Abstract
Coronavirus disease 2019 (COVID-19) has spread worldwide since 2019, and mRNA vaccines for the disease have been rapidly delivered to limit the severity of infection. However, while these vaccines are effective in reducing the morbidity and severity of the disease, some patients develop severe adverse drug reactions and new-onset autoimmune phenomena, such as myocarditis, thrombosis with thrombocytopenia, and vasculitis. In addition, some patients develop arthritis following vaccination, including rheumatoid arthritis (RA). We herein report a case of new-onset seropositive RA following COVID-19 mRNA vaccination. Although tests for rheumatoid factor and anti-cyclic citrullinated peptide antibody had been negative three years before vaccination, the patient developed seropositive RA following COVID-19 mRNA vaccination.
Keywords: autoimmune phenomena, COVID-19, seropositive, mRNA vaccine, rheumatoid arthritis
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has spread worldwide since 2019. COVID-19 mRNA vaccines, including “BNT162b2” (Pfizer-BioNTech) and “mRNA-1273” (Moderna), have been rapidly delivered to ensure disease prevention in Japan. However, while these vaccines are effective in reducing the morbidity and severity of the disease with minor adverse drug reactions, such as a fever, fatigue, muscle pain, and swollen arms, some patients develop severe adverse drug reactions, such as anaphylactic shock and new-onset autoimmune phenomena, including myocarditis, thrombosis with thrombocytopenia, and vasculitis (1-3). Specifically, some patients develop arthritis following vaccination, including reactive arthritis and rheumatoid arthritis (RA) (4).
We herein report a case of new-onset seropositive RA following COVID-19 mRNA vaccination in a patient with combined pulmonary fibrosis and emphysema (CPFE). Although tests for rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (ACPA) had been negative three years before vaccination, the patient developed seropositive RA following COVID-19 mRNA vaccination.
Case Report
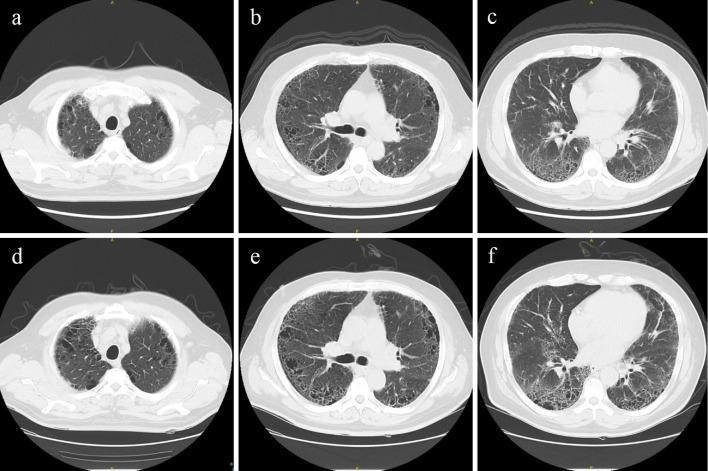
A 54-year-old man was referred to our hospital with joint pain. Three years prior to consultation with our department, he had been diagnosed with CPFE based on a 30-year smoking history and computed tomography (CT) findings with upper-lobe emphysema and lower-lobe interstitial fibrotic changes (Fig. 1). A respiratory function test showed a forced vital capacity (FVC) of 4.15 L (108.1%) and forced expiratory volume in 1 second as percent of FVC (FEV1%) of 97.9. Blood tests showed lactate dehydrogenase level (LDH), 247 U/L; Krebs von den Lungen-6 (KL-6), 1,356.8 U/mL; and pulmonary surfactant protein-D (SP-D), 216 ng/mL; however, RF and ACPA were negative.
Figure 1.
Chest CT findings. Upper CT findings (a-c) were obtained at the initial diagnosis of CPFE (3 years ago). Lower CT findings (d-f) were observed on admission. CPFE: combined pulmonary fibrosis and emphysema, CT: computed tomography
About 15 weeks prior to consulting with our department, he received a second dose of the Pfizer vaccine. One day after vaccination, the patient developed swelling and pain in the joints that did not resolve. He had shown no other chronic diseases, allergies, or infectious symptoms, nor had he received any new medications before vaccination. His respiratory symptoms had remained unchanged for three years. He had been to the dentist several times over the past year because of a decayed tooth and gum disease. The patient and his family had no documented history of COVID-19. He was referred to our department 107 days after vaccination because of joint pain.
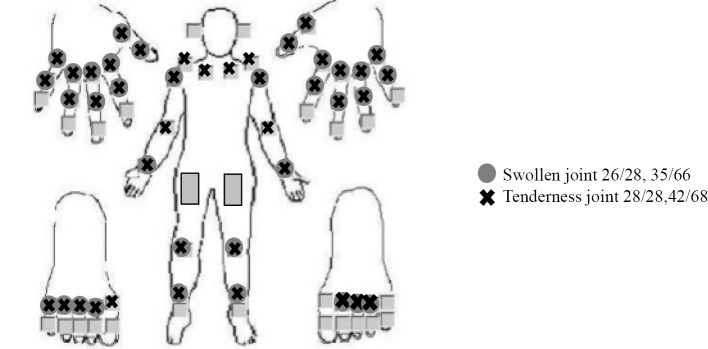
On admission, his body temperature, blood pressure, pulse, and SpO2 were 36.4°C, 145/98 mmHg, 86 beats/min, and 97% (room air), respectively. A physical examination revealed fine crackles in his lungs along with polyarthritis with swelling in 26/28 joints and tenderness in 28/28 (Fig. 2). However, he did not have skin lesions or numbness, muscle weakness, or difficulty moving his upper or lower extremities. His Disease Activity Score 28 C-reactive protein (CRP), Simplified Disease Activity Index, and Clinical Disease Activity Index scores were 7.68, 74.89, and 71.2, respectively. Blood tests showed white blood cells of 13,240 /μL, neutrophils of 7,931 /μL, lymphocytes of 4,250 /μL, red blood cells of 543×104/μL, hemoglobin of 15.9 g/dL, hematocrit of 48.5%, platelets of 36.0×104/μL, prothrombin time of 14.0 s, activated partial thromboplastin time of 30.2 s, fibrinogen of 544 mg/dL, D-dimer of 4.9 μg/mL, total protein of 7.3 g/dL, albumin of 3.3 g/dL, aspartate transaminase of 15 U/L, alanine transaminase of 11 U/L, LDH of 221 U/L, creatinine kinase of 21 U/L, creatinine of 0.66 mg/dL, total cholesterol of 235 mg/dL, low density lipoprotein cholesterol of 176 mg/dL, high density lipoprotein cholesterol of 40 mg/dL, triglyceride of 75 mg/dL, blood sugar level of 109 mg/dL, hemoglobin A1c level of 6.0%, CRP level of 3.69 mg/dL, matrix metalloproteinase-3 of 104.5 ng/mL, RF of 1,712 IU/mL, ACPA of 162 U/mL, antinuclear antibody level of 40 (homogenous+speckled pattern), KL-6 of 1,356.8 U/mL, and SP-D of 216 ng/mL, and a urinalysis showed no blood, 1-4 red blood cells/high-power field, 1-4 white blood cells/high-power field, and no protein. Tests for anti-streptolysin O, Mycoplasma, Chlamydia trachomatis, T-SPOT.TB, and polymerase chain reaction for human parvovirus B19 and other autoantibodies were negative. The COVID-19 sputum polymerase chain reaction test was negative. X-ray of the hands and feet did not show erosion or joint space-narrowing lesions.
Figure 2.
Joint findings. Polyarthritis was noted.
Chest CT showed no remarkable changes compared to the results obtained three years earlier (Fig. 1). A respiratory function test showed an FVC of 4.18 L (111.2%), FEV1% of 96.3, and % diffusing capacity for carbon monoxide (DLCO) of 61.3. He was diagnosed with RA based on the 2010 RA classification criteria and treated with methylprednisolone 8 mg/day and iguratimod 25 mg/day, showing a good clinical response (5).
Discussion
To our knowledge, this is the first report of new-onset seropositive RA following COVID-19 mRNA vaccination in a patient with a seronegative status before vaccination.
The mechanism underlying RA development after vaccination is unclear, mRNA vaccine presents as both antigen and adjuvant, and is identified by endosomal Toll-like receptors and cytosolic inflammasome components (4,6). The adjuvant may activate the NLR pyrin domain containing 3 (NLRP3) inflammasome (7). NLRP3 is a protein expressed on antigen-presenting cells, such as dendritic cells, monocytes, and macrophages (8). NLRP3 is an important mediator of innate immunity and T cell priming and is involved in the formation of the NLRP3 inflammasome (9,10). The NLRP3 inflammasome plays an important role in innate and adaptive immune system and is implicated in the pathogenesis of several autoimmune diseases, including RA (11). However, a previous report indicated that autoinflammation occurs in genetically predisposed individuals (12). In addition, ACPA is a predictive factor for the development of RA, and the ACPA status is associated with specific environmental factors, including the smoking history and the oral cavity condition (13).
In our case, although the patient’s tests for RF and ACPA had been negative three years before vaccination, he was a heavy smoker with a gum disease and eventually developed seropositive RA following COVID-19 mRNA vaccination, suggesting that the COVID-19 mRNA vaccine might have triggered seropositive RA in this patient with a predisposition to RA. However, there is no objective, clear evidence that the COVID-19 vaccination triggered the onset of RA in this case, as we were unable to examine previous blood samples to determine whether or not serological signs of RA had developed within one year before the vaccination.
Several investigators have reported flare-up or the new onset of RA after COVID-19 vaccination (4). However, the US Food and Drug Administration reported that arthritis in patients following COVID-19 vaccination was not related to vaccinations (14). Another report also showed that full vaccination with COVID-19 vaccines may not be associated with arthritis flares (15). The European League against Rheumatic Diseases and the American College of Rheumatology recommend COVID-19 vaccination for patients with systemic rheumatic diseases (SRD), even though vaccination has the potential to induce the new onset or relapse of SRD.
Factors associated with the development of severe adverse drug reactions to COVID-19 mRNA vaccines have not yet been clarified. A larger prospective study is thus warranted to investigate new-onset autoimmune diseases following COVID-19 vaccination.
In conclusion, COVID-19 vaccines may induce autoimmune diseases, including RA. Clinicians should be alert for the occurrence of these diseases after COVID-19 vaccination.
Written informed consent for this case report was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. ; the C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. ; the COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 16: 413-414, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Xu Z, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 165: 386-401, 2022. [DOI] [PubMed] [Google Scholar]
- 5. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69: 1580-1588, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21: 195-197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reinke S, Thakur A, Gartlan C, Bezbradica JS, Milicic A. Inflammasome-mediated immunogenicity of clinical and experimental vaccine adjuvants. Vaccines (Basel) 8: 554, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guarda G, Zenger M, Yazdi AS, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol 186: 2529-2534, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Dupaul-Chicoine J, Arabzadeh A, Dagenais M, et al. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity 43: 751-763, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat Med 15: 1170-1178, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Li Z, Guo J, Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed Pharmacother 130: 110542, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev 269: 60-75, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol 21: 279-283, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document Moderna COVID-19 Vaccine. 2020 [Internet]. [cited 2020 Dec 17]. Available from: https://www.fda.gov/media/144434/download
- 15. Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis 81: 564-568, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]