Abstract
Introduction
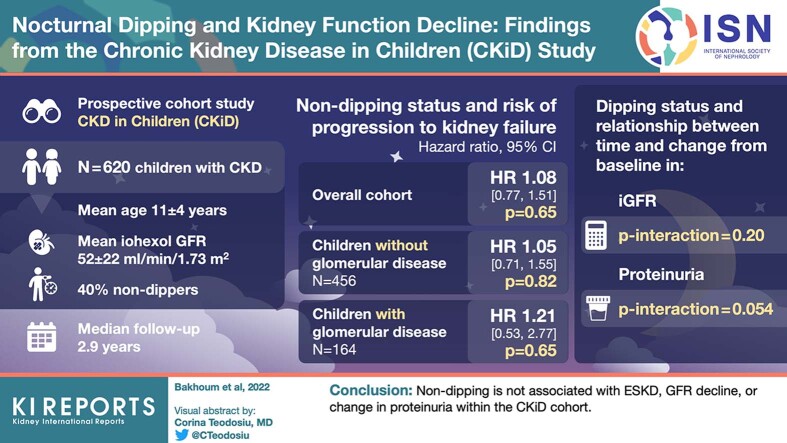
Normally, blood pressure (BP) declines by at least 10% from daytime to nighttime. In adults, blunted nocturnal dipping has been associated with more rapid decline in kidney function. Nondipping is prevalent in children with chronic kidney disease (CKD). We sought to determine whether nondipping is associated with proteinuria and progression to kidney failure in children with CKD.
Methods
In the prospective CKD in children (CKiD) cohort, Cox proportional hazards models were used to evaluate the relationship between baseline nondipping and progression to kidney failure. Linear mixed effects models were used to evaluate the relationship between nondipping and changes in iohexol glomerular filtration rate (GFR) and urine protein-to-creatinine ratio (log-UPCR, mg/mg) over time.
Results
Among 620 participants, mean age was 11 (± 4) years, mean iohexol GFR was 52 (± 22) ml/min per 1.73 m2, and 40% were nondippers at baseline. There were 169 kidney failure events during 2.9 years (median) of follow-up. Dipping status was not significantly associated with kidney failure overall (hazard ratio [HR] 1.08; 95% confidence interval [CI] 0.77, 1.51) or in those with (HR 1.21; 95% CI 0.53, 2.77) or without (HR 1.05; 95% CI 0.71, 1.55) glomerular disease. Dipping status did not modify the relationship between time and change in iohexol GFR or log (UPCR) from baseline (interaction P values = 0.20 and 0.054, respectively).
Conclusion
Nondipping is not associated with end-stage kidney disease, GFR decline, or change in proteinuria within the CKiD cohort.
Keywords: blood pressure, children, chronic kidney disease, dipping, hypertension, pediatrics
Graphical abstract
See Commentary on Page 2327
The use of ambulatory BP monitoring in various patient populations has consistently revealed that BP follows a circadian rhythm.1,2 The exact mechanism underlying this diurnal variation is not fully understood, but it is thought to be multifactorial, with dietary sodium, volume retention, sympathetic nervous system activation, and other factors hypothesized to play a role.1 At night, BP normally declines by 10% or more relative to daytime.3,4 Blunting of this decline, or “nondipping,” is associated with increasing microalbuminuria and more rapid decline in kidney function in adults, independent of overall BP.5,6 It has also been associated with increased risk of cardiovascular disease in adults.7,8
Although it is known that nondipping is prevalent in children with CKD, there are limited data on the relationship of nondipping with clinical outcomes in this population.9 Children with CKD represent a population with a high prevalence of hypertension, who are at risk for kidney failure and cardiovascular disease in adulthood.10,11 In a previous study in the CKiD cohort, the largest cohort of children with CKD in North America, we did not detect an association of nondipping with left ventricular mass index, a marker of subclinical cardiovascular disease.12 Whether nondipping may be associated with other important outcomes in this population remains uncertain. To that end, we sought to determine whether nondipping of BP would be associated with greater proteinuria and more rapid progression to kidney failure over time in children with CKD.
Methods
Study Population and Design
The CKiD study protocol has been previously described.13 Briefly, CKiD is a prospective, multicenter, observational cohort study that recruited children aged 6 months to 16 years with Schwartz estimated GFR of 30 to 90 ml/min per 1.73 m2.13 Children and adolescents were recruited from 59 different centers across North America.13,14 Among the exclusion criteria were a history of dialysis within 3 months before recruitment, history of transplantation, malignancy, and structural heart disease.13 The Institutional Review Board of each participating center reviewed and approved the study protocol. The study was conducted in adherence with the tenets of the Declaration of Helsinki. Parental permission and age-appropriate assent were obtained from a parent or guardian and each participant, respectively. Deidentified data were obtained from the CKiD data coordinating center and analyzed for this study. Our study was deemed as not human subjects research by the Yale University Institutional Review Board.
Participants were included in this analysis if they had a successful ambulatory BP monitoring study at baseline. A study was deemed successful if (i) it was worn for ≥21 hours with ≥1 valid BP measured per hour for at least 18 hours and (ii) it had ≥1 successful BP recording in ≥75% of wake hours and ≥75% in sleep hours.15 Overall, 198 subjects were excluded for an inadequate ambulatory BP monitoring study. Participants were also excluded if their time to end-stage kidney disease preceded their baseline ambulatory BP monitoring study (n = 37). Thus, we identified 620 participants who were included in this analysis. For time-to-event and linear mixed models, baseline was set at the visit in which the first successful ambulatory BP measurement was obtained.
Ambulatory BP Monitoring Protocol
The specifics of the standardized procedure for ambulatory BP monitoring in CKiD have been previously described in detail.15 Briefly, SpaceLabs 90217 monitors (SpaceLabs Healthcare, Issaquah, WA) were used to obtain ambulatory BP monitoring data. BP was measured every 20 minutes in a 24-hour period at a bleed step of 8 mm Hg.15 Families recorded sleep and awake times in a diary. All data were centrally analyzed.15 For participants with an unsuccessful study, at least 1 repeat attempt was made.
Dipping Calculations and Definitions
The percentage change in the average BP measurements from the wake period to the sleep period was used to calculate both systolic and diastolic dipping (%). A nondipping pattern was defined as a decrease of <10% in either systolic or diastolic dipping and was the exposure of interest.3
Other Variables
Time to kidney failure was defined as time, in years, from the date of baseline ambulatory BP monitoring to either dialysis or kidney transplant (whichever came first) or was censored at the last follow-up date. This was captured through direct follow-up of subjects within the cohort. Plasma clearance of iohexol was used to measure GFR at baseline, 12 months, and biannually thereafter.16 A spot UPCR was measured at baseline and then annually in all subjects thereafter.13 All laboratory analyses were performed at the CKiD central laboratory at the University of Rochester (Rochester, NY). Additional demographic and medical history was obtained as part of the CKiD protocol, including the following: age at entry, sex, body mass index z-score, etiology of CKD, self-reported race, and use of antihypertensive medications in the past 30 days (including categories of medications used).13
Statistical Analysis
Descriptive statistics were calculated for participants by their baseline dipping status (nondipper vs. dipper). For continuous variables that were normally distributed, mean and SD are presented. For non-normally distributed continuous variables, median and interquartile range are presented. Frequency and percent are used to describe categorical variables.
Cox proportional hazards models were used to evaluate the relationship between baseline dipping status (both as a categorical and a continuous predictor) and progression to kidney failure. The analysis was performed in the entire cohort, as well as stratified by etiology of CKD (glomerular vs. nonglomerular), given the established differences in progression of disease between these groups.17 Covariates included age, sex, race, body mass index z-score, 24-hour mean systolic BP at baseline, use of angiotensin-converting enzyme inhibitor, baseline UPCR, and baseline iohexol GFR. The Supremum test was used to check the proportional hazards assumption in the multivariable model. Interaction with time terms were added into the model for covariates found to violate the proportional hazards assumption. Kaplan-Meier curves were generated to visualize kidney failure-free survival probability over time in nondippers versus dippers overall and by etiology of CKD. Incidence rates of kidney failure were calculated in nondippers and dippers, and incidence rate ratios were calculated as the incidence rate in nondippers divided by the incidence rate in dippers.
In a sensitivity analysis, we identified participants who had at least 2 successful consecutive ambulatory BP monitoring studies before developing kidney failure or censoring. We categorized participants into the following 3 groups based on their first 2 ambulatory BP studies: never nondipper, nondipper once, or nondipper twice. A Cox proportional hazards model was used to evaluate the relationship of serial dipping status (as a categorical variable) and its relationship with progression to kidney failure, using the same covariates as described previously.
Linear mixed effects models, with random intercept and slope, were used to evaluate the relationship between dipping status and change in iohexol GFR and change in UPCR over time. UPCR was log-transformed, given its right-skewed distribution. Covariates included age, sex, etiology of CKD, race, body mass index z-score, 24-hour mean systolic BP at baseline, use of angiotensin-converting enzyme inhibitor, baseline UPCR, and baseline iohexol GFR. Dipping status was included as a time-varying covariate. We tested for interactions between dipping status and time in all models. Main effects were interpreted after removing nonsignificant interactions.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). P values of ≤0.05 were considered significant for all analyses, including interaction terms.
Results
Overall, 620 children were included in this study and 40% were nondippers at baseline. The mean age was 10.8 (SD 4.3) years, and the mean iohexol GFR was 52 ml/min per 1.73 m2 (SD 22). The etiology of CKD was nonglomerular disease (n = 456) in 74% of the subjects, 39% were female, and 13% were Black. Antihypertensive medication use was reported in 66% of the participants, with 51% on angiotensin-converting enzyme inhibitors. The median systolic dipping percentage was 11.2% (interquartile range 7.8–14.6), and the median diastolic dipping was 17.5% (interquartile range 13.2–21.7) (Table 1).
Table 1.
Characteristics of chronic kidney disease in children participants by baseline dipping status
| Characteristic | Nondippers (n = 251) | Dippers (n = 369) | Overall (N = 620) |
|---|---|---|---|
| Age (yr) | 10.4 (4.5) | 11.0 (4.1) | 10.8 (4.3) |
| Female | 110 (44) | 134 (36) | 244 (39) |
| Race: Black | 40 (16) | 40 (11) | 80 (13) |
| White | 168 (67) | 275 (74) | 443 (71) |
| Other | 43 (17) | 54 (15) | 97 (16) |
| Glomerular disease | 69 (27) | 95 (26) | 164 (26) |
| BMI z-score | 0.33 (1.20) | 0.42 (1.10) | 0.38 (1.14) |
| Birthweight (kg) | 3.10 (0.73) | 3.12 (0.73) | 3.11 (0.73) |
| Iohexol GFR (ml/min per 1.73 m2) | 51 (24) | 52 (21) | 52 (22) |
| eGFR (ml/min per 1.73 m2) | 54 (19) | 55 (20) | 55 (20) |
| Urine protein/creatininea (mg/mg) | 0.3 (0.1–0.9) | 0.3 (0.1–0.8) | 0.3 (0.1–0.9) |
| ACE inhibitor use | 119 (47) | 197 (53) | 316 (51) |
| Antihypertensive use | 161 (64) | 249 (67) | 410 (66) |
| BP stage: normal BP | 165 (67) | 232 (65) | 397 (66) |
| Elevated BP | 35 (14) | 46 (13) | 81 (13) |
| Stage 1 HTN | 36 (15) | 66 (18) | 102 (17) |
| Stage 2 HTN | 9 (4) | 13 (4) | 22 (4) |
| 24-hour mean systolic BP (mm Hg) | 112 (12) | 110 (10) | 110 (11) |
| 24-hour mean diastolic BP(mm Hg) | 66 (8) | 65 (8) | 66 (8) |
| Systolic dippinga % | 7.0 (3.5–8.5) | 13.8 (11.8–16.6) | 11.2 (7.8–14.6) |
| Diastolic dippinga % | 12.3 (7.7–16.1) | 20.1 (17.2–23.9) | 17.5 (13.2–21.7) |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate; HTN, hypertension.
Median with interquartile range is reported, as it is non-normally distributed.
Relationship of Baseline Dipping Status With Progression to End-Stage Kidney Disease
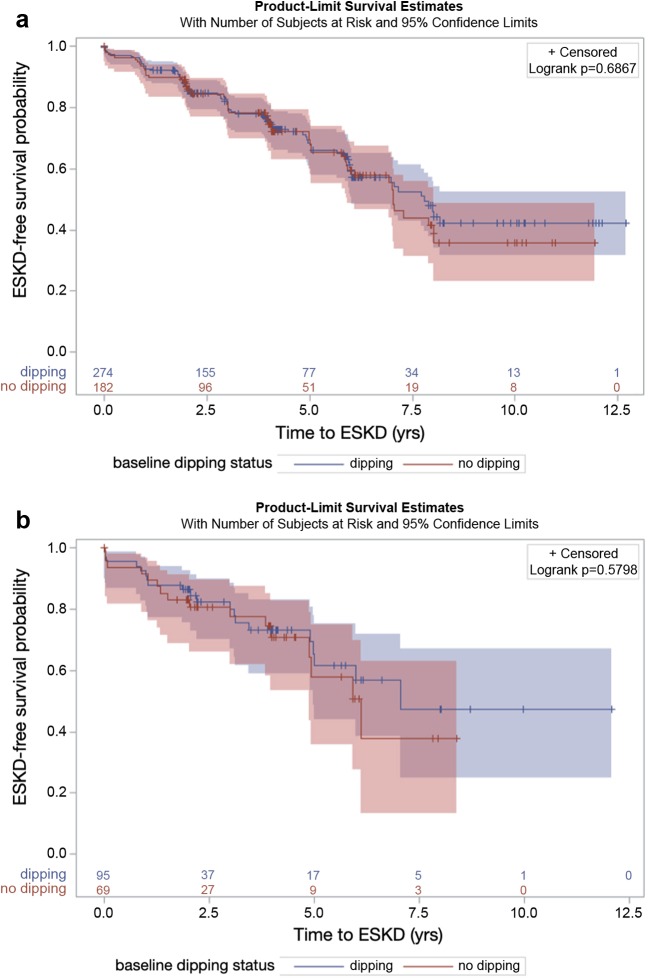
There were 169 kidney failure events during a median of 2.9 years of follow-up. The unadjusted incidence rate ratio was 1.13 in the overall cohort, 1.09 in subjects with nonglomerular disease and 1.26 in subjects with glomerular disease. There was no significant difference in kidney failure-free survival probability between dippers and nondippers overall (P = 0.54) or in strata among those with nonglomerular disease (P = 0.69) or with glomerular disease (P = 0.58) (Figure 1). In multivariable models, dipping status was not significantly associated with the risk of kidney failure overall (HR 1.08; 95% CI 0.77, 1.51; Table S1) or in either subjects with or without glomerular disease (Table 2). To maximize power, we also evaluated dipping as a continuous predictor variable within the overall cohort (Table S2). In this multivariable analysis, each percent systolic dipping was associated with a HR of 1.01 (95% CI 0.98, 1.04) for kidney failure, and each percent diastolic dipping was associated with a HR of 1.00 (95% CI 0.98, 1.02) for kidney failure.
Figure 1.
Kidney failure-free survival by dipping status in participants with (a) nonglomerular CKD and (b) glomerular CKD. Nondippers are represented in red and dippers in blue. Shading represents 95% confidence interval. Log-rank P values presented for difference in kidney failure-free survival between groups. ESKD, end-stage kidney disease.
Table 2.
Multivariable Cox proportional hazards models for the relationship between nondipping and progression to kidney failure, stratified by etiology of chronic kidney disease
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Nonglomerular disease (n = 456) | ||
| Nondipping status (vs. dipping) | 1.05 (0.71, 1.55) | 0.82 |
| 24-h mean systolic BP (per 10 mm Hg) | 0.87 (0.64, 1.20) | 0.39 |
| Age (per yr) | 1.10 (1.05, 1.16) | <0.001 |
| Female | 0.69 (0.46, 1.06) | 0.69 |
| Black | 1.94 (1.09, 3.48) | 0.03 |
| BMI z-score | 0.98 (0.83, 1.15) | 0.78 |
| ACE inhibitor use | 0.96 (0.66, 1.48) | 0.96 |
| Baseline iohexol GFR (per ml/min per 1.73 m2) | 0.92 (0.90, 0.93) | <0.001 |
| Baseline UPCR (per mg/mg) | 1.22 (1.10, 1.36) | <0.001 |
| 24-hour mean systolic BP ∗ time | 1.01 (1.0, 1.02) | 0.01 |
| Glomerular disease (n = 164) | ||
| Nondipping status (vs. dipping) | 1.21 (0.53, 2.77) | 0.65 |
| 24-h mean systolic BP (per 10 mm Hg) | 1.10 (0.66, 1.63) | 0.79 |
| Age (per yr) | 1.13 (1.0, 1.28) | 0.06 |
| Female | 0.51 (0.22, 1.16) | 0.11 |
| Black | 2.45 (0.91, 6.59) | 0.07 |
| BMI z-score | 0.98 (0.71, 1.35) | 0.88 |
| ACE inhibitor use | 1.26 (0.48, 3.32) | 0.64 |
| Baseline iohexol GFR (per ml/min per 1.73 m2) | 0.95 (0.92, 0.97) | <0.001 |
| Baseline UPCR (per mg/mg) | 1.61 (1.25, 2.07) | <0.001 |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; CI, confidence interval; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; UPCR, urine protein/creatinine ratio (mg/mg).
Relationship of Serial Dipping Status With Progression to Kidney Failure
We identified 198 participants who had at least 2 successful sequential ambulatory BP monitoring studies before kidney failure or censoring. Of those, 44% were never nondippers (n = 87), 38% were nondippers once (n = 76), and 18% were nondippers twice (n = 35). In a multivariable Cox proportional hazards model, nondipping twice was not associated with risk of kidney failure, as compared with never nondipping (P = 0.73; Table S3).
Relationship of Dipping Status With Change in Iohexol GFR and UPCR
For both dippers and nondippers, there was a median of 1 iohexol GFR and UPCR measurement after baseline (interquartile range 1–2). Dipping status did not modify the relationship between time and change in iohexol GFR (Table 3, P interaction dipping ∗ time = 0.20). Similarly, dipping status did not modify the relationship between time and change in log (UCPR) (Table 4, P interaction dipping ∗ time = 0.054).
Table 3.
Linear mixed effects model for the relationship of time-varying dipping status with change in iohexol GFR from baseline (n = 368)
| Variable | Estimate (95% CI) | P value |
|---|---|---|
| Dipping status ∗ time: nondippers vs. dippers | 0.57 (−0.31, 1.44) | 0.20 |
| Dipping status: nondippers vs. dippers | −0.78 (−4.35, 2.78) | 0.66 |
| Baseline 24-h mean systolic BP (per mm Hg) | −0.21 (−0.33, −0.08) | 0.002 |
| Age at entry (per yr) | −0.01 (−0.33, 0.30) | 0.93 |
| Sex: female vs. male | −1.33 (−3.71, 1.05) | 0.27 |
| Race: Black vs. Caucasian | 0.96 (−3.21, 5.13) | 0.64 |
| Etiology of CKD: glomerular vs. nonglomerular | 2.03 (−1.03, 5.09) | 0.19 |
| Baseline BMI z-score | 0.28 (−0.72, 1.27) | 0.58 |
| Baseline ACE inhibitor use | −1.76 (−4.17, 0.64) | 0.15 |
| Baseline UPCR (per mg/mg) | −3.54 (−5.14, −1.94) | <0.001 |
| Baseline iohexol GFR (per ml/min per 1.73 m2) | −0.18 (−0.24, −0.11) | <0.001 |
| Time | −1.66 (−2.23, −1.09) | <0.001 |
| Intercept | 33.7 (20.9, 46.6) | <0.001 |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; GFR, glomerular filtration rate; UPCR, urine protein/creatinine ratio (mg/mg).
Table 4.
Linear mixed effects model for the relationship of time-varying dipping status with change in log (urine protein/creatinine) from baseline (n = 368)
| Variable | Estimate (95% CI) | P value |
|---|---|---|
| Dipping status ∗ time: nondippers vs. dippers | 0.06 (−0.001, 0.13) | 0.054 |
| Dipping status: nondippers vs. dippers | 0.04 (−0.24, 0.32) | 0.79 |
| Baseline 24-h mean systolic BP (per mm Hg) | −0.01 (−0.02, 0.001) | 0.09 |
| Age at entry (per yr) | 0.07 (0.04, 0.09) | <0.001 |
| Sex: female vs. male | −0.18 (−0.38, 0.03) | 0.09 |
| Race: Black vs. Caucasian | −0.01 (−0.37, 0.34) | 0.94 |
| Etiology of CKD: glomerular vs. nonglomerular | 0.08 (−0.18, 0.33) | 0.55 |
| Baseline BMI z-score | 0.03 (−0.06, 0.11) | 0.51 |
| Baseline ACE inhibitor use | 0.05 (−0.16, 0.25) | 0.66 |
| Baseline iohexol GFR (per ml/min per 1.73 m2) | −0.01 (−0.01, 0) | 0.001 |
| Baseline UPCR (per mg/mg) | −0.27 (−0.40, −0.14) | <0.001 |
| Time | 0.12 (0.08, 0.16) | <0.001 |
| Intercept | 0.66 (−0.41, 1.75) | 0.23 |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; GFR, glomerular filtration rate; UPCR, urine protein/creatinine ratio (mg/mg).
Discussion
In a large and well-characterized cohort of children with mild-to-moderate CKD, we evaluated the relationship of nocturnal BP dipping status with risk of kidney failure, progressive GFR decline, and proteinuria. We did not identify a significant association between nocturnal dipping status and progression to kidney failure overall or in those with or without glomerular disease when evaluated separately. Furthermore, we did not identify a significant relationship between dipping status and decline in measured GFR or change in proteinuria over time.
Nondipping has been extensively studied in adults and found to be consistently associated with increased cardiovascular disease risk, greater proteinuria, and lower estimated GFR.5,6,8,18, 19, 20, 21 As such, interventional studies have been conducted in adults with hypertension to determine whether chronotherapy, or modifying timing of BP medications, can improve dipping profile and outcomes. These clinical trials have revealed that chronotherapy may improve dipping profile and overall 24-hour BP control in certain populations.22,23 Despite advances in the understanding of the clinical effect of nondipping in adults, little is known about its consequences in children and adolescents, particularly those with CKD where identification of modifiable risk factors is of critical importance. In a small, cross-sectional study of children with CKD, less systolic dipping was associated with lower estimated GFR.24 In addition, in a study of adolescents with type 1 diabetes, nondipping at baseline was associated with microalbuminuria at follow-up.25 In a cross-sectional analysis of a pediatric cohort with hypertension, proteinuria was significantly associated with nondipping.26 With regard to cardiovascular outcomes, analyses of pediatric cohorts have reported significant associations between BP nondipping and pulse wave velocity, carotid-intima medial thickness, and coronary artery calcium presence.27, 28, 29 However, within the CKiD study, nondipping was not found to be significantly associated with left ventricular mass index in a cross-sectional analysis.12 Similarly, here, in a longitudinal analysis within the CKiD cohort, nondipping was not associated with progression to end-stage kidney disease, decline in iohexol GFR, or change in proteinuria in a median follow-up time of 2.9 years.
The discrepancy between our findings and those described in adults may stem from the fact that the reliability of ambulatory BP monitoring in children may be different than in adults, particularly at nighttime. A recent study evaluating reproducibility of BP measurements in children revealed a disagreement of 40% in determining dipping status on serial ambulatory BP monitoring studies.30 This may be secondary to high levels of movement or poor tolerance of BP measurements during sleep in children. In a subanalysis of our study, we attempted to improve reliability of nondipping by assessing 2 consecutive ambulatory BP monitoring studies. Although the HR for progression to kidney failure seemed higher for those who were nondippers twice (as compared with never nondippers), this was not statistically significant. This may be partially reflective of the small sample size in the subanalysis. To date, systolic and diastolic dipping percentages and presence of nondipping are reported as part of a standard ambulatory BP analysis and report in pediatrics.3 On the basis of data thus far from the CKiD study, nondipping does not seem to be a risk factor for subclinical cardiovascular disease or progression to kidney failure. This brings to question its clinical value and whether pediatric nephrologists should interpret and follow this parameter over time. Future studies are needed to further evaluate the reliability of ambulatory BP monitoring in pediatric CKD populations and to determine whether dipping status is still meaningful in this high-risk group.
Our study has important limitations. First, ambulatory BP monitoring studies were obtained at 12 months and then biannually in CKiD. Many factors may change in the course of 2 years (such as medications and estimated GFR) in a CKD cohort, and thus, we were not able to sufficiently evaluate the reliability of the ambulatory BP monitoring data. Second, there may be selection bias, as we only included ambulatory BP data if the study was deemed adequate by criteria detailed previously. Thus, we are likely selecting children who were more cooperative with the study (had less movement or alarms), and their BP dipping profiles may be more normal than those who were excluded. This might bias our results toward the null. Strengths of our study include its large sample size, longitudinal nature, and detailed characterization of a diverse group of study participants with available data on a wide variety of covariates, improving the generalizability of our findings. Furthermore, in a subgroup of CKiD subjects with serial ambulatory BP monitoring studies, we were able to evaluate consistent nondipping status in 2 measurements performed 2 years apart.
In conclusion, in a large cohort of children and adolescents with mild-to-moderate CKD, we did not identify an association between nocturnal BP dipping and progression to kidney failure in either those with or without glomerular disease. In addition, we did not identify a significant relationship between BP dipping and longitudinal change in measured GFR or proteinuria. Further studies are needed to investigate the reliability of ambulatory BP monitoring studies in children with CKD and to clarify the clinical utility of dipping profile as a parameter in this population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (principal investigators) at the Children’s Mercy Hospital and the University of Missouri—Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD, and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD website is located at https://statepi.jhsph.edu/ckid, and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/.
Sources of Funding
The CKiD study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (U01DK66143, U01DK66174, U24DK082194, and U24DK066116). The work for this ancillary study was supported by the NIDDK through grant K23 DK129836 (CYB) and K24 DK110427 (JHI). This work was also supported by the American Heart Association Grant # 857722 (CYB).
Data Sharing Statement
The CKiD cohort deidentified participant data and data dictionaries used in our analyses are available through the NIDDK Central Repository.
Footnotes
Table S1. Multivariable Cox proportional hazards models for the relationship between nondipping and progression to kidney failure in the overall CKiD cohort.
Table S2. Multivariable Cox proportional hazards models for the relationship between systolic and diastolic dipping (%) and progression to kidney failure.
Table S3. Multivariable Cox proportional hazards models for the relationship between serial dipping status and progression to kidney failure in the overall CKiD cohort (N = 198).
STROBE Statement.
Supplementary Material
Table S1. Multivariable Cox proportional hazards models for the relationship between nondipping and progression to kidney failure in the overall CKiD cohort.
Table S2. Multivariable Cox proportional hazards models for the relationship between systolic and diastolic dipping (%) and progression to kidney failure.
Table S3. Multivariable Cox proportional hazards models for the relationship between serial dipping status and progression to kidney failure in the overall CKiD cohort (N = 198).
STROBE Statement.
References
- 1.Kanbay M., Turgut F., Uyar M.E., Akcay A., Covic A. Causes and mechanisms of nondipping hypertension. Hypertens. 2008;30:585–597. doi: 10.1080/10641960802251974. [DOI] [PubMed] [Google Scholar]
- 2.Biaggioni I. Circadian clocks, autonomic rhythms, and blood pressure dipping. Hypertension. 2008;52:797–798. doi: 10.1161/HYPERTENSIONAHA.108.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn J.T., Daniels S.R., Hayman L.L., et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/hyp.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano Y., Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- 5.Davidson M.B., Hix J.K., Vidt D.G., Brotman D.J. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 6.Afsar B., Elsurer R. Urinary albumin excretion among nondipper hypertensive patients is closely related with the pattern of nondipping. J Am Soc Hypertens. 2010;4:196–202. doi: 10.1016/j.jash.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Hoshide S., Kario K., Hoshide Y., et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–438. doi: 10.1016/s0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla M., Caughey M.C., Tanner R.M., et al. Associations of blood pressure dipping patterns with left ventricular mass and left ventricular hypertrophy in blacks: the Jackson heart study. J Am Heart Assoc. 2017;6:e004847. doi: 10.1161/jaha.116.004847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsnefes M., Flynn J., Cohn S., et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warady B.A., Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson A.C., Flynn J.T. Blood pressure in children with chronic kidney disease: lessons learned from the chronic kidney disease in children cohort study. Pediatr Nephrol. 2020;35:1203–1209. doi: 10.1007/s00467-019-04288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakhoum C.Y., Katz R., Samuels J.A., et al. Nocturnal dipping and left ventricular mass index in the chronic kidney disease in children cohort. Clin J Am Soc Nephrol. 2022;17:75–82. doi: 10.2215/cjn.09810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furth S.L., Cole S.R., Moxey-Mims M., et al. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/cjn.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson M.A., Ng D.K., Warady B.A., Furth S.L., Flynn J.T. The CKiD study: overview and summary of findings related to kidney disease progression. Pediatr Nephrol. 2020;36:527–538. doi: 10.1007/s00467-019-04458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels J., Ng D., Flynn J.T., et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43–50. doi: 10.1161/HYPERTENSIONAHA.111.189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz G.J., Abraham A.G., Furth S.L., Warady B.A., Muñoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furth S.L., Pierce C., Hui W.F., et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018;71:783–792. doi: 10.1053/j.ajkd.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuspidi C., Giudici V., Negri F., Sala C. Nocturnal nondipping and left ventricular hypertrophy in hypertension: an updated review. Expert Rev Cardiovasc Ther. 2010;8:781–792. doi: 10.1586/erc.10.29. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R., Light R.P. GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant. 2009;24:2400–2406. doi: 10.1093/ndt/gfp074. [DOI] [PubMed] [Google Scholar]
- 20.Choi H.Y., Lee C.J., Lee J.E., et al. Loss of nighttime blood pressure dipping as a risk factor for coronary artery calcification in nondialysis chronic kidney disease. Medicine (Baltimore) 2017;96:e7380. doi: 10.1097/md.0000000000007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman M., Griffin V., Heyka R., Hoit B. Diurnal variation of blood pressure; reproducibility and association with left ventricular hypertrophy in hemodialysis patients. Blood Press Monit. 2005;10:25–32. doi: 10.1097/00126097-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zhao P., Xu P., Wan C., Wang Z. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;2011:Cd004184. doi: 10.1002/14651858.CD004184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowles N.P., Thosar S.S., Herzig M.X., Shea S.A. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97. doi: 10.1007/s11906-018-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsnefes M.M., Kimball T.R., Daniels S.R. Office and ambulatory blood pressure elevation in children with chronic renal failure. Pediatr Nephrol. 2003;18:145–149. doi: 10.1007/s00467-002-1030-z. [DOI] [PubMed] [Google Scholar]
- 25.Lovshin J.A., Škrtić M., Bjornstad P., et al. Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with type 1 diabetes mellitus. Am J Physiol Ren Physiol. 2018;314:F667–F674. doi: 10.1152/ajprenal.00400.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakhoum C.Y., Vuong K.T., Carter C.E., Gabbai F.B., Ix J.H., Garimella P.S. Proteinuria and nocturnal blood pressure dipping in hypertensive children and adolescents. Pediatr Res. 2021;90:876–881. doi: 10.1038/s41390-020-01315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correia-Costa A., Correia-Costa L., Caldas Afonso A., et al. Determinants of carotid-femoral pulse wave velocity in prepubertal children. Int J Cardiol. 2016;218:37–42. doi: 10.1016/j.ijcard.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 28.Chang J.C., Xiao R., Meyers K.E., et al. Nocturnal blood pressure dipping as a marker of endothelial function and subclinical atherosclerosis in pediatric-onset systemic lupus erythematosus. Arthritis Res Ther. 2020;22:129. doi: 10.1186/s13075-020-02224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viera A.J., Lin F.C., Hinderliter A.L., et al. Nighttime blood pressure dipping in young adults and coronary artery calcium 10–15 years later: the coronary artery risk development in young adults study. Hypertension. 2012;59:1157–1163. doi: 10.1161/hypertensionaha.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stergiou G.S., Bountzona I., Alamara C., Vazeou A., Kollias A., Ntineri A. Reproducibility of office and out-of-office blood pressure measurements in children: implications for clinical practice and research. Hypertension. 2021;77:993–1000. doi: 10.1161/HYPERTENSIONAHA.120.16531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.