Abstract
Primary cardiac lymphomas (PCLs) are extremely rare tumors with a poor prognosis. They usually involve the pericardium and the right side of the heart. PCLs arising from the left side of the heart are relatively rare, with bilateral cases being even rarer. We herein report a case of PCL arising from both the right and left sides of the heart in a 65-year-old man. Multiple imaging modalities clearly demonstrated the tumors at the initial evaluation. The pathological diagnosis was diffuse large B-cell lymphoma.
Keywords: diffuse large B-cell lymphoma, right atrium, left atrium, left ventricle
Introduction
Primary cardiac lymphomas (PCLs) account for 1-2% of all surgically resected heart tumors (1). PCLs have a very poor prognosis, with 60% of patients dying within 2 months of the diagnosis (2). PCLs were initially defined as any extranodal lymphoma involving only the heart and/or pericardium. However, the definition was later expanded to include cases of lymphoma with cardiac manifestations where the bulk of the disease is located in the heart. The World Health Organization defines PCLs as cases of lymphoma presenting with cardiac symptoms, with most of the tumors being intrapericardial at the time of presentation (1). The most common location of PCLs is the right atrium, followed by the right ventricle, with left heart involvement being rare.
We herein report a case of PCL affecting both the right and left sides of the heart.
Case Report
A 65-year-old man with a history of exertional dyspnea for 2 weeks and massive pericardial effusion (Fig. 1) detected by computed tomography (CT) at another hospital was referred to our hospital for a further evaluation of his pericardial effusion. His medical history included hypertension, intracranial hemorrhaging, and Parkinson's syndrome. Upon admission, he had an arterial blood pressure of 83/66 mmHg; heart rate, 90 beats/min; arterial blood oxygen saturation, 95% in room air; and body temperature, 37.1°C. Cardiac auscultation was normal, and mild pitting edema was observed at both lower extremities, without any sign of lymphadenopathy. Electrocardiography revealed no remarkable findings. Chest radiography revealed marked cardiomegaly with a cardiothoracic ratio of 78%. Transthoracic echocardiography demonstrated pericardial effusion as well as two low-echoic masses that were spatially separate (Fig. 2): one in the lateral wall of the right atrium (35×37 mm) and one extending from the posterior wall of the left atrium to the basal part of the left ventricle (36×55 mm).
Figure 1.

Chest computed tomography performed at another hospital the day before admission, revealing marked pericardial effusion (arrow) and mild bilateral pleural effusion (arrowheads).
Figure 2.

An initial two-dimensional transthoracic echocardiogram in the apical view, revealing a mass in the lateral wall of the right atrium (yellow arrows) and another mass lesion extending from the posterior wall of the left atrium to the left ventricle (white arrows). Pericardial effusion was also observed. RA: right atrium, RV: right ventricle, LA: left atrium, LV: left ventricle
The patient's laboratory data were as follows: white blood cells, 6,300/μL; platelets, 294,000/μL; aspartate aminotransferase, 37 U/L; alanine aminotransferase, 31 U/L; lactic dehydrogenase, 428 U/L; blood urea nitrogen, 17.2 mg/dL; creatinine, 0.9 mg/dL; uric acid, 5.5 mg/dL; brain natriuretic peptide, 66.2 pg/mL; and soluble interleukin-2 receptor, 1,354.3 U/L.
On hospitalization day 2, cardiac positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) was performed following proper dietary preparation (i.e., prolonged fasting and a carbohydrate-free diet). PET revealed the marked accumulation of FDG in the heart, two mediastinal lymph nodes, and two mesenteric lymph nodes. An FDG-PET and CT image fusion analysis revealed the cardiac accumulation of FDG to be located in the right atrium and along the left atrial and ventricular walls (Fig. 3).
Figure 3.
FDG-PET/CT findings following proper dietary preparation. (A) Maximum intensity projection of whole-body FDG-PET, revealing the FDG accumulation in the heart (black arrows), two mediastinal lymph nodes (red arrows), and two mesenteric lymph nodes (blue arrows). (B) FDG-PET and CT image fusion, (B) FDG-PET and CT image fusion, demonstrating that the accumulation of FDG in the heart was located in the right atrium (black arrow) and along the left atrial and ventricular walls (white arrow). FDG: 18F-fluorodeoxyglucose, PET: positron emission tomography, CT: computed tomography
Furthermore, magnetic resonance imaging demonstrated two cardiac masses (Fig. 4A) with isointensity on T1- (Fig. 4B) and mildly hyperintensity on T2-weighted images (Fig. 4C). These signal intensity findings were considered to be consistent with previous findings on PCLs (3).
Figure 4.
A: Cine-mode magnetic resonance imaging, demonstrating the two cardiac tumor masses (yellow arrows). B: Axial T1-weighted magnetic resonance imaging, demonstrating isointense masses (yellow arrows). C: Axial T2-weighted magnetic resonance imaging, demonstrating mildly hyperintense masses (yellow arrows). RA: right atrium, RV: right ventricle, LA: left atrium, LV: left ventricle
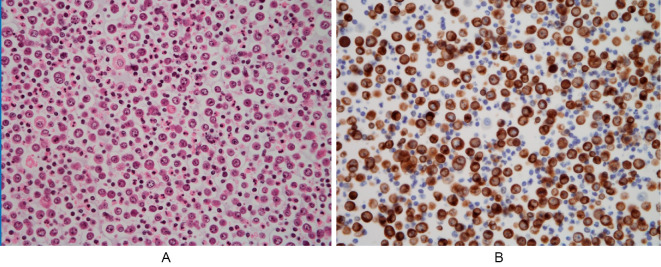
During percutaneous pericardiocentesis, 440 mL of bloody fluid was aspirated from the pericardial cavity. A cytological examination (cell block) of the pericardial effusion revealed many atypical lymphoid cells with enlarged nuclei and nucleoli. Immunohistochemistry demonstrated positive staining for cluster of differentiation (CD) 20 and CD79a, whereas no staining was detected for CD3 or CD5 (Fig. 5). We therefore suspected diffuse large B-cell-lymphoma.
Figure 5.
Cell block cytology of pericardial effusion, showing many atypical lymphoid cells with enlarged nuclei and nucleoli. (A) Hematoxylin and Eosin staining (×200). (B) Positive immunohistochemical staining for CD79a (×200). CD: cluster of differentiation
Since the patient and his family declined chemotherapy despite our recommendations, only supportive care was provided. Contrast-enhanced CT performed on hospitalization day 22 revealed marked enlargement of the cardiac tumors (Fig. 6). His general condition rapidly deteriorated, and he died on hospitalization day 34. Autopsy findings revealed that despite the marked enlargement of the cardiac tumors, no continuity of the tumors on either side of the heart was evident. The diagnosis of diffuse large B-cell lymphoma was confirmed by a pathological analysis.
Figure 6.

Contrast-enhanced computed tomography findings obtained on hospitalization day 22, revealing marked enlargement of the cardiac tumors (arrows).
Immunohistochemistry showed positive staining for CD20, CD79a, and Bcl-6, whereas no staining was detected for CD3, CD5, CD10, or MUM-1 (Fig. 7). This result was similar for the tumors in both the right atrium and left side of the heart, suggesting that the tumors in both sides of the heart were likely derived from the same cell. Based on the immunohistochemical findings, this tumor was subclassified as germinal center B-cell-like (GCB) (4,5).
Figure 7.
An immunohistochemical examination of the tumor tissue obtained by an autopsy showing positive staining for Bcl-6 (A) and negative staining for MUM-1 (B) (×200).
Discussion
PCLs are rare and account for 1-2% of all primary malignant cardiac tumors (2). They were initially defined as any extranodal lymphoma involving only the heart and/or pericardium; however, this definition was later broadened to include cases of lymphoma with cardiac manifestations where the bulk of the disease is in the heart (1).
According to previous reports, right heart involvement was far more common than left heart involvement. The most affected chamber was the right atrium>right ventricle>LA>left ventricle, in that order (6). PCLs of the left side of the heart are rare (7-9), with bilateral PCL cases being even rarer. There are several reports of PCL in both the right and left sides of the heart (Table) (10-22). However, in most cases, tumors of the right and left sides of the heart are connected, exist as a mass, and are frequently observed in the right and left atria across the interatrial septum. It has therefore been postulated that the tumor in the right atrium might have invaded the atrial septum and subsequently spread to the left atrium, or vice versa. Zyssman et al. reported a case of Burkitt lymphoma with separate tumors in the right atrium, right ventricle, and left atrium (10). Furthermore, Chao et al. reported a case of PCL affecting the right and left atria in which whether the tumor growths were continuous or discontinuous could not be confirmed due to the lack of clear images (11). To our knowledge, there are no other reports of PCL with discontinuous tumors in the right and left sides of the heart. At the very least, there are no reports of diffuse large B-cell lymphoma with separately located tumors in both sides of the heart where clear imaging findings have been presented.
Table.
Case Reports of PCLs Involving Both Sides of the Heart in the English Literature.
| Age | Sex | Location | Tumor | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| 29 | Unknown | RA, RV, LA | Apart | 6 | ||||
| 57 | M | RA, LA | Not confirmed | 7 | ||||
| 69 | M | RA, Other | Contact | 8 | ||||
| 48 | M | RA, LA | Contact | 9 | ||||
| 40 | F | RA, LA | Contact | 10 | ||||
| 67 | M | RA, LA | Contact | 11 | ||||
| 42 | M | RA, LA | Contact | 12 | ||||
| 63 | F | RA, LA | Contact | 13 | ||||
| 48 | F | RA, LA, LV | Contact | 14 | ||||
| 70 | Unknown | RA, LA | Contact | 15 | ||||
| 87 | M | RV, LV | Contact | 16 | ||||
| 57 | M | RA, LA | Contact | 17 | ||||
| 48 | M | RA, LA | Contact | 18 |
PCL: primary cardiac lymphoma, RA: right atrium, RV: right ventricle, LA: left atirium, LV: left ventricle, M: male, F: female
In our case, various imaging modalities employed at the initial assessment revealed that the tumor in the right atrium and that extending from the left atrium to the base of the left ventricle were completely separate. One possible mechanism behind the bilateral formation of tumor masses in this case, other than the two masses that appeared multifocally, is a mechanism mediated by pericardial effusion. In the present case, we observed pericardial fluid retention and lymphoma cells in the pericardial fluid. There is a possibility that the lymphoma cells in the pericardial fluid might have infiltrated the myocardium, leading to multiple tumor formations. Nevertheless, we believe that this mechanism is unlikely. Although pericardial effusion is a relatively common manifestation in malignancies, metastatic cardiac tumors that involve the myocardium are very rare (23). Similarly, pericardial fluid retention is known to be highly frequent in PCL patients, occurring in about one-third of cases. Thus, since there are few reports of multiple cardiac tumors despite the high frequency of pericardial fluid retention, it is plausible to suggest that the lymphoma cells in the pericardial fluid are unlikely to form tumor masses in the myocardium.
Another possible mechanism is via coronary tumor embolism. In this case, the tumor on the left side of the heart was larger than that in the right atrium, so it is highly possible that the tumor on the left side of the heart was older than the one on the right. A left-side tumor forming a tumor mass in the right atrium by coronary tumor embolism might result in a situation similar to the present case. Petrich et al. reported that 12 out of 197 patients (6%) presented with embolic phenomena that were attributed to PCL (6); however, there were no cases of coronary embolization. To our knowledge, tumor embolism related to PCL has been reported in only one case of myxoma, including malignant lymphoma (24). Therefore, the mechanism of coronary micro-embolism does not appear to be frequent, and it is difficult to prove.
We encountered a case that was considered to be extremely rare. Furthermore, at the time of the evaluation, clear images were obtained with various imaging modalities. Therefore, we consider this to be a valuable case and reported it.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol 11: 441-452, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Burazor I, Aviel-Ronen S, Imazio M, et al. Primary malignancies of the heart and pericardium. Clin Cardiol 37: 582-588, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoey ET, Shahid M, Ganeshan A, Baijal S, Simpson H, Watkin RW. MRI assessment of cardiac tumours: part 2, spectrum of appearances of histologically malignant lesions and tumour mimics. Quant Imaging Med Surg 4: 489-497, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 29: 4079-4087, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275-282, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer 117: 581-589, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Matsunaga K, Kobayashi T, Takahashi M, Gohra H. Huge primary cardiac malignant lymphoma in the left ventricle. Ann Thorac Surg 110: e115-e118, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Sharma A, Minhas HS, Sakhuja P, Satsangi DK. Left atrial primary B cell lymphoma presenting with mitral regurgitation. J Card Surg 27: 300-303, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Thiagaraj A, Kalamkar P, Rahman R, Farah V, Poornima I. An unprecedented case report of primary cardiac lymphoma exclusive to left ventricle: a diagnostic and therapeutic challenge. Eur Heart J Case Rep 2: 1-6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zyssman I, Cantor A, Steyn M, Meyer T. Multiple intracavitary cardiac masses; an uncommon presentation of African Burkitt's lymphoma. Int J Cardiol 137: 421-423, 1992. [DOI] [PubMed] [Google Scholar]
- 11. Chao TY, Han SC, Nieh S, Lan GY, Lee SH. Diagnosis of primary cardiac lymphoma. Report of a case with cytologic examination of pericardial fluid and imprints of transvenously biopsied intracardiac tissue. Acta Cytol 39: 955-959, 1995. [PubMed] [Google Scholar]
- 12. Saotome M, Yoshitomi Y, Kojima S, Kuramochi M. Primary cardiac lymphoma - a case report. Angiology 53: 239-241, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Zakynthinos E, Tassopoulos G, Haritos C, et al. Huge biatrial primary cardiac B-cell lymphoma resulting in bilateral atrioventricular valve obstruction. Leuk Lymphoma 45: 2339-2342, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol 31: 1344-1350, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Santini F, Innocente F, Gilioli E, et al. Primary bi-atrial Burkitt lymphoma with severe inflow impairment in an immunocompetent patient. Cardiovasc Pathol 18: 123-125, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Lin JN. Cardiac lymphoma with first manifestation of recurrent syncope - a case report and literature review. Int Med Case Rep J 3: 1-6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho JS, Her SH, Park MW, et al. A butterfly-shaped primary cardiac lymphoma that showed bi-atrial involvement. Korean Circ J 42: 46-49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jonavicius K, Salcius K, Meskauskas R, Valeviciene N, Tarutis V, Sirvydis VJ. Primary cardiac lymphoma: two cases and a review of literature. J Cardiothorac Surg 10: 138, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hajj-Chahine J, Uzan C, Corbi P. Primary B-cell cardiac lymphoma presenting as a biatrial mass. Asian Cardiovasc Thorac Ann 24: 221, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Yoshihara S, Matsunaga M, Tanioka F, Naito M. Primary cardiac diffuse large B-cell lymphoma. Circ J 82: 2919-2920, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Ömeroğlu SN, Balkanay OO, Göksedef D, Öz B, İpek G. The surgical treatment of primary cardiac B-cell lymphoma of clinically unstable patient. Ann Thorac Surg 105: e215-e217, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Zhou Z, Xin F, et al. Primary cardiac lymphoma in both atria: a case report. J Clin Ultrasound 47: 561-563, 2019. [DOI] [PubMed] [Google Scholar]
- 23. Imazio M, Colopi M, De Ferrari GM. Pericardial diseases in patients with cancer: contemporary prevalence, management and outcomes. Heart 106: 569-574, 2020. [DOI] [PubMed] [Google Scholar]
- 24. Pineda AM, Mihos CG, Nascimento FO, Santana O, Lamelas J, Beohar N. Coronary Embolization from a left atrial myxoma containing malignant lymphoma cells. Tex Heart Inst J 42: 565-568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]