies were eliminated based on titles and abstracts. After intensively reading full text, we eventually retained 11 articles that met our eligibility criteria (Fig. 1).3,5,14, 15, 16, 17, 18, 19, 20, 21, 22
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines selection process.
The 11 studies involved 34,159 knee arthroplasties in total. Of these, 20,997 received ALBC, and 13,162 received PBC and served as controls. The details of these 11 articles were summarized in Table 1, which consisted of two randomized controlled trials, one prospective comparative trial and eight retrospective comparative trials. According to the criteria of the modified Jadad scale, two studies5,16 and nine articles3,14,15,17, 18, 19, 20, 21, 22 were considered high-quality and low-quality studies, respectively.
Table 1.
Patient demographics and study characteristics.
| Study (year) | Country | Study design | Group | Knees | Male/ Female |
Mean age years (SD) |
Follow-up (month, range) |
DI | SI | Antibiotic type and dose | Modified Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiu et al.5 (2002) | China | RCT | ALBC | 178 | 124/54 | 70 (7.4) | 49 (26–80) | 0 | 2 | Cefuroxime, 2 g | 4 |
| PBC | 162 | 112/50 | 68 (6.9) | 49 (26–80) | 5 | 2 | |||||
| Eveillard et al.14 (2003) | France | RE | ALBC | 83 | 59/108 | – | 12 | 1 | – | Gentamicin, - | 2 |
| PBC | 84 | – | – | 12 | 8 | – | |||||
| Gandhi et al.15 (2009) | Canada | PRO | ALBC | 814 | 285/529 | 65.1 (15.4) | 12 | 18 | 0 | Tobramycin, - | 3 |
| PBC | 811 | 286/543 | 67.2 (10.8) | 12 | 25 | 0 | |||||
| Hinarejos et al.16 (2013) | Spain | RCT | ALBC | 1483 | 346/1137 | 75.8 (7.44) | 12 | 20 | 27 | Erythromycin, 0.5 g | 6 |
| PBC | 1465 | 353/1112 | 76.0 (7.22) | 12 | 20 | 18 | |||||
| Qadir et al.19 (2014) | New Orleans | RE | ALBC | 1486 | 560/926 | 68.13 (10.34) | 1–24 | 20 | – | gentamicin, - tobramycin, - Gentamicin, - |
2 |
| PBC | 1025 | 381/644 | 68.18 (9.84) | 1–24 | 15 | – | |||||
| Gutowski et al.17 (2014) | USA | RE | ALBC | 4826 | 2219/2611 | 65.7 | 24 | 40 | – | Tobramycin, 1 g | 1 |
| PBC | 3048 | 1465/1573 | 65.9 | 24 | 23 | – | |||||
| Hansen et al.18 (2014) | USA | RE | ALBC | 8441 | – | – | >24 | 59 | – | Tobramycin, 1.2 g | 2 |
| PBC | 3053 | – | – | >24 | 61 | – | |||||
| Wang et al.20 (2015) | China | RE | ALBC | 256 | 38/218 | 63.32 (11.13) | – | 1 | – | Gentamicin, 0.5–0.8 g | 2 |
| PBC | 2037 | 351/1686 | 64.97 (10.63) | – | 9 | – | |||||
| Wu et al.21 (2016) | China | RE | ALBC | 2790 | 693/2459 | 69.7 (7.8) | – | 18 | 23 | cefazolin,- vancomycin, - cefuroxime, - gentamycin, - Tobramycin, - |
2 |
| PBC | 362 | – | – | 7 | |||||||
| Sanz-Ruiz et al.22 (2017) | Spain | RE | ALBC | 555 | 183/372 | 77.9 | >24 | – | – | Gentamycin, 0.5 g | 1 |
| PBC | 695 | 250/445 | 76.4 | >24 | – | – | |||||
| Sadullah Turhan3 (2019) | Turkey | RE | ALBC | 85 | 91/415 | 62.8 (10.3) | 1–12 | 1 | 0 | Gentamicin, 1 g | 3 |
| PBC | 421 | – | 1–12 | 6 | 3 |
SD: standard deviation, DI: deep infection, SI: superficial infection, RCT: randomized controlled trail, ALBC: antibiotic-loaded bone cement, PBC: plain bone cement, RE: retrospective trail, PRO: prospective trail.
-: not mentioned.
Efficacy for avoiding deep incisional SSI
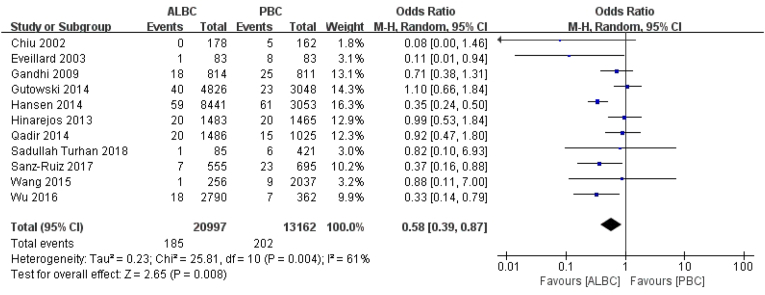
As for postoperative deep incisional SSI, 387 knees in 11 articles were described, of which 185 knees (185/20997 knees) were treated with ALBC, and 202 knees (202/13162 knees) with PBC. Using ALBC prophylactically could reduce the prevalence of deep incisional PJI after PTKA (RR = 0.58, 95% CI 0.39–0.87, p = 0.008, I2 = 61%, Fig. 2).
Fig. 2.
The RR and 95% CI for the incidence of deep incisional SSI among patients treated with ALBC vs. PBC. ALBC: antibiotic-loaded bone cement, PBC: plain bone cement, SSI: surgical site infection.
Efficacy for avoiding superficial incisional SSI
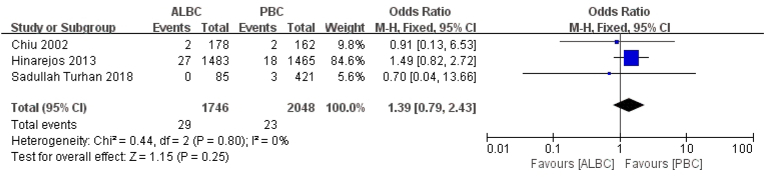
However, regarding superficial infection, three articles representing 52 knees reported on postoperative superficial incisional SSI, among which cases of knee infection in the ALBC and PBC groups were 29 knees (29/1746 knees) and 23 knees (23/2048 knees) after PTKA, respectively. There was no significant reduction in superficial incisional SSI rate between two groups (RR = 1.39, 95% CI 0.79–2.43, p = 0.25, I2 = 0, Fig. 3).
Fig. 3.
The RR and 95% CI for the incidence of superficial incisional SSI among patients treated with ALBC vs. PBC. SSI: surgical site infection, ALBC: antibiotic-loaded bone cement, PBC: plain bone cement. Efficacy of different antibiotic types for avoiding SSI in the ALBC group.
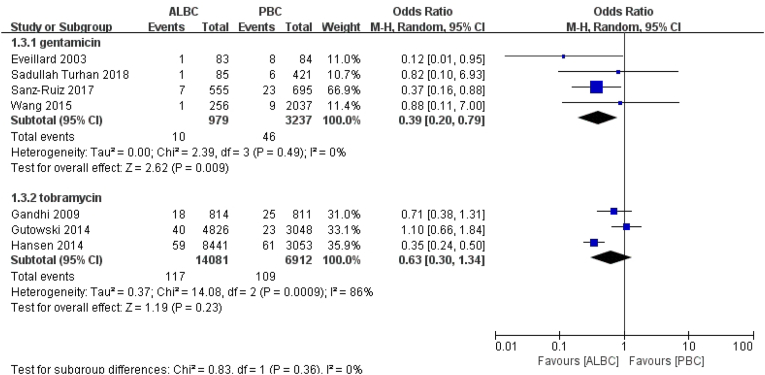
Regarding the different antibiotic types in the ALBC group, seven studies were included. Four studies reported gentamicin-loaded cement, while three studies represented tobramycin-loaded cement. Fifty-six knees experienced deep incisional SSI postoperatively with gentamicin-loaded cement in the ALBC group. Ten knees (10/979 knees) were treated with ALBC, while 46 knees (46/3237 knees) were treated with PBC. According to the result, gentamicin-loaded cement was effective in preventing deep incisional PJI (RR = 0.39, 95% CI 0.20–0.79, p = 0.009, I2 = 0, Fig. 4).
Fig. 4.
The RR and 95% CI for the incidence of deep incisional SSI among patients treated with ALBC vs. PBC in term of different antibiotics.
SSI: surgical site infection, ALBC: antibiotic-loaded bone cement, PBC: plain bone cement. Efficacy of different antibiotic dosage for avoiding SSI in the ALBC group.
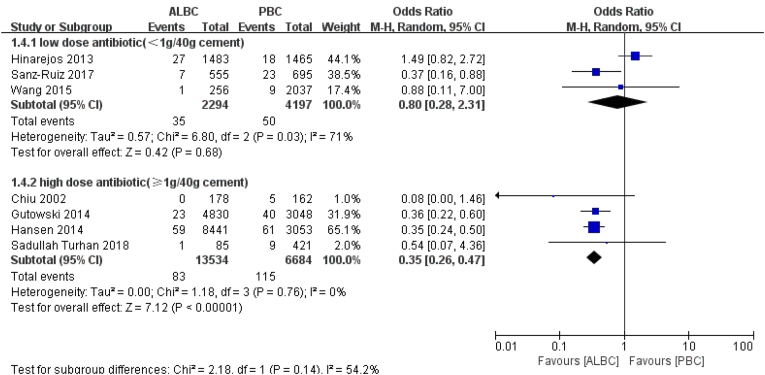
In the ALBC group, regarding antibiotic dosage, three articles reported the use of low-dose (<1 g of antibiotic powder per 40 g cement) antibiotic, and four studies reported the use of high-dosage (≥1 g antibiotic powder per 40 g cement) antibiotic. According to the result, using high-dose antibiotic in ALBC could significantly reduce the rate of deep incisional PJI (RR = 0.35, 95% CI 0.26–0.47, p < 0.01, I2 = 0, Fig. 5).
Fig. 5.
The RR and 95% CI for the incidence of deep incisional SSI among patients treated with ALBC vs. PBC in term of different antibiotic-dosing.
SSI: surgical site infection, ALBC: antibiotic-loaded bone cement, PBC: plain bone cement.
Discussion
Although ALBC has been demonstrated to have a certain therapeutic effect on preventing PJI for a long time, using ALBC in routine for non-high-risk PTKA remains controversial. In our research, the results demonstrate that the prophylactic use of ALBC during PTKA can reduce the incidence of deep incisional infection. Moreover, gentamicin-loaded bone cement and high-dosage ALBC could significantly decrease the incidence of deep incisional PJI after PTKA.
Several clinical researches also recommended using ALBC to prevent PJI following TJA,5,6,23 which was in accordance with our results. And our results suggested that the incidence of deep infection was lower when ALBC was used. A prospective and randomized study of Chiu et al.5 included 178 knees undergoing PTKA with ALBC and 162 knees with PBC. No infection was observed in the ALBC group, while 5 PJIs were observed in the PBC group, which showed a significantly lower rate of deep infection in the former (0 vs. 3.1%, p = 0.0238). Besides, Engesaeter et al.23 even reported that the efficacy of intravenous antibiotic was related to significant benefits from the use of ALBC during PTKA. In contrast, an article retrospectively reviewed 22,889 knees using PBC in TKA and 2030 knees using ALBC in TKA.24 PJI rates in the ALBC and PBC groups were 1.4% and 0.7%, respectively (p = 0.002), which revealed that the efficacy of ALBC was worse than that of PBC. Nonetheless, we did not include this study because the sample sizes between the ALBC and PBC groups were very different. And there was significant bias in patient screening. For example, there was more diabetic patients and more patients with American Society of Anesthesiologists classification greater than or equal to three in the ALBC group.
For the prevention of superficial infections, our study showed no significant effect of ALBC, which was consistent with previous studies,10,16 probably because antibiotics in bone cement was difficult to reach the incision surface and thus was not able to accumulate the effective doses to eradicate pathogenic bacteria.
We also evaluated the efficacy of antibiotic type utilized in bone cement. Recently, antibiotic was recommended to be loaded into the cement, although it remained controversial. Our result concluded that bone cement containing gentamicin could significantly reduce the rate of periprosthetic infection following PTKA (p < 0.05). However, bone cement containing tobramycin showed no statistical difference in preventing infection. Gentamicin is possibly used in joint arthroplasty because it is a wide-spectrum antibacterial agent, which has features of low sensitization potential, low protein-binding activity and high water solubility.25 Jiranek et al.26 indicated that staphylococcal species of bacteria were the primary cause of infection in knee replacement, and they reported that gentamicin-loaded bone cement could have bactericidal effects. However, according to Hanssen et al.27, gentamicin was possibly more effective within three months after surgery. Moreover, Eveillard et al.14 demonstrated that using gentamicin-loaded bone cement might be more beneficial than cefuroxime-loaded one, when a high prevalence of methicillin-resistant Staphylococcus aureus is prevalent in a hospital. Additionally, gentamicin has a more unique characteristic, i.e., it is thermally and chemically stable, compared to other antibiotics.28
Our result concluded that high-dose ALBC could significantly decrease the probability of deep incisional PJI following PTKA (p < 0.05). However, low-dose ALBC showed no statistical difference in preventing infection. In fact, adequate antibiotic dose depends on the release of antibiotics in bone cement and veins. Nevertheless, two in vitro articles demonstrated that the release of antibiotic in bone cement was only increased within three days.29,30 In addition, late release level was lower, resulting in antibiotic resistance.29,30 Although the above experiments have raised concerns about antibiotic resistance, we did not find any clinical trials proving the relation between ALBC and bacterial resistance. Hansen et al.18 reviewed the patients who had implanted ALBC during primary TJA, and found that there was no significant addition of bacterial resistance during PJI revision.
Other significant adverse events after the application of ALBC including aseptic loosening in arthroplasties, allergic reaction and toxicity were not reported in the analyzed studies. Although our results indicated that ALBC could significantly reduce deep incisional SSI, the addition of antibiotic in bone cement remains controversial due to additional costs. King et al.7 reported that using PBC instead of ALBC can save at least $155,000 per 1000 TKAs. Gutowski et al.17 found that prophylactic use of ALBC would increase the cost of each PTKA by more than $2112.72. However, Lavernia et al.31 found that it would cost $109,805 to treat PJI in a TKA revision. The hidden commercial cost of ALBC was higher than that of PBC. Compared with the hidden cost of ALBC used in PTKA, a revision TKA was much more expensive. Therefore, it is important to strike an appropriate balance between the cost of using ALBC in PTKA to prevent PJI and the cost of PJI that may occur after PTKA in the future.8
This study has some limitations. Firstly, there are limited eligible long-term randomized controlled trials to evaluate the efficacy of ALBC for preventing PJI. Of all the included studies, only two articles were considered high-quality, of which modified Jadad score were ≥4. Secondly, the concluded articles were conducted in hospitals around the world. There was a variation among studies in the antibiotic dose and type and other approaches to preventing PJI. Therefore, we have fully considered the major aspects to evaluate the efficacy of antibiotic dose and gentamicin in avoiding deep infection with ALBC. Finally, the diagnostic criteria of deep and superficial incisional SSI were not identical in the included articles. Some authors employed the system of Center for Disease Control, while others employed the Musculoskeletal Infection Society Criteria.20
In conclusion, the prophylactic use of ALBC in PTKA can reduce the incidence of deep incisional SSI. Furthermore, bone cement containing gentamicin and high-dose antibiotic can significantly prevent deep infection after PTKA. We concluded that ALBC was a safe and effective measure in preventing deep incisional SSI after PTKA. However, several high-level evidence-based clinical studies comprising a large number of patients were needed for confirmation in the future.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical statement
Not applicable.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Author contributions
Conceptualization: Ke Jie; data curation: Ting Xu, Ke-Liang Wu; risk Assessment: Ting Xu, Ke-Liang Wu; statistical analyses: Ting Xu, Ke-Liang Wu; supervision: Ke Jie; writing-review & editing: Ke Jie, Ting Xu, Ke-Liang Wu.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjtee.2022.06.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bourne R.B. Prophylactic use of antibiotic bone cement: an emerging standard--in the affirmative. J Arthroplasty. 2004;19:69–72. doi: 10.1016/j.arth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Rorabeck C.H. Session IV: salvage of the infected total knee replacement. Infection: the problem. Clin Orthop Relat Res. 2002:113–115. doi: 10.1097/00003086-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Turhan S. Does the use of antibiotic-loaded bone cement have an effect on deep infection in primary total knee arthroplasty practice. Surg Infect. 2019;20:244–246. doi: 10.1089/sur.2018.123. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz H.W., Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg. 1970;41:511–515. [PubMed] [Google Scholar]
- 5.Chiu F.Y., Chen C.M., Lin C.F., et al. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg Am. 2002;84:759–762. [PubMed] [Google Scholar]
- 6.Parvizi J., Saleh K.J., Ragland P.S., et al. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335–341. doi: 10.1080/17453670810016984. [DOI] [PubMed] [Google Scholar]
- 7.King J.D., Hamilton D.H., Jacobs C.A., et al. The hidden cost of commercial antibiotic-loaded bone cement: a systematic review of clinical results and cost implications following total knee arthroplasty. J Arthroplasty. 2018;33:3789–3792. doi: 10.1016/j.arth.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Wang J.X., Zhu C., Cheng T., et al. A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph T.N., Chen A.L., Di Cesare P.E. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg. 2003;11:38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y.Q., Li L.T., Zhou Q., et al. Lack of efficacy of prophylactic application of antibiotic-loaded bone cement for prevention of infection in primary total knee arthroplasty: results of a meta-analysis. Surg Infect. 2015;16:183–187. doi: 10.1089/sur.2014.044. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz E.M., Parvizi J., Gehrke T., et al. International Consensus meeting on musculoskeletal infection: research priorities from the general assembly questions. J Orthop Res. 2018;37:997–1006. doi: 10.1002/jor.24293. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari M., Richards R.R., Sprague S., et al. Quality in the reporting of randomized trials in surgery: is the Jadad scale reliable? Contr Clin Trials. 2001;22:687–688. doi: 10.1016/s0197-2456(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 14.Eveillard M., Mertl P., Tramier B., et al. Effectiveness of gentamicin-impregnated cement in the prevention of deep wound infection after primary total knee arthroplasty. Infect Control Hosp Epidemiol. 2003;24:778–780. doi: 10.1086/502134. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi R., Razak F., Pathy R., et al. Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. J Arthroplasty. 2009;24:1015–1018. doi: 10.1016/j.arth.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Hinarejos P., Guirro P., Leal J., et al. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am. 2013;95:769–774. doi: 10.2106/JBJS.L.00901. [DOI] [PubMed] [Google Scholar]
- 17.Gutowski C.J., Zmistowski B.M., Clyde C.T., et al. The economics of using prophylactic antibiotic-loaded bone cement in total knee replacement. Bone Joint Lett J. 2014;96:65–69. doi: 10.1302/0301-620X.96B1.31428. [DOI] [PubMed] [Google Scholar]
- 18.Hansen E.N., Adeli B., Kenyon R., et al. Routine use of antibiotic laden bone cement for primary total knee arthroplasty: impact on infecting microbial patterns and resistance profiles. J Arthroplasty. 2014;29:1123–1127. doi: 10.1016/j.arth.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Qadir R., Sidhu S., Ochsner J.L., et al. Risk stratified usage of antibiotic-loaded bone cement for primary total knee arthroplasty: short term infection outcomes with a standardized cement protocol. J Arthroplasty. 2014;29:1622–1624. doi: 10.1016/j.arth.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Qiu G.X., Lin J., et al. Antibiotic bone cement cannot reduce deep infection after primary total knee arthroplasty. Orthopedics. 2015;38:462–466. doi: 10.3928/01477447-20150603-52. [DOI] [PubMed] [Google Scholar]
- 21.Wu C.T., Chen I.L., Wang J.W., et al. Surgical site infection after total knee arthroplasty: risk factors in patients with timely administration of systemic prophylactic antibiotics. J Arthroplasty. 2016;31:1568–1573. doi: 10.1016/j.arth.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Sanz-Ruiz P., Matas-Diez J.A., Sanchez-Somolinos M., et al. Is the commercial antibiotic-loaded bone cement useful in prophylaxis and cost saving after knee and hip joint arthroplasty? The transatlantic paradox. J Arthroplasty. 2017;32:1095–1099. doi: 10.1016/j.arth.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Engesaeter L.B., Lie S.A., Espehaug B., et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644–651. doi: 10.1080/00016470310018135. [DOI] [PubMed] [Google Scholar]
- 24.Namba R.S., Chen Y.X., Paxton E.W., et al. Outcomes of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty. 2009;24:44–47. doi: 10.1016/j.arth.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Bertazzoni Minelli E., Caveiari C., Benini A. Release of antibiotics from polymethylmethacrylate cement. J Chemother. 2002;14:492–500. doi: 10.1179/joc.2002.14.5.492. [DOI] [PubMed] [Google Scholar]
- 26.Jiranek W.A., Hanssen A.D., Greenwald A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 27.Hanssen A.D., Osmon D.R. The use of prophylactic antimicrobial agents during and after hip arthroplasty. Clin Orthop Relat Res. 1999:124–138. doi: 10.1097/00003086-199912000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Wahlig H., Dingeldein E. Antibiotics and bone cements. Experimental and clinical long-term observations. Acta Orthop Scand. 1980;51:49–56. doi: 10.3109/17453678008990768. [DOI] [PubMed] [Google Scholar]
- 29.van de Belt H., Neut D., Schenk W., et al. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop Scand. 2000;71:625–629. doi: 10.1080/000164700317362280. [DOI] [PubMed] [Google Scholar]
- 30.Dunne N., Hill J., McAfee P., et al. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007;78:774–785. doi: 10.1080/17453670710014545. [DOI] [PubMed] [Google Scholar]
- 31.Lavernia C., Lee D.J., Hernandez V.H. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446:221–226. doi: 10.1097/01.blo.0000214424.67453.9a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.