Abstract
In this study, we demonstrate that the mechanism by which Staphylococcus aureus induces apoptosis in bovine mammary epithelial (MAC-T) cells involves caspases 8 and 3, two key components of a proteolytic cascade leading to apoptosis. In addition, internalized S. aureus induces expression of the inflammatory cytokines tumor necrosis factor alpha and interleukin-1β by MAC-T cells. These data suggest that the internalization of S. aureus could induce specific cellular responses in vivo that may ultimately impact the course of infection.
Staphylococcus aureus is internalized by a variety of nonprofessional mammalian phagocytes (1, 8, 19). Work in our laboratories has shown that internalization requires a specific interaction between fibronectin binding protein and the host cell, presumably followed by signal transduction events that ultimately lead to rearrangement of the host cell cytoskeleton (4). We have also shown that internalization by bovine mammary epithelial cells induces apoptosis and that metabolically active intracellular S. aureus are needed for apoptosis to occur (1, 20).
Assessment of caspase activity.
The induction of apoptosis, also called programmed cell death, often progresses through an ordered series of events involving a family of cysteine aspartyl proteases known as caspases. This family of proteins is divided into two major groups: initiator caspases, which are involved in triggering the cascade of events leading to cell death, and effector caspases, which, when activated by initiator caspases, catalyze the disassembly of cell structures (17). The caspase cascade can be initiated in response to the binding of an appropriate ligand to receptors on target cells or through self-aggregation of receptors in response to their increased density on the cell surface. To determine whether caspases are involved in apoptosis induction, two major caspase pathways were examined; the first is mediated through the most apical of the initiator caspases, caspase 8 (10), and the second is mediated through caspase 1. Either caspase 8 or 1 can activate caspase 3 and result in the cell disassembly associated with apoptosis.
Bovine mammary epithelial cells, designated MAC-T (9), were infected with S. aureus (wild-type strain RN6390) essentially as described previously (1, 20). After either 3 or 6 h of infection, MAC-T cell culture supernatants were discarded, and the monolayers were washed three times with sterile phosphate-buffered saline (PBS, pH 7.2). The monolayers were harvested with sterile cell scrapers (Nalge Nunc International, Naperville, Ill.), and cells were collected by centrifugation at 450 × g for 5 min at 4°C. Each cell pellet was washed once with sterile PBS and resuspended in 10 ml of sterile PBS. Cells were counted with a hemacytometer, pelleted again by centrifugation, and resuspended to a concentration of 108 cells/ml in cell lysis buffer (25 mM HEPES [pH 7.5], 5 mM MgCl2, 5 mM EDTA, 5 mM dithiothreitol, 2 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml; all reagents from Sigma, St. Louis, Mo.). Cells were lysed by freezing and thawing four times, and lysates were clarified by centrifugation at 16,000 × g and 4°C for 30 min. The supernatants of the lysates were retained, and the protein concentration of each was determined with the Bio-Rad protein assay (Bio-Rad, Hercules, Calif.).
Caspase 8 (Fas-associated death domain-like interleukin-1β-[IL-1β]-converting enzyme) activity was evaluated with the caspase-8 fluorometric assay from R & D Systems (Minneapolis, Minn.) according to the manufacturer's protocol. Caspase 3 (32-kDa cysteine protease; also called CPP32) and caspase 1 (interleukin-1β-converting enzyme) activities were assayed with the fluorometric CaspACE assay system from Promega (Madison, Wis.) according to the supplier's recommendations. Briefly, the assays involved testing the MAC-T cell lysate for caspase activity by the addition of a caspase substrate coupled to a reporter molecule (7-amino-4-trifluoromethyl coumarin). Caspase inhibitors were used to confirm substrate cleavage specificity. To determine whether the caspase activity was derived from infected MAC-T cells and not from a product expressed by staphylococci, filter-sterilized supernatants from overnight S. aureus cultures were assayed for caspase activity. No caspase 8, 1, or 3 activity was detected (data not shown).
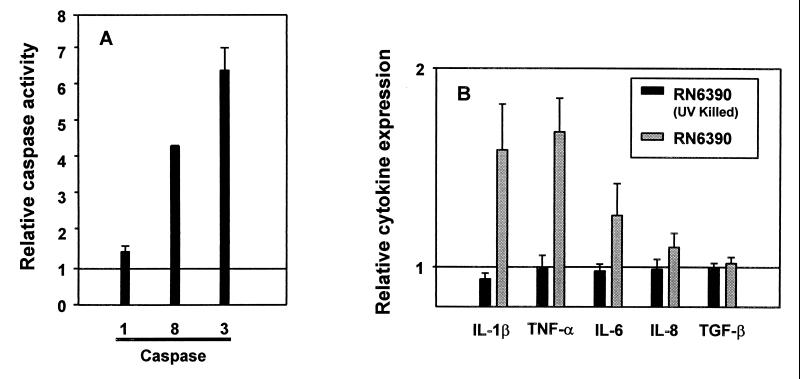
As shown in Fig. 1A, caspase 8 activity in MAC-T cells increased 4.4-fold after 6 h of infection (Fig. 1A). As a control, the agr virulence mutant RN6911, which was previously shown to be internalized but not to induce apoptosis (1, 20), did not stimulate caspase 8 activity (unpublished data). Based on the activation of caspase 8 demonstrated in our system, and because caspase 8 can cleave caspase 3 but not caspase 1 (11), we would predict the activation of caspase 3 but not caspase 1. Although caspase 1 has been shown to be the target for the induction of apoptosis by Shigella (3) and Salmonella (7), we found only minimal stimulation of caspase 1 activity in MAC-T cells after 6 h of infection (Fig. 1A) and no caspase 1 activity at 3 h after infection (data not shown). Thus, unlike the situation in Shigella and Salmonella, data from this study indicate that the induction of apoptosis by S. aureus in our system does not involve activation of caspase 1. In contrast, internalized S. aureus caused a 6.6-fold increase in caspase 3 activity by MAC-T cells compared with the uninfected control after 6 h of infection (Fig. 1A). As observed in the caspase 8 assays, the agr mutant did not show increased caspase 3 activity. It should be noted that the numbers of intracellular bacteria remain relatively constant over the time period in which caspase activity was assayed (20), indicating that the changes in caspase activity observed are likely not a function of alterations in the number of intracellular bacteria present.
FIG. 1.
(A) Caspase 1, 8, and 3 activities in MAC-T cells infected with S. aureus. MAC-T cells were harvested at 6 h after infection with S. aureus and evaluated for caspase activity. The increase in caspase activity (fold) was calculated by dividing the relative fluorescence value obtained from analysis of infected cells by that of the uninfected control. The value obtained from uninfected cells is represented as 1. In all experiments, specific caspase inhibitors reduced the fluorescent signal to the level of the uninfected control cells. Values from four (caspases 1 and 3) and two (caspase 8) separate experiments were used to calculate the mean relative activity (± standard errors of the mean). The differences in caspase 3 and 8 activities observed in control and infected MAC-T cells were analyzed by Student's t test and found to be significant (P values of 0.03 and <0.001, respectively). (B) Cytokine expression by MAC-T cells infected with S. aureus. The increase in expression of cytokine transcript levels (fold) was calculated by dividing the normalized S. aureus-induced cytokine value by the normalized cytokine value from uninfected MAC-T cells. A value of 1 indicates basal transcript levels. Values from four separate experiments were used to calculate the mean relative expression (± standard errors of the mean). The differences in IL-1β and TNF-α transcript levels between the control and infected MAC-T cells were statistically significant (P = 0.07 and 0.02, respectively), while differences in IL-6, IL-8, or TGF-β transcript levels were not (P = 0.34, 0.27, and 0.54, respectively).
The caspase 8 substrate used in this study has an absolute specificity for caspase 8 (18). The caspase 1 and 3 substrates have preferential but not absolute specificity (16, 18). Since caspase 8 is the most apical caspase in the initiation of a cascade leading to activation of caspase 3 (11), these data strongly suggest that S. aureus induces apoptosis in MAC-T cells through a mechanism involving caspases 8 and 3. This mechanism is similar to that induced by Sendai virus (2) and by Legionella pneumophila (6) in macrophages.
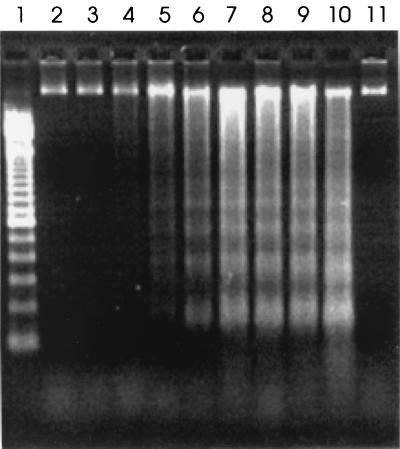
The precise pathways leading from caspase activation through DNA fragmentation to apoptotic cell death are not fully characterized. However, in apoptotic events involving caspases, the general sequence of events involves an initial stimulus followed by activation of caspase and finally cleavage by activated caspase of protein substrates with apoptotic functions (10, 15). The time required for the various events of apoptosis to occur depends on the type of initial stimulus and the type of cell (10). To determine the correlation between the time of measurable caspase activation and the earliest detection of apoptotic DNA laddering in MAC-T cells, we infected MAC-T cells with S. aureus RN6390 and assessed DNA laddering as described previously (1). Samples for DNA extraction were taken at various times postinfection. As shown in Fig. 2, apoptotic laddering of MAC-T DNA in increments of 180 bp was evident at 7 h postinfection. Since DNA laddering is a terminal event in apoptosis, this time is consistent with the observed prior onset of caspase activation (Fig. 1A).
FIG. 2.
Agarose gel (1.8%) electrophoretic separation of DNA extracted from infected MAC-T cells. Lane 1, 100-bp DNA marker ladder; lanes 2 through 10, samples taken 5, 6, 7, 8, 9, 10, 11, 12, and 24 h after infection with S. aureus, respectively; lane 11, uninfected control MAC-T cells harvested after 24 h of incubation.
Quantitation of cytokine transcripts.
Since the internalization of bacteria often leads to altered cytokine expression, which, in turn, can initiate apoptosis, the expression of both pro- and anti-inflammatory cytokines during the course of infection with S. aureus in epithelial cell monolayers was also evaluated. Moreover, the pathway initiated by caspase 8 is also known as “death receptor-mediated,” since binding of tumor necrosis factor (TNF) or Fas ligand to their cell surface receptors can result in the proteolytic activation of associated caspase 8 (10). Thus, the induction of cytokines can play an important role in the induction of apoptosis.
To examine cytokine expression in MAC-T cells infected with S. aureus, a quantitative reverse transcriptase-PCR technique was used. Trizol reagent (Gibco BRL, Grand Island, N.Y.) was used to isolate RNA from MAC-T cell monolayers according to the manufacturer's instructions. First-strand cDNA was prepared for the PCR with 5 μg of total RNA and Superscript II reverse transcriptase (Gibco BRL) according to the manufacturer's procedures. All reagents were prepared using diethylpyrocarbonate (Sigma)-treated water (0.01%, vol/vol). Primers, designed from sequences in GenBank, were used to amplify bovine transcripts specific for IL-1β, IL-6, IL-8, transforming growth factor β1 (TGF-β1), TNF-α, and β-actin (Table 1). The β-actin transcript was coamplified and used as an internal standard. A bulk PCR reagent mixture was prepared for each cytokine primer pair. The final concentrations of reagents in 50 μl of PCR mixture were 1× PCR buffer (Gibco BRL), 2.5 mM MgCl2, deoxynucleoside triphosphates (5 mM each A, T, C, and G), 0.3 μM β-actin primer pair, 0.3 μM cytokine primer pair, and 0.5 U of Taq DNA polymerase (Gibco BRL). Forty-nine microliters of the bulk mixture was added to 1.0 μl of cDNA solution and overlaid with mineral oil. PCR conditions consisted of an initial 95°C denaturation for 4 min followed by repeated cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, and ended with a terminal extension step of 72°C for 4 min. The total number of cycles varied from 28 to 39 depending on the cytokine transcript being amplified. Aliquots (10 μl) of each PCR mixture were loaded on a 2% agarose gel (Tris-borate-EDTA buffer) containing ethidium bromide (0.3 μg/ml), and the coamplified cytokine and β-actin products were separated by electrophoresis. PCR products were detected by UV fluorescence, and fluorescent band intensity (pixel density units) was measured using the Multi-Analyst program from Bio-Rad. Cytokine values were normalized by dividing by the coamplified β-actin value.
TABLE 1.
PCR primers for detection of bovine cytokine transcripts in MAC-T cells
| Primer | Sequence (5′ → 3′) | Predicted size (bp) | GenBank accession no. |
|---|---|---|---|
| 5′ IL-1β | CACCTGGACCTCGGTTCCATGGGA | 370 | M37211 |
| 3′ IL-1β | CAAAGCTCATGCAGAACACCACTT | ||
| 5′ IL-6 | AATGAGAAAGGAGATATGTGAGAA | 405 | X57317, S49716 |
| 3′ IL-6 | CTGAACTGCAGGAAATTCTCAAGG | ||
| 5′ IL-8 | ATGACTTCCAAACTGGCTGTTGCT | 293 | S82598 |
| 3′ IL-8 | TTCTCAGCTCTCTTCACAAATACC | ||
| 5′ TGF-β1 | CTACTACGCCAAGGAGGTCACCC | 319 | M36271 |
| 3′ TGF-β1 | CAGCCACTGCCGCACAACTCCAG | ||
| 5′ TNF-α | ACTTCGGGGTAATCGGCCCCCAGA | 345 | AF011926 |
| 3′ TNF-α | CCTTGGTCTGGTAGGAGACTGCAA | ||
| 5′ β-actin | TACCACCACAGCCGAGCGGGAAAT | 455 | K00622 |
| 3′ β-actin | GGAAGGTGGACAGGGAGGCCAGGA |
In this study, internalization of S. aureus consistently increased the transcript levels of the proinflammatory cytokines TNF-α and IL-1β (1.68- and 1.59-fold, respectively) (Fig. 1B) compared with the levels in the uninfected controls. Viable staphylococci were required to induce cytokine expression, because equivalent numbers of UV-killed RN6390 cells were unable to elevate any cytokine transcript accumulation above basal levels. Although it is currently unknown whether activation of caspases 8 and 3 occurs through signaling by TNF-α, the observation that TNF-α transcripts accumulate in cells infected with S. aureus makes this an intriguing possibility. After infection with S. aureus, transcripts for the proinflammatory cytokine IL-6 were slightly elevated (1.26-fold increase), whereas levels of transcripts encoding the anti-inflammatory cytokines IL-8 and TGF-β were similar to those in the uninfected controls (Fig. 1B).
The results of this analysis suggest that infection of MAC-T cells with S. aureus causes a significant increase in expression of the proinflammatory cytokines TNF-α and IL-1β (and possibly IL-6), while expression of the anti-inflammatory cytokines IL-8 and TGF-β appears to be unaffected. The effects of other pathogens on cytokine expression in response to internalization have been well documented and, in some cases, characterized. Shigella flexneri causes release of IL-1β through an interaction with caspase 1 to initiate an inflammatory response (3). In contrast, Yersinia spp. inhibit production of TNF-α to suppress inflammation (12). Interestingly, strains of S. aureus are variable in their ability to induce inflammation. For example, S. aureus is typically considered to be pyogenic, and many infections with S. aureus increase the level of proinflammatory cytokines in different cell types (13, 21). However, the lack of an inflammatory response frequently associated with certain types of S. aureus infections, such as subclinical bovine mastitis (14) and human toxic shock syndrome (5), might suggest that mechanisms to suppress the inflammatory response also exist. Work is currently in progress to determine whether certain isolates differ in their ability to induce apoptosis and inflammatory cytokines and to characterize the staphylococcal factor(s) responsible for the induction of these events.
Acknowledgments
Grant support was received from National Institutes of Health grant R29-AI38901 (K.W.B.), National Science Foundation-Idaho Experimental Program to Stimulate Competitive Research grant EPS-9720634 (K.W.B.), National Research Initiative Competitive Grants Program U.S. Department of Agriculture grant 9402399 (G.A.B.), Public Health Service grant AI28401 (G.A.B.), and the United Dairymen of Idaho (G.A.B.).
REFERENCES
- 1.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitzer M, Prinz F, Bauer M, Spiegel M, Neubert W J, Gregor M, Schulze-Osthoff K, Lauer U. Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32) J Virol. 1999;73:702–708. doi: 10.1128/jvi.73.1.702-708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 4.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fast D J, Schlievert P M, Nelson R D. Nonpurulent response to toxic shock syndrome toxin 1-producing Staphylococcus aureus. Relationship to toxin-stimulated production of tumor necrosis factor. J Immunol. 1988;140:949–953. [PubMed] [Google Scholar]
- 6.Gao L Y, Abu Kwaik Y. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect Immun. 1999;67:4886–4894. doi: 10.1128/iai.67.9.4886-4894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 9.Hyunh H T, Robitaille G, Turner J D. Establishment of bovine mammary epithelial cells (MAC-T): an in vivo model for bovine lactation. Exp Cell Res. 1991;197:191–199. doi: 10.1016/0014-4827(91)90422-q. [DOI] [PubMed] [Google Scholar]
- 10.Kidd V J. Proteolytic activities that mediate apoptosis. Annu Rev Physiol. 1998;60:533–573. doi: 10.1146/annurev.physiol.60.1.533. [DOI] [PubMed] [Google Scholar]
- 11.Muzio M, Salvesen G S, Dixit V M. FLICE induced apoptosis in a cell-free system: cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 12.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 13.Persson-Waller K. Accumulation of leucocytes and cytokines in the lactating ovine udder during mastitis due to Staphylococcus aureus and Escherichia coli. Res Vet Sci. 1997;62:63–66. doi: 10.1016/s0034-5288(97)90182-x. [DOI] [PubMed] [Google Scholar]
- 14.Pyorala S. Staphylococcal and streptococcal mastitis. In: Sandholm M, Honkanen-Buzalski T, Kaartineu L, Pyoral S, editors. The bovine udder and mastitis. Helsinki, Finland: University of Helsinki; 1995. pp. 143–148. [Google Scholar]
- 15.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 16.Talanian R V, Quinlan C, Trautz S, Hackett M C, Mankovich J A, Banach D, Ghayur T, Brady K D, Wong W W. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 17.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 18.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B: functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 19.Vann J M, Proctor R A. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and dose-dependent damage to endothelial cell monolayers. Infect Immun. 1987;55:2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesson C A, Liou L E, Todd K M, Bohach G A, Trumble W R, Bayles K W. The Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect Immun. 1998;66:5238–5243. doi: 10.1128/iai.66.11.5238-5243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao L, Bengualid V, Lowy F D, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]