Abstract
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a rare inherited disorder characterized by progressive loss of kidney function, nonsignificant urinalysis and tubulointerstitial fibrosis. ADTKD progresses to end stage renal disease (ESRD) in adulthood. The classification of ADTKD is an evolving concept and the agreement is now that, due to the overlap in terms of phenotype characteristics, this should be based on the involved gene. The umbrella term ADTKD therefore includes different conditions as follows: ADTKD-UMOD, ADKTD-MUC1, ADTKD-REN, and ADTK-HNF1B, with ADTKD-SEC61A1 and ADTKD-DNAJB11 as a further rare and atypical diagnosis recently described.
The employment of next-generation sequencing (NGS) as a diagnostic tool in patients with familial kidney disease has improved the diagnostic accuracy in this field with ADTKD now being considered the third genetic cause of renal disease worldwide after autosomal dominant polycystic kidney disease (ADPKD) and Alport syndrome.
On average, the disease pathogenesis is similar across the different subtypes, With the exception of HNF1B, the different mutated genes give rise to misfolded proteins leading to cellular stress and cytotoxicity. Research is now focused in better defining the underlying mechanism of fibrosis to guide therapeutic interventions.
The aim of this review is to discuss how the knowledge of ADTKD has evolved in the last decades, with emphasis on the clinical features, molecular diagnosis, and pathogenic aspects of the different diseases included under the ADTKD term.
Keywords: ADTKD, DNAJB11, HNF1B, MUC1, REN, UMOD
ADTKD is a rare genetic renal disorder characterized by nonsignificant urinalysis and tubulointerstitial fibrosis, in the absence of significant glomerular lesions, leading to ESRD in adulthood.1,2
In the last century, the most frequent term to describe this clinical entity was medullary cystic kidney disease (MCKD), first introduced in the 1945 by Smith, who described a girl with anemia, medullary cysts and uremia.3 In the 1970s, due to clinicopathologic similarities with nephronophthisis (NPH), MCKD was considered to be part of the same disease spectrum, named NPH-MCKD complex, despite some important differences such as age of onset of the renal disease (childhood in NPH; adulthood in MCKD), extrarenal involvement (tapeto-retinal degeneration in NPH; gout in MCKD), and inheritance pattern (dominant in MCKD; recessive in NPH).4,5 In the 1990s, the identification of a different genetic background for MCKD and NPH definitively clarified the 2 entities. Most recessive NPH cases carried a homozygous deletion of the NPHP1 gene6 and additional causing genes were subsequently identified; all NPH genes encode for proteins of the primary cilia.7 In the same decade, 2 loci for autosomal dominant MCKD, MCKD1 and MCKD2, were mapped on chromosome 1q21 and chromosome 16p12, respectively.8,9 Of interest, a locus for FJHN1, a clinical phenotype very similar to MCKD, was mapped on 16p12 overlapping the MCKD2 locus.10 In 2001, UMOD disease variants were found to be associated with both FJHN1 and MCKD2, providing evidence that MCKD2 and FJHN1 were allelic disorders.11
Of note, the genetic cause of MCKD1 remained unidentified for a long time and only in 2013 were variants of the MUC1 gene detected as a cause for this disease.12 To add complexity, a wide genetic heterogeneity of FJHN was subsequently discovered as follows: FJHN2, caused by pathogenic variant in the REN13; FJHN3, mapped to chromosome 2p22.1-p21 (gene not yet identified)14; and FJHN4 caused by pathogenic variants in the SEC61A1 gene.15 Finally, an atypical form of FJHN, associated with renal cysts and diabetes, was found to be caused by pathogenic variant in the HNF1B gene.16
Because the common feature of these diseases is tubulointerstitial fibrosis, the unifying definition of ADTKD was proposed (Figure 1). In 2015, a subclassification, according to the detected pathogenic variants in the 4 known causative genes (ADTKD-UMOD, ADTKD-MUC1, ADTKD-REN, ADTKD-HNF1B) was adopted, following a consensus KDIGO conference.1 After this KDIGO conference, other rare ADTKD genes were identified, namely SEC61A1 and DNAJB11, the latter being associated with a phenotype overlapping ADPKD and ADTKD.2,15 Because pathogenic variants in UMOD, MUC1, REN, and HNF1B are detected only in about 50% of the ADTKD probands, other causative genes are yet to be identified; for such cases the term ADTKD-not otherwise specified for “not otherwise specified” was proposed.
Figure 1.
Shared and hallmark clinical features of the different types of ADTKD. ADTKD, autosomal dominant tubulointerstitial kidney disease; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; ESRD, end stage renal disease; NDD, neurodevelopmental delay; yo, year.
For an uncommon disease such as ADTKD, the prevalence is difficult to establish because cases are few and hard to diagnose. Recent new molecular diagnostic approaches with NGS analysis and large international cohort studies led to the identifying new cases of ADTKD. It is estimated that ADTKD-UMOD alone accounts for 3% of genetic cause of renal disease worldwide, ranking as the third genetic cause of nephropathy after ADPKD and Alport syndrome.17 Moreover, ADTKD-MUC1 is the second most prevalent ADTKD form, thus, considering the difficulty of identifying MUC1 pathogenic variants through genetic testing because it escapes NGS techniques, total ADTKD prevalence could be higher.18
In this review we will discuss genetic, clinical features and disease mechanisms of the following 6 different forms of ADTKD, further classified according to the underlying genetic defect: ADTKD-UMOD, ADTKD-MUC1, ADTKD-REN, ADTKD-HNF1B, ADTKD-SEC61A1, and ADTKD-DNAJB11. Common and disease-specific clinical features of known ADTKD forms are illustrated in Figure 1 and 2.
Figure 2.
Age at onset of renal and extrarenal manifestation in different types of ADTKD according to the mutated gene. ADTKD, autosomal dominant tubulointerstitial kidney disease; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; ESRD, end stage renal disease; RTI, respiratory tract infections; yo, year.
ADTKD-UMOD
Previously known as FJHN1 or MCKD2, ADTKD-UMOD (OMIM #162000) is the most frequent form of ADTKD. A nation-wide epidemiologic survey conducted in Austria revealed a prevalence of 1.7 cases of ADTKD-UMOD per million in the population.19 In a recent large exome-sequencing study, 0.3% of patients with chronic kidney disease (CKD) were found to have ADTKD-UMOD disease.17 Recently, data from 2 large ADTKD registries, including 726 ADTKD patients suggested that ADTKD-UMOD is the most frequent subtype of ADTKD, showing a prevalence of 37%.20
Clinical Manifestations
ADTKD-UMOD patients usually develop slowly progressive asymptomatic elevations of serum creatinine concentration between the second and the fourth decade of life. Urinalysis is negative with bland urinary sediment and mild proteinuria.2 A defective urinary concentration is present although not clinically relevant. Histology is usually unspecific and may mimic focal segmental glomerulosclerosis21; electron microscopy may reveal uromodulin accumulation as large intracellular deposits (fibrillar or amorphous material) in the endoplasmic reticulum (ER) of the thick ascending limb (TAL) of Henle’s loop22,23 (Figure 3). Ultrasonography may reveal renal cysts whereby the kidney size is usually normal but declines with disease progression.2 Heterogeneity in ESRD age of onset has been reported. In a recent large international study, the median age at onset was 47 years (range 18-87) and women had significantly better renal survival.24 In the ADTKD cohort, including the European and USA ADTKD registries, the median age at onset of ESRD was 54 years (range 25 to >70 years); prognosis was confirmed to be worse in men with a more rapid progression to ESRD.20 Hyperuricemia is frequent (70–80% of patients) and usually precedes CKD. Gout develops at a median age of 27 years and more frequently affects men, with a prevalence variable from 24% to >70% of patients.18,20,24, 25, 26
Figure 3.
ADTKD-UMOD kidney biopsy. (a) Light microscopy shows lymphocytic infiltrate associated with interstitial fibrosis and tubular atrophy (hematoxylin and eosin stain). (b) Immunohistochemical examination with anti-UMOD antibody shows intracellular aggregates of mutant UMOD in tubular cells.
Diagnosis
The definitive diagnosis can be established by genetic testing showing a heterozygous pathogenic variant in UMOD gene (11 exons).1 In the past, molecular testing approach included single-gene testing performed by Sanger sequencing. Recently, NGS analysis of a multigene panel, including UMOD and other genes of interest have been more frequently employed (Table 1). To date, >130 pathogenic variants in UMOD gene have been described with the most frequently reported being missense changes, clustered in exons 3 and 4, resulting in the replacement of cysteine residues and leading to misfolding of the uromodulin protein (Table 1).2,18,24
Table 1.
Genetics and molecular diagnosis of ADTKD
| Gene | OMIM# | Chr | Exons | Pathogenic variants | Molecular analysis methods |
|---|---|---|---|---|---|
| UMOD | 191845 | 16p12.3 | 11 | Single nucleotide variants: - 95.3% missense pathogenic variants - 89.6% located in exon 3 |
Sanger sequencing and/or NGS analysis |
| MUC1 | 158340 | 1q22 | 8 | Mostly a cytosine duplication within a domain of VNTR leading to a frameshift pathogenic variant | Mass spectometry or SNaPshot mini-sequencing |
| REN | 179820 | 1q32.1 | 10 | Single nucleotide variants in the prosegment, signal peptide and mature peptide | Sanger sequencing and/or NGS analysis |
| HNF1B | 189907 | 17q12 | 9 | 42% sequence substitutions 49% whole gene deletions 6% duplications |
Sanger sequencing and/or NGS and MLPA or copy number variation analysis |
| SEC61A1 | 617056 | 3q21.3 | 12 | Single nucleotide variants | Sanger sequencing and/or NGS analysis |
| DNAJB11 | 618061 | 3q27.3 | 10 | Single nucleotide variants | Sanger sequencing and/or NGS analysis |
ADTKD, autosomal dominant tubulointerstitial kidney disease; Chr, chronosome; MLPA, Multiplex Ligation-dependent Probe Amplification; NGS, next-generation sequencing; VNTR, variable number of tandem repeats.
Disease Mechanism
UMOD is a kidney-specific protein, produced by the epithelial cells in the TAL of Henle and by the initial part of the distal convoluted tubule.27,28 UMOD plays a key role in water homeostasis. In the tubule, UMOD polymerizes into gel-like structures contributing to water permeability of TAL; moreover, UMOD regulates the activity of the renal outer medullary potassium channel29 and of the sodium-potassium-chloride cotransporter.30 Of note, by inhibiting calcium oxalate crystallization, UMOD exerts a protective role against nephrolithiasis31 and its biochemical properties make it a candidate for being a host defense factor involved in clearing bacteria from the urinary tract.32 UMOD seems also to have an immunomodulatory function, trapping pro-inflammatory molecules and modulating neutrophilic migration in the setting of acute kidney injury.33,34 Finally, recent studies suggest that serum and urinary levels of UMOD can be considered surrogate markers of kidney function and nephron mass.35,36
UMOD disease-causing variants affect the biosynthesis of the protein, leading to protein misfolding, aberrant intracellular trafficking and ER retention of the mutant protein, supporting the designation of ADTKD-UMOD as a storage disease.22,23 In renal biopsy of ADTKD-UMOD patients UMOD accumulates in large intracellular aggregates that colocalize with ER markers, leading to expansion of ER stacks.22,23 Intracellular retention is consistent with the reduced level of uromodulin in urine and blood of ADTKD-UMOD patients.37, 38, 39 Several in vitro and in vivo models of mutant uromodulin expression have clearly established ER retention of mutant uromodulin as the primary effect of UMOD pathogenic variants. In particular, several models reported the induction of ER stress and of the unfolded protein response (UPR), a cellular response aimed at restoring ER homeostasis.38, 39, 40, 41, 42, 43 The accumulation of the misfolded protein leading to chronic ER stress of TAL cells is likely to be at the basis of the observed defect in mitochondria biogenesis and function and of a maladaptive suppression of autophagy.42 In all mouse models of ADTKD-UMOD, ER retention and aggregation of mutant uromodulin is followed by progressive renal damage, i.e., tubulointerstitial fibrosis with inflammatory cell infiltration and tubule dilation, urinary concentrating defect, and renal failure, hence recapitulating the clinicopathologic features of ADTKD-UMOD.39,42,44 This phenotype is not reproduced in UMOD-knockout mices establishing the mechanism of action of UMOD pathogenic variants as mostly a gain-of-toxic function.45 This effect could be exerted intracellularly due to ER retention but also extracellularly. Mutant uromodulin excretion was indeed detected in patients carrying uromodulin pathogenic variants and the presence of mutant uromodulin on the plasma membrane was shown to interfere with the polymerization of the wild-type isoform through formation of large extracellular aggregates.46 Nevertheless, a loss-of-function effect possibly secondary to loss of the integrity of TAL cells, may determine a defect in urinary concentration and a reduction in sodium reabsorption, leading to hypovolemia. The volume depletion can subsequently increase the reabsorption of uric acid in the proximal tubule, causing a lower fractional excretion of urate and hyperuricaemia, as observed in UMOD knockout mice.45
Though the primary effect of UMOD pathogenic variants (ER retention and ER stress) has been largely documented, its downstream effects are less known. Inflammation is likely to play an important role in disease onset because inflammatory markers have been recognized in ADTKD mouse model at very early stage well before the induction of fibrosis and renal damage.47
Onset and progression of the disease in mouse models depend on the pathogenic variant type and its allelic status.40 In human,s there is high interfamilial and intrafamilial variability of clinical parameters, such as the age at ESRD, making it difficult to establish a clear genotype-phenotype correlation. Nevertheless, gene dosage effect seems to be present also in humans, because the few individuals carrying UMOD pathogenic variants in homozygosity present a more severe disease.48 Moreover, a recent study analyzing the clinical characteristics associated with 125 UMOD pathogenic variants identified the male sex and the severity of trafficking defect of mutant uromodulin (scored in cell models) as risk factors for earlier age at ESRD.24
Management
No specific therapy is available for ADTKD-UMOD. Treatment includes management of gout and of CKD. No conclusive evidence suggests a benefit on CKD progression of allopurinol, which can prevent gouty arthritis attacks. The management of hypertension, hyperphosphatemia, anemia, and ESRD is similar to that proposed to patients with other causes of CKD. ADTKD patients are good candidates for renal transplantation, because the genetic disease does not recur.1,2 Only few trials of pre-clinical therapeutic interventions have been reported so far. Interestingly disease progression was reduced in a knock-in mouse model of ADTKD-UMOD after anti-inflammatory treatment with TNFR:Fc, a tumor necrosis factor-α inhibitor,41 supporting a role for inflammation in disease onset and progression. More recently, BRD4780, a small molecule first identified for ADTKD-MUC1 treatment (see below), was shown to induce mutant uromodulin degradation in cellular models.49 The effectiveness of this molecule still needs to be tested in vivo.
ADTKD-MUC1
The true prevalence of ADTKD-MUC1, previously known as MCKD1, is difficult to estimate, as pathogenic variants in MUC1, not detected by NGS analysis, require specialized genetic testing not routinely performed.2 For these technical difficulties, the disease was mapped in 1998 on chromosome 1q21,8 but the causative gene MUC1 (7 exons) was identified only 15 years later.12 According to the 2 large ADTKD registries from Europe and United States, ADTKD-MUC1 is the second most frequent form of ADTKD, representing 35% of UMOD-negative families, with an estimated overall prevalence of 21%.20
Clinical Manifestations
Main clinical features are indistinguishable from ADTKD-UMOD, including slowly progressive CKD, nonsignificant urinalysis, dominant inheritance, and hyperuricemia or gout.1,2 In the first large study performed, Bleyer et al. Described a variable age of onset of ESRD (mean age 45 years; range 16–80 years) and a rapid progression to dialysis in the third decade of life.50 In the cohort derived from European and USA ADTKD registries, the renal disease appeared to be more severe in patients with ADTKD-MUC1 when compared with ADTKD-UMOD, with higher prevalence of ESRD (58% vs. 44%) and earlier onset of ESRD (36 years vs. 46 years). In addition, gout was less frequent in ADTKD-MUC1 (26%) compared with ADTKD-UMOD (79%).20
Diagnosis
The diagnosis of ADTKD-MUC1 is based on the identification of a heterozygous MUC1 pathogenic variant. The molecular testing for ADTKD-MUC1 is challenging because the mutational hotspot is a large variable number of tandem repeats of MUC1 gene that escapes detection by standard sequencing techniques.12 The most common pathogenic variant is a cytosine duplication within a stretch of 7 cytosines within 1 unit of variable number of tandem repeats sequence. Other mutations involve the addition of a guanosine residue or loss of 2 cytosine residues. All disease-causing variants result in the same frameshift (fs) change leading to the synthesis of a frameshifted protein that is prematurely terminate (MUC1-fs). MUC1 pathogenic variants are not identified using gene panels or whole exome sequencing and alternative methods of detection (mass spectrometry or SnaPshot mini-sequencing) have been developed (Table 1).12,51, 52, 53 Unfortunately, current MUC1 testing techniques are able to identify only the most frequent pathogenic variant that involves cytosine but not the others. To our knowledge, MUC1 testing is available in few laboratories in Europe and US (Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States; First Faculty of Medicine, Charles University, Prague, Czech Republic; Fundaciò Puigvert, Barcelona, Spain; and Laboratorio di Biochimica Clinica, Azienda Ospedaliera Universitaria di Novara, Italy).
An alternative nongenetic disease diagnostic tool has been suggested, using immunohistochemistry for the specific detection of the MUC1-fs peptide on kidney biopsy specimens or on urinary exfoliated cells of patients. The immunodetection of MUC1-fs could be applicable to selected patients for molecular diagnosis and can provide diagnosis in patients in whom the pathogenic variant is different from the cytosine insertion as well as in renal transplant patients, because mucin 1 is also produced by the bladder cells of transplant recipient.54,55
Disease Mechanism
The MUC1gene encodes mucin1, a transmembrane glycoprotein expressed on the apical surface of many epithelial cells, including breast, lung, intestine, and kidney, providing a protective barrier.2 Mucin1 is also involved in cellular signaling.2 In the kidney, mucin 1 is expressed on TAL, distal convoluted tubule, and collecting duct.2,56 The abnormal MUC1-fs neo-peptide accumulates in the cytoplasm of tubular epithelial cells, where it accelerates cell death, determining tubulointerstitial fibrosis and CKD.2,57 All MUC1 pathogenic variants lead to the same aberrant protein, suggesting that MUC1-fs protein is central in the pathogenesis. Because knockout Muc1 mices are characterized by the absence of a developmental phenotype,2,58 ADTKD-MUC1 is likely caused by a gain-of-function effect of pathogenic variants. How the abnormal MUC1-fs protein causes progressive tubulointerstitial renal damage is poorly understood, as well as why the pathogenic variant produces an abnormal phenotype only in the kidney, despite its presence in several tissues. An important step toward the elucidation of the mechanisms of pathogenesis in ADTK—MUC1 was recently achieved in in vitro and in vivo models of MUC1-fs expression, demonstrating that MUC1-fs accumulates intracellularly in the ER-Golgi intermediate compartment, leading to activation of the ATF6 branch of the unfolded protein response. Through small molecule screening, a new lead molecule, BRD4780, was identified, able to selectively bind TMED9 receptor, which has a key role in the retention of MUC1-fs in the ER-Golgi intermediate compartment. The binding BRD4780-TMED9 releases MUC1-fs that is re-routed toward the lysosome for degradation, thus clearing MUC1-fs from the cells.49
Management
No specific therapy is available for ADTKD-MUC1. The newly identified lead molecule BRD4780 which, by selectively binding TMED9, induces the removal of mutant protein MUC1-fs could represent a potential approach for ADTKD-MUC1. Importantly, this molecule was found to be effective (at least in vitro) in other toxic proteinopathies characterized by intracellular retention of misfolded proteins, such as ADTKD-UMOD.49
ADTKD-REN
ADTKD-REN, previously known as FJHN2, is due to disease-causing variants in the REN gene (10 exons, chromosome 1).2,59 The human renin precursor is synthesized in the juxtaglomerular cells in the juxtaglomerular apparatus of the kidney as a 406 amino acid pre-prorenin, composed of a 23 amino acid N-terminal signal peptide, a 43 amino acid prosegment, and a 340 amino acid mature renin peptide. Renin has a key role in the renin-angiotensin-aldosterone system, which exhibits multiple biological functions, including vascular tone modulation, renal sodium and potassium handling, erythropoiesis, and cardiac hypertrophy.60 ADTKD-REN is a very rare condition because so far, 30 families, including 111 affected individuals have been described.61
Clinical Manifestations
ADTKD-REN is a condition with onset in childhood; rarely, REN pathogenic variants can cause an adult, milder form of the disease.61,62 Most clinical, laboratory, ultrasonographic, and histologic findings are nonspecific, except for a decreased immunostaining for renin in the juxtaglomerular apparatus.2 Nevertheless, some features appear to be relatively characteristic, being attributed to a decreased secretion of renin. Due to low renin and aldosterone levels, ADTKD-REN patients may experience some degrees of hypotension and hyperkalemia.60, 61, 62 Children are at increased risk of acute kidney injury, particularly in case of volume depletion and use of NSAIDs, as a consequence of the combination of low renin activity, volume depletion, and prostaglandin inhibition.2,61 Recently, a large international retrospective cohort provided clinical and genetic data on 111 individuals from 30 families with heterozygous REN disease-causing variants; 69 patients harbored a REN pathogenic variant in the signal peptide region; 27 in the prosegment; and 15 in the mature renin peptide. Differences in the severity of the clinical manifestation of the disease were observed according to the position of the pathogenic variant. Patients harboring the pathogenic variant in the signal peptide and prosegment region were most severely affected, with clinical onset during childhood of kidney failure and anemia. In these groups most patients have estimated glomerular filtration rate <60 ml/min per 1.73 m2 at earliest presentation, suggesting that decreased kidney function is present at birth and therefore can be noted in the first weeks of life. Despite early onset, childhood and adolescence renal function tended to be relatively stable, although decreased.61 The presence of hypoproliferative anemia is thought to be secondary to low erythropoietin levels. Interestingly, anemia resolves as the child enters adolescence, probably due to the rise in sex steroid production enhancing erythropoietin production.2,61 Patients with REN disease-causing variants in the mature peptide showed a milder course of the disease with gout in early adulthood or CKD later in life.62 Differences in the median age at ESRD were also observed among the 3 groups of patients, with patients with pathogenic variants in the signal peptide and prosegment reaching ESRD earlier (57 years and 62 years, respectively) than the patients with pathogenic variants located in the mature protein (68 years).61
Diagnosis
Findings suggestive of ADTKD-REN are very early onset of CKD, which can be found at 3 or 4 years of age or even earlier; hypoproliferative anemia beginning early in life (1 year or 3 years) and resolving during adolescence; and hyperuricemia and gout, usually in the second decade of life. Urinalysis reveals a bland urinary sediment and low degree proteinuria. Renal ultrasonography is nondiagnostic, showing normal or small kidneys. Plasma renin and plasma aldosterone are low; serum potassium is mildly elevated.2,61 Though renal biopsy may show nonspecific features of tubulointerstitial fibrosis, immunostaining for renin in the granular cells of the juxtaglomerular apparatus may be decreased.2,13,61 The final diagnosis may be established by genetic testing. Because pathogenic REN disease-causing variants are mostly single nucleotide variants, standard methods of genetic testing may be used, including both Sanger and NGS sequencing (Table 1).2,61,62 To date, a total of 16 disease-causing variants have been identified as follows: 8 (50% of total pathogenic variants, 70% of the families) map in the signal peptide; 3 in the prosegment (19% of total pathogenic variants, 13% of the families); and 5 (31% of total pathogenic variants, 17% of the families) in the mature part of renin.61
Disease Mechanism
Renin is synthesized as pre-prorenin that is translocated into the ER where it is processed to prorenin by cleavage of the signal peptide. In addition to the signal peptide (which directs the insertion of the nascent pre-prorenin into the translocation channel in the ER), pre-prorenin contains a prosegment, located after the signal peptide that assists in protein folding and the mature renin peptide.2,60 Homozygosity or compound heterozygosity for loss-of-function REN pathogenic variants causing complete loss of renin synthesis were first described in 2005 in autosomal recessive renal tubular dysgenesis, a very rare condition leading to perinatal mortality.63 In 2009, Zivná et al.13 reported heterozygous dominant pathogenic variants in the REN gene, resulting in reduced prorenin and renin biosynthesis and secretion. In this setting, production of wild-type renin occurs via 1 allele, enabling kidney development but causing symptoms of low renin activity, such as mild hypotensive state, hyperkalemia, anemia and renal tubulointerstitial fibrosis.13,64 The molecular mechanisms of renal fibrosis are not fully understood. Pathogenic variants affecting the signal sequence are associated with an impaired co-translational insertion in the ER of the mutated pre-prorenin, which is aberrantly located in the cytoplasm.2,13,59,61 Mutant renin due to pathogenic variants affecting the mature renin is retained in the ER, likely due to protein misfolding, and induces ER stress and activation of the unfolded protein response in vitro that may possibly lead in vivo to accelerated cell death, tubulointerstitial fibrosis, and progressive loss of kidney function.2,59,62 REN pathogenic variants in the prosegment led to deposition of prorenin and renin in the ER-Golgi intermediate compartment, decreasing prorenin secretion.2,59,61
Management
In ADTKD-REN patients, in addition to the management of progressive CKD and gout, anemia, hypotension and hyperkalemia can be treated with erythropoiesis-stimulating agents and fludrocortisone.2,64 Moreover, considering that part of renin pathogenic variants lead to prorenin accumulation in the early secretory pathway, similarly to MUC1-fs, a potential role for BRD4780 in this context may have a rational.49
ADTKD-HNF1B
HNF1B gene is a developmental gene located on chromosome 17q12, coding for hepatocyte nuclear factor 1 homeobox B protein, which is required for tissue specific gene expression in the epithelial cells of many organs, including kidney, pancreas, liver, and genitourinary tract. Heterozygous pathogenic variants of HNF1B gene are responsible for a dominant inherited disease with several renal and extrarenal phenotypes as exocrine pancreatic failure, pancreatic hypoplasia, fluctuating liver test abnormality, early-onset gout, and genital tract malformations.1,2,16 HNF1B-nephropathy is the umbrella term used to include the various kidney phenotypes of the disease, ranging from congenital anomalies of the kidney and urinary tract, tubular transport abnormalities, cystic renal disease and ADTKD.65, 66, 67, 68, 69
Clinical Manifestations
HNF1B-disease is a condition with renal phenotype varying according to the age at recognition. The prevalence of HNF1B pathogenic variants in children with structurally abnormal kidneys is approximately 20%. In the fetus, the most frequent phenotype is hyperechogenic kidneys.68 In childhood, HNF1B-nephropathy usually shows renal cystic hypodysplasia, congenital anomalies of the kidney and urinary tract and tubular transport disorders (hypomagnesemia, hypokalemia).68,70 In the adult, the renal disease manifests as ADTKD, associated with hyperuricemia and gout, and varying degrees of renal impairment, from mild slow progressive CKD to ESRD.68,69 The renal phenotype described in adulthood is very similar to the one observed in patients with ADTKD-UMOD, ADTKD-MUC1, and ADTKD-REN. Nevertheless, whereas the clinical manifestations of diseases caused by pathogenic variants in UMOD, MUC1, and REN are usually confined to the kidney, HNF1B pathogenic variants can result in variable and associated renal manifestations, including congenital anomalies of the kidney and urinary tract, renal cysts, hypomagnesemia and hypokalemia. Therefore, some authors suggested to confine the term ADTKD only to those rare HNF1B-related cases in which ubule-interstitial fibrosis is the leading renal manifestation.1 A large spectrum of extrarenal manifestations can be associated, such as MODY5, pancreatic hypoplasia, liver test abnormalities, and genital tract malformations.65, 66, 67, 68 Neurodevelopmental disorders have also been described, particularly in patients with chromosome 17q12 deletion encompassing HNF1B gene.2,67,68
Recently the mnemonic MAGIC LUCID has been suggested to summarize the possible clinical manifestations of HNF1B disease, including renal and extrarenal manifestations (M= Hypomagnesemia; A= Autosomal dominant; G= Genital tract abnormalities, including bicornuate uterus, absent uterus, vaginal hypoplasia; I= Incomplete penetrance; C= Cysts of the kidney and other structural abnormalities, including multicystic kidneys, fetal bilateral hyperechogenic kidneys, kidney agenesis, and hypoplastic kidneys; L= Liver test abnormalities; U= Uric acid elevation; C= Chronic kidney disease; I= Inherited; D= Diabetes and pancreatic anomalies.).71
Diagnosis
Clinical diagnosis of HNF1B-nephropathy is challenging, because the disease may mimic a variety of renal disorders. The presence of extrarenal manifestations is an important tool for early diagnosis. Although the association of MODY with a heterogenous clinical kidney disease is the core phenotype of HFN1B-associated disease, a patient can present only 1 of the possible clinical features. Moreover, it is possible to observe in the same family a different clinical characteristic for each member. The extreme variability, also intrafamilial, and incomplete penetrance of clinical features makes diagnosis of HNF1B-disease even more challenging.2,65, 66, 67, 68, 69 For the final molecular diagnosis, conventional sequence analysis for point pathogenic variants should be combined with Multiplex Ligation-dependent Probe Amplification or copy number variation analysis, to identify whole gene deletion, which is the molecular defect identified in 40% to 50% of patients, occurring also in the context of the 17q12 Recurrent Deletion Syndrome.2,67 In the remaining cases, point pathogenic variant can be detected, 50% being missense (Table 1). Interestingly, de novo pathogenic variants are up to 30% to 50% of cases; thus, the presence or not of a positive family history is less significant for diagnosis than in other forms of ADTKD.2,67
Mechanisms of Disease
HNF1B coordinates transcriptional networks regulating nephrogenesis, renal tubular ion transport, and transcription of numerous cystic disease genes (PKHD1, PKD2, UMOD, and GLIS2).2,67,72 This may explain, in patients with HNF1B gene anomalies, the frequent occurrence of congenital anomalies of the kidney and urinary tract, renal cystic disease, hypomagnesemia and hypokalemia. The mechanism whereby mutated HNF1B leads to renal fibrosis is not fully understood.73 However, recent data showed that HNF1B is implicated in epithelial–mesenchymal transition,74 a process by which epithelial cells acquire mesenchymal characteristics. Epithelial–mesenchymal transition is essential for tissue regeneration and, when sustained, is associated with fibro-genesis. Because 50% of ADTKD-HNF1B patients are heterozygous for full gene deletion, and the remaining have truncating or missense pathogenic variants,2 ADTKD-HNF1B phenotype is likely to be caused by haplo-insufficiency.
Management
In ADTKD-HNF1B patients, management should pay attention to extrarenal manifestations, including pancreatic dysfunction, genital tract malformations, and neuropsychiatric symptoms. Basic knowledge on how to modulate HNF1B expression or activity is still very limited. Strategies aiming at increasing wild-type allele expression or activity could be very valuable in view of therapeutic intervention, because nowadays no specific therapy is available.
ADTKD-SEC61A1
ADTKD-SEC61A1 (previously FJHN4) is a very rare disease, being reported in 2 families.15 Two additional families with SEC61A1 disease-causing variants have also been reported with primary antibody deficiency and unknown renal phenotype.75
Clinical Manifestations
In 2016, Bolar et al. described 2 ADTKD families harboring missense pathogenic variants in SEC61A1 gene, disrupting the function of the translocon pore.15 The first family presented slow progressive ADTKD, anemia, hyperuricemia, and several extrarenal phenotypes. Renal ultrasound revealed small cystic dysplastic kidneys. Renal biopsy showed multiple foci of tubulointerstitial lesions and glomerular sclerosis. The affected members of the second ADTKD-SEC61A1 family presented CKD, anemia, neutropenia, and early gout. In 2018, Schubert et al.75 described 2 families with heterozygous SEC61A1 pathogenic variants showing hypogammaglobulinemia and early-onset respiratory tract infections; the renal phenotype was not provided.
Diagnosis
ADTKD-SEC61A1 should be considered when, in addition to the presence of ADTKD, anemia, neutropenia, and recurrent respiratory tract infections are observed.2 The confirmatory diagnosis must be obtained by the demonstration of the underlying genetic defect of SEC61A1 gene, using Sanger or NGS sequencing (Table 1).2,15,75
Mechanism of Disease
SEC61A1 gene (chromosome 3q21.3) encodes the subunit alpha 1 of the heterotrimeric protein-conducting channel SEC61, also, including beta (SEC61B) and gamma (SEC61G) subunits. SEC61A1 is the major component of the mammalian translocon, a complex needed to transport newly synthesized secretory proteins into the ER.2,75 The pathogenic variants affect the selectivity and permeability of the pore of the translocon channel, leading to SEC61-channelopathy. In vitro studies showed that altered structural properties of SEC61A1 induced by pathogenic variants cause aggregation of the mutated SEC61α in the ER and misrouting to the Golgi apparatus, leading to alterations in post-translational modifications and folding of various secretory and transmembrane proteins, including uromodulin, mucin1, and renin.15,76 A recent study demonstrated that ADTKD-SEC61A1 mutations lead to a signal peptide dependent impairment of protein ER translocation, renin being one of the defective substrates, and to a reduction of regulatory calcium transporters (i.e. Ora1 and SERCA2) interacting with the SEC61 complex.77 These events may induce ER stress (as in ADTKD-REN, ADTKD-UMOD, and ADTKD-MUC1), leading to apoptosis and ultimately to interstitial fibrosis.15
Finally, animal studies (zebrafish) suggested that the SEC61 complex and its translocon function are necessary for normal renal development.15 Of note, a similar phenotype consisting of pronephros development defect was also observed on expression of renin mutants associated with ADTKD.62 Taken together, these data expand the genetic spectrum of the tubulointerstitial kidney disorders, allowing to include pathogenic variants of SEC61A1 (determining protein translocation defects across the ER membrane) as a possible cause of ADTKD.
Management
No specific therapy is available for ADTKD-SEC61A1. Recently, it has been shown that treatment with the small molecule sodium phenylbutyrate (an US Food and Drug Administration approved drug for treatment of urea cycle disorders) may reverse the impairment of renin transport in ADTKD-SEC61A1, suggesting that phenylbutyrate could be a potential early therapy for ADTKD-SEC61A1.77
ADTKD-DNAJB11
ADTKD-DNAJB11 is an atypical and very rare cause of tubulointerstitial kidney diseases. Recently 7 families were reported with 5 different heterozygous pathogenic variants in DNAJB11 gene. The transmission pattern is consistent with autosomal dominant inheritance. Renal phenotype in affected family members mostly overlap clinical features of ADPKD with bilateral small renal cysts, slightly enlarged kidneys, liver cysts, and slowly progressive renal failure with ESRD in the sixth to seventh decade. In some patients, renal histologic examination revealed tubulointerstitial fibrosis and in 1 family at least 3 patients showed gout. Thus, the authors suggested aphenotype partly overlapping with ADPKD and ADTKD.78 Due to overlapping clinical phenotype with cystic kidney diseases, the diagnosis could be established only with genetic analysis confirming the presence of pathogenic heterozygous variant in DNAJB11 gene, using Sanger or NGS sequencing (Table 1).2,15,78
Mechanism of Disease
The DNAJB11 gene encodes a cofactor of GRP78/BiP, a major ER chaperone regulating folding, trafficking and degradation of secreted and membrane protein, thus playing a major role in ER homeostasis.
Evidence linking DNAJB11 with ADPKD and ADTKD have been found. Studies of DNAJB11-null human renal cortical tubular epithelial cells showed a defective maturation process of the PKD1 protein. Moreover, histologic analysis of renal samples from affected patients revealed abnormal intracellular retention of uromodulin and mucin1 linking DNAJB11 disease with pathogenic mechanism of tubulointerstitial disease.78
Genetic Counseling
ADTKD is a Mendelian autosomal dominant disorder where at-risk individuals have a 50% chance of inheriting the disease. Genetic counseling should be offered to all patients to discuss potential risks to offspring, reproductive options, possible genotype-phenotype correlation (i.e. location of pathogenic variants in REN: in the signal peptide and prosegment vs. mature protein) and challenging intrafamilial phenotypic variability mainly for HNF1B disease.61,71
Genetic testing is currently the only way to confirm ADTKD and its subtypes. The most widely shared genetic testing strategy consists of a stepwise approach as follows: first, NGS analysis of a gene panel, including ADTKD genes (UMOD, REN, HNF1B, SEC61A11, DNAJB11) and Multiplex Ligation-dependent Probe Amplification analysis for HNF1B gene, in case of negative result MUC1 analysis if available should be performed as last step.2,18 It is important to explain during genetic counseling that a negative result does not exclude the diagnosis of ADTKD, because rare variants could be missed with conventional analysis and not all ADTKD genes have yet been identified.
In all confirmed genetic cases, at-risk adult family members, even if apparently healthy, can exclude or confirm the presence of the disease through genetic testing. Genetic screening should be mandatory for family members who wish to donate a kidney.1 In young adults with childhood onset diseases (i.e. HNF1B disease) it is appropriate to inform them that the knowledge of causative gene variant may allow preimplantation genetic testing.
ADTKD-UMOD and ADTKD-MUC1 become symptomatic in adult age, thus presymptomatic genetic screening in minors is not generally recommended. Instead, presymptomatic screening of at-risk children should be evaluated in families with pathogenic variants in REN, HNF1B, and SEC61A1 that could be associated with childhood onset disease.71
Conclusion
ADTKD includes at least 6 different genetic forms (ADTKD-UMOD, ADTKD-MUC1, ADTKD-REN, ADTKD-HNF1B, ADTKD-SEC61A1, and ADTKD-DNAJB11) of slowly progressive kidney disease, characterized by tubulointerstitial fibrosis and tubular damage. In the past, due to the bland urinary sediment and the nonspecific features, many patients with ADTKD remained undiagnosed with these diseases being considered extremely rare. With the identification of ADTKD causing genes and the increasing availability for genetic analysis, a number of new ADTKD families has been identified; recent studies suggest that ADTKD may be the third most common genetic cause of ESRD after ADPKD and Alport syndrome.17 The identification of ADTKD causing genes also encouraged the exploration of intracellular pathways activated by the encoded protein, revealing that most associated ADTKD gene pathogenic variants effect on the early secretory pathway (Figure 4). Currently, no approved therapeutic option is available for ADTKD, other than kidney replacement therapy when ESRD is established. Because in the most common forms of ADTKD (UMOD, MUC1, and REN) the pathogenesis can be mainly explained by a gain-of-toxic function mechanism, therapies aiming at decreasing mutant protein accumulation may be effective. Alternatively, strategies aimed at increasing expression or activity of the wild-type protein wild-type could be beneficial for ADTKD-SEC61 and ADTKD-HNF1B subtypes for which pathogenesis is mainly related to a loss-of-function mechanism. The recent advances in disease awareness and in the understanding of the disease mechanisms together with the creation of international networks will help clinicians in improving the diagnostic rates, the clinical management, as well as the genetic counseling while allowing the proper management of the disease and the prompt enrolment in new therapeutic protocols.
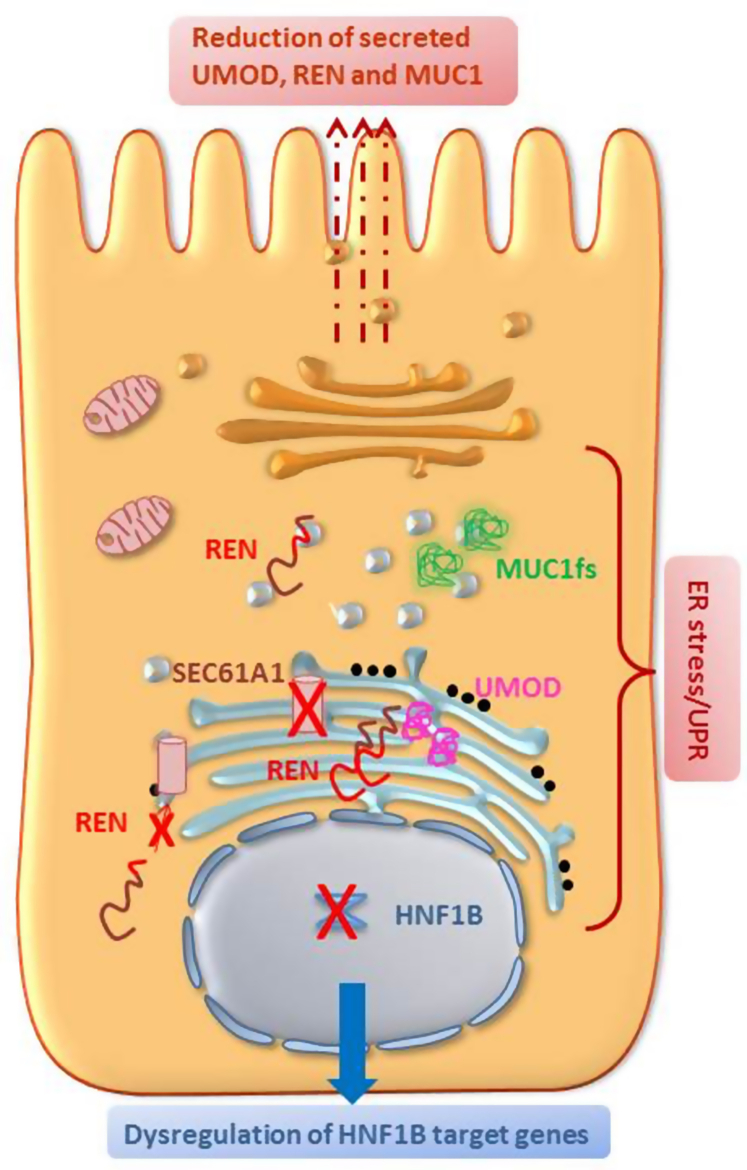
Figure 4.
Common cellular pathway in ADTKD. SEC61A1, REN, UMOD, MUC1 disease-causing variants effect on the early secretory pathway, ranging from ER translocation defect (SEC61, REN), to retention of mutant protein in the ER (UMOD, REN) or ER-Golgi intermediate compartment (MUC1-fs, REN). ER stress and induction of the Unfolded Protein Response have been reported on expression of the mutated genes, with the exception of renin mutations in the prosegment for which no data are available yet. Intracellular accumulation of UMOD, REN and MUC1-fs lead to a subsequent decrease of their delivery to the apical plasma membrane (UMOD,MUC1-fs) and/or of their secretion (UMOD,REN). Of note, mutant uromodulin reaching the plasma membrane interferes with the polymerization of wild-type protein. HNF1B coordinates several transcriptional networks. It seems likely that the ADTKD-like phenotype due to HNF1B pathogenic variants can be explained by the absence of transcriptional activation of HNF1B target genes. ADTKD, autosomal dominant tubulointerstitial kidney disease; ER, endoplasmic reticulum; fs, frameshift.
Disclosure
FA declares serving on the advisory boards of AstraZeneca, GSK, Vifor, and Trevere Therapeutics; also declares consultancy fees from Baxter and Otsuka. All the other authors declared no competing interests.
Acknowledgments
LR is supported by the Italian Ministry of Health (RF-2016- 02362623) and the Italian Society of Nephrology (SIN) (Adotta un progetto di Ricerca).
References
- 1.Eckardt K.U., Alper S.L., Antignac C., et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int. 2015;88:676–683. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 2.Devuyst O., Olinger E., Weber S., et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers. 2019;5:1–20. doi: 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 3.Smith C.H., Graham J.B. Congenital medullary cysts of the kidneys with severe refractory anemia. Am J Dis Child. 1945;69:369–377. [Google Scholar]
- 4.Strauss M.B., Sommers S.C. Medullary cystic disease and familial juvenile nephronophthisis: clinical and pathological identity. N Engl J Med. 1967;277:863–864. doi: 10.1056/NEJM196710192771606. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt F., Waldherr R., Kutt R., Brandis M. The nephronophthisis complex: clinical and genetic aspects. Clin Investig. 1992;70:802–808. doi: 10.1007/BF00180751. [DOI] [PubMed] [Google Scholar]
- 6.Konrad M., Saunier S., Heidet L., et al. Large homozygous deletions of the 2q13 region are a major cause of juvenile nephronophthisis. Hum Mol Genet. 1996;5:367–371. doi: 10.1093/hmg/5.3.367. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava S., Molinari E., Raman S., Sayer J.A. Many genes—one disease? Genetics of nephronophthisis (NPHP) and NPHP-associated disorders. Front Pediatr. 2018;5:287. doi: 10.3389/fped.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christodoulou K., Tsingis M., Stavrou C., et al. Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease. Hum Mol Genet. 1998;7:905–911. doi: 10.1093/hmg/7.5.905. [DOI] [PubMed] [Google Scholar]
- 9.Scolari F., Puzzer D., Amoroso A., et al. Identification of a new locus for medullary cystic disease, on chromosome 16p12. Am J Hum Genet. 1999;64:1655–1660. doi: 10.1086/302414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahan K., Fuchshuber A., Adamis S., et al. Familial juvenile hyperuricemic nephropathy and autosomal dominant medullary cystic kidney disease type 2: two facets of the same disease? J Am Soc Nephrol. 2001;12:2348–2357. doi: 10.1681/ASN.V12112348. [DOI] [PubMed] [Google Scholar]
- 11.Hart T.C., Gorry M.C., Hart P.S., et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby A., Gnirke A., Jaffe D.B., et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet. 2013;45:299–303. doi: 10.1038/ng.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zivná M., Hůlková H., Matignon M., et al. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet. 2009;85:204–213. doi: 10.1016/j.ajhg.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piret S.E., Danoy P., Dahan K., et al. Genome-wide study of familial juvenile hyperuricaemic (gouty) nephropathy (FJHN) indicates a new locus, FJHN3, linked to chromosome 2p22. 1-p21. Hum Genet. 2011;129:51–58. doi: 10.1007/s00439-010-0897-1. [DOI] [PubMed] [Google Scholar]
- 15.Bolar N.A., Golzio C., Živná M., et al. Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet. 2016;99:174–187. doi: 10.1016/j.ajhg.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhave J.C., Bech A.P., Wetzels J.F., Nijenhuis T. Hepatocyte nuclear factor 1β-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345–353. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groopman E.E., Marasa M., Cameron-Christie S., et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142–151. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mabillard H., Sayer J.A., Olinger E. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab268. Published online September 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lhotta K., Piret S.E., Kramar R., et al. Epidemiology of uromodulin-associated kidney disease–results from a nation-wide survey. Nephron Extra. 2012;2:147–158. doi: 10.1159/000339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olinger E., Hofmann P., Kidd K., et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int. 2020;98:717–731. doi: 10.1016/j.kint.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Chun J., Wang M., Wilkins M.S., et al. Autosomal dominant tubulointerstitial kidney disease-uromodulin misclassified as focal segmental glomerulosclerosis or hereditary glomerular disease. Kidney Int Rep. 2020;5:519–529. doi: 10.1016/j.ekir.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scolari F., Caridi G., Rampoldi L., et al. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis. 2004;44:987–999. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Nasr S.H., Lucia J.P., Galgano S.J., Markowitz G.S., D’Agati V.D. Uromodulin storage disease. Kidney Int. 2008;73:971–976. doi: 10.1038/sj.ki.5002679. [DOI] [PubMed] [Google Scholar]
- 24.Kidd K., Vylet’al P., Schaeffer C., et al. Genetic and clinical predictors of age of ESKD in individuals with autosomal dominant tubulointerstitial kidney disease due to UMOD mutations. Kidney Int Rep. 2020;5:1472–1485. doi: 10.1016/j.ekir.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayasreh N., Bullich G., Miquel R., et al. Autosomal dominant tubulointerstitial kidney disease: clinical presentation of patients with ADTKD-UMOD and ADTKD-MUC1. Am J Kidney Dis. 2018;72:411–418. doi: 10.1053/j.ajkd.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Bollée G., Dahan K., Flamant M., et al. Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin J Am Soc Nephrol. 2011;6:2429–2438. doi: 10.2215/CJN.01220211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scolari F., Izzi C., Ghiggeri G.M. Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant. 2015;30:1250–1256. doi: 10.1093/ndt/gfu300. [DOI] [PubMed] [Google Scholar]
- 28.Tokonami N., Takata T., Beyeler J., et al. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int. 2018;94:701–715. doi: 10.1016/j.kint.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Renigunta A., Renigunta V., Saritas T., Decher N., Mutig K., Waldegger S. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem. 2011;286:2224–2235. doi: 10.1074/jbc.M110.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutig K., Kahl T., Saritas T., et al. Activation of the bumetanide-sensitive Na+,K+,2Cl− cotransporter (NKCC2) is facilitated by tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem. 2011;286:30200–30210. doi: 10.1074/jbc.M111.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudbjartsson D.F., Holm H., Indridason O.S., et al. Association of variants at UMOD with chronic kidney disease and kidney stones—role of age and comorbid diseases. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates J.M., Jr., Raffi H.M., Prasadan K., et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection Rapid Communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 33.Micanovic R., Chitteti B.R., Dagher P.C., et al. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol. 2015;26:2172–2182. doi: 10.1681/ASN.2014070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Achkar T.M., Wu X.R., Rauchman M., McCracken R., Kiefer S., Dagher P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Ren Physiol. 2008;295:F534–F544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pivin E., Ponte B., de Seigneux S., et al. Uromodulin and nephron mass. Clin J Am Soc Nephrol. 2018;13:1556–1557. doi: 10.2215/CJN.03600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaeffer C., Devuyst O., Rampoldi L. Uromodulin: Roles in health and disease. Annu Rev Physiol. 2021;83:477–501. doi: 10.1146/annurev-physiol-031620-092817. [DOI] [PubMed] [Google Scholar]
- 37.Bleyer A.J., Hart T.C., Shihabi Z., Robins V., Hoyer J.R. Mutations in the uromodulin gene decrease urinary excretion of tamm-Horsfall protein. Kidney Int. 2004;66:974–977. doi: 10.1111/j.1523-1755.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 38.Bernascone I., Vavassori S., Di Pentima A., et al. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic. 2006;7:1567–1579. doi: 10.1111/j.1600-0854.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 39.Bernascone I., Janas S., Ikehata M., et al. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet. 2010;19:2998–3010. doi: 10.1093/hmg/ddq205. [DOI] [PubMed] [Google Scholar]
- 40.Kemter E., Prueckl P., Sklenak S., et al. Type of uromodulin mutation and allelic status influence onset and severity of uromodulin-associated kidney disease in mice. Hum Mol Genet. 2013;22:4148–4163. doi: 10.1093/hmg/ddt263. [DOI] [PubMed] [Google Scholar]
- 41.Johnson B.G., Dang L.T., Marsh G., et al. Uromodulin p.Cys147Trp mutation drives kidney disease by activating ER stress and apoptosis. J Clin Invest. 2017;127:3954–3969. doi: 10.1172/JCI93817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemter E., Fröhlich T., Arnold G.J., Wolf E., Wanke R. Mitochondrial dysregulation secondary to endoplasmic reticulum stress in autosomal dominant tubulointerstitial kidney disease-UMOD (ADTKD-UMOD) Sci Rep. 2017;7:42970. doi: 10.1038/srep42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffer C., Merella S., Pasqualetto E., Lazarevic D., Rampoldi L. Mutant uromodulin expression leads to altered homeostasis of the endoplasmic reticulum and activates the unfolded protein response. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piret S.E., Olinger E., Reed A.A.C., et al. A mouse model for inherited renal fibrosis associated with endoplasmic reticulum stress. Dis Model Mech. 2017;10:773–786. doi: 10.1242/dmm.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raffi H., Bates J.M., Laszik Z., Kumar S. Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Int. 2006;69:1914–1915. doi: 10.1038/sj.ki.5000411. [DOI] [PubMed] [Google Scholar]
- 46.Schaeffer C., Cattaneo A., Trudu M., et al. Urinary secretion and extracellular aggregation of mutant uromodulin isoforms. Kidney Int. 2012;81:769–778. doi: 10.1038/ki.2011.456. [DOI] [PubMed] [Google Scholar]
- 47.Trudu M., Schaeffer C., Riba M., et al. Early involvement of cellular stress and inflammatory signals in the pathogenesis of tubulointerstitial kidney disease due to UMOD mutations. Sci Rep. 2017;7:7383. doi: 10.1038/s41598-017-07804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards N., Olinger E., Adam J., et al. A novel homozygous UMOD mutation reveals gene dosage effects on uromodulin processing and urinary excretion. Nephrol Dial Transplant. 2017;32:1994–1999. doi: 10.1093/ndt/gfx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dvela-Levitt M., Kost-Alimova M., Emani M., et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse Proteinopathy. Cell. 2019;178:521–535.e23. doi: 10.1016/j.cell.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Bleyer A.J., Kmoch S., Antignac C., et al. Variable clinical presentation of an MUC1 mutation causing medullary cystic kidney disease type 1. Clin J Am Soc Nephrol. 2014;9:527–535. doi: 10.2215/CJN.06380613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekici A.B., Hackenbeck T., Morinière V., et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int. 2014;86:589–599. doi: 10.1038/ki.2014.72. [DOI] [PubMed] [Google Scholar]
- 52.Blumenstiel B., DeFelice M., Birsoy O., et al. Development and validation of a mass spectrometry–based assay for the molecular diagnosis of mucin-1 kidney disease. J Mol Diagn. 2016;18:566–571. doi: 10.1016/j.jmoldx.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Wenzel A., Altmueller J., Ekici A.B., et al. Single molecule real time sequencing in ADTKD-MUC1 allows complete assembly of the VNTR and exact positioning of causative mutations. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-22428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knaup K.X., Hackenbeck T., Popp B., et al. Biallelic expression of Mucin-1 in autosomal dominant tubulointerstitial kidney disease: implications for nongenetic disease recognition. J Am Soc Nephrol. 2018;29:2298–2309. doi: 10.1681/ASN.2018030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Živná M., Kidd K., Přistoupilová A., et al. Noninvasive immunohistochemical diagnosis and novel MUC1 mutations causing autosomal dominant tubulointerstitial kidney disease. J Am Soc Nephrol. 2018;29:2418–2431. doi: 10.1681/ASN.2018020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-bataineh M., Sutton T.A., Hughey R.P. Novel roles for mucin 1 in the kidney. Curr Opin Nephrol Hypertens. 2017;26:384–391. doi: 10.1097/MNH.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu S.M.W., Bleyer A.J., Anis K., et al. Autosomal dominant tubulointerstitial kidney disease due to MUC1 mutation. Am J Kidney Dis. 2018;71:495–500. doi: 10.1053/j.ajkd.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 58.Spicer A.P., Rowse G.J., Lidner T.K., Gendler S.J. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 59.Kmoch S., Živná M., Bleyer A.J. University of Washington: Seattle; Seattle, WA: 2017. Autosomal dominant tubulointerstitial kidney disease, REN-related. Source GeneReviews® [Internet] [Google Scholar]
- 60.Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 61.Živná M., Kidd K., Zaidan M., et al. An international cohort study of autosomal dominant tubulointerstitial kidney disease due to REN mutations identifies distinct clinical subtypes. Kidney Int. 2020;98:1589–1604. doi: 10.1016/j.kint.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaeffer C., Izzi C., Vettori A., et al. Autosomal dominant tubulointerstitial kidney disease with adult onset due to a novel renin mutation mapping in the mature protein. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-48014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gribouval O., Gonzales M., Neuhaus T., et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 64.Bleyer A.J., Zivná M., Hulková H., et al. Clinical and molecular characterization of a family with a dominant renin gene mutation and response to treatment with fludrocortisone. Clin Nephrol. 2010;74:411–422. doi: 10.5414/cnp74411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horikawa Y., Iwasaki N., Hara M., et al. Mutation in hepatocyte nuclear factor–1β gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 66.Bingham C., Bulman M.P., Ellard S., et al. Mutations in the hepatocyte nuclear factor-1β gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68:219–224. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faguer S., Decramer S., Chassaing N., et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]
- 68.Clissold R.L., Hamilton A.J., Hattersley A.T., Ellard S., Bingham C. HNF1B-associated renal and extra-renal disease—an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 69.Izzi C., Dordoni C., Econimo L., et al. Variable expressivity of HNF1B nephropathy, from renal cysts and diabetes to medullary sponge kidney through tubulo-interstitial kidney disease. Kidney Int Rep. 2020;5:2341–2350. doi: 10.1016/j.ekir.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heidet L., Decramer S., Pawtowski A., et al. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol. 2010;5:1079–1090. doi: 10.2215/CJN.06810909. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bleyer A.J., Wolf M.T., Kidd K.O., et al. Autosomal dominant tubulointerstitial kidney disease: more than just HNF1β. Pediatr Nephrol. 2021;37:933–946. doi: 10.1007/s00467-021-05118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hiesberger T., Bai Y., Shao X., et al. Mutation of hepatocyte nuclear factor–1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan S.C., Zhang Y., Shao A., et al. Mechanism of fibrosis in HNF1B-related autosomal dominant tubulointerstitial kidney disease. J Am Soc Nephrol. 2018;29:2493–2509. doi: 10.1681/ASN.2018040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carew R.M., Wang B., Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347:103–116. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 75.Schubert D., Klein M.C., Hassdenteufel S., et al. Plasma cell deficiency in human subjects with heterozygous mutations in Sec61 translocon alpha 1 subunit (SEC61A1) J Allergy Clin Immunol. 2018;141:1427–1438. doi: 10.1016/j.jaci.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang S., Pfeffer S., Lee P.H., et al. An update on Sec61 channel functions, mechanisms, and related diseases. Front Physiol. 2017;8:887. doi: 10.3389/fphys.2017.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sicking M., Živná M., Bhadra P., et al. Phenylbutyrate rescues the transport defect of the Sec61α mutations V67G and T185A for renin. Life Sci Alliance. 2022;5 doi: 10.26508/lsa.202101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cornec-Le Gall E., Olson R.J., Besse W., et al. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet. 2018;102:832–844. doi: 10.1016/j.ajhg.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]