Abstract
A 51-year-old man was admitted to the hospital with a diagnosis of Listeria monocytogenes meningitis. Diffuse cerebral edema appeared after improvement of meningitis with appropriate treatment and worsened for two months. Due to brain herniation, brain tissue leaked through the incision made during the drain insertion in a hydrocephalus surgery. We found pathological evidence of significant neutrophil infiltration with a few lymphocytes without bacterial detection in the degraded brain tissue. The present case indicates that fatal cerebral edema with significant neutrophil infiltration may develop even after appropriate treatment for L. monocytogenes meningitis.
Keywords: Listeria monocytogenes, bacterial meningitis, cerebral edema, pathology, post-infectious immune-mediated inflammation
Introduction
Listeria monocytogenes is one of the most common causes of bacterial meningitis in adults and has a higher affinity for the brain than other bacteria, which may contribute to the high mortality rate of L. monocytogenes meningitis (1). Cerebral edema is frequently accompanied by communicating hydrocephalus as a major complication of L. monocytogenes meningitis (2). However, the pathogenesis of cerebral edema following L. monocytogenes meningitis is still unknown.
We herein report a case in which severe cerebral edema with significant neutrophil infiltration persisted for a long period of time after appropriate treatment.
Case Report
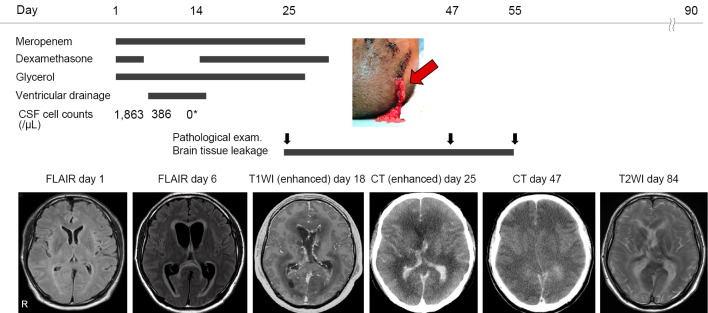
A previously healthy 51-year-old man who had a headache for 5 days was admitted to our hospital due to a high fever of 40.5°C. He was drowsy and showed disturbance of consciousness and abnormal behavior of trying to put on his pants as a jacket. On admission, brain magnetic resonance imaging (MRI) showed no abnormalities (Fig. 1). Blood tests showed an elevated white blood cell count of 15,600 cells/μL and C-reactive protein (CRP) level of 20 mg/L. A cerebrospinal fluid (CSF) analysis revealed 1,863 cells/μL (72% polymorphonucleocytes) with high protein (187 mg/dL) and low glucose (46 mg/dL) levels. Due to allergy to ampicillin, the patient was treated with meropenem, vancomycin, dexamethasone and glycerol for possible bacterial meningitis. Laboratory tests reported positive CSF and blood cultures and Gram staining of CSF for L. monocytogenes.Due to an allergy to ampicillin, he was treated with Sulfamethoxazole Trimethoprim for Listeria meningitis (3). The patient had eaten no foods that were likely to cause Listeria spp. infection, and he was negative for human immunodeficiency virus (HIV) infection. Chest and abdominal computed tomography (CT) showed no tumors or abnormalities.
Figure 1.
Clinical course. A patient with Listeria monocytogenes meningitis was initially treated with meropenem and dexamethasone. Although the CSF cell count decreased after antibiotic treatment, brain MRI revealed hydrocephalus with ventriculitis and diffuse cerebral edema on day 6. He underwent external ventricular drainage but was ventilated due to worsening cerebral edema that persisted for two months. On day 25, brain tissue had leaked through the incision site of the external drain (red arrow) due to the severe cerebral edema lasting over a month. While cerebral edema persisted, the pathology of the leaked specimen showed significant neutrophil infiltration. On day 55, the brain tissue stopped leaking, indicating that the cerebral edema had finally subsided. A specimen revealed no neutrophils. *The CSF cell count (0 cells/μL) shown at hospital day 14 was corrected by the red blood cell count. CSF: cerebrospinal fluid, MRI: magnetic resonance imaging, FLAIR: fluid-attenuated inversion-recovery, T1WI: T1-weighted imaging, T2WI: T2-weighted imaging, CT: computed tomography
The next day, the patient regained consciousness and was able to speak. However, on hospital day 4, the patient fell unconscious again. Worsening of bacterial meningitis was suspected, but the CSF cell count had decreased to 386 cells/μL, and CSF cultures were negative. The IgG index was 0.62. Repeat MRI revealed diffuse cerebral edema with tentorial herniation, lateral ventricle enhancement, and hydrocephalus, probably due to ventriculitis. He underwent external ventricular drain insertion; however, he developed mydriasis and respiratory failure. He was ventilated due to worsening cerebral edema. The external drain was ineffective and thus removed. On day 14, the CSF cell count decreased to 72 cells/μL (23% polymorphonucleocytes) with 43,000 red blood cells/μL, which suggested that the cell count was nearly 0 cells/μL by correction. The CSF glucose level also improved (76 mg/dL), indicating that the antibiotics had effectively treated the bacterial infection. Thus, we resumed dexamethasone administration because the cerebral edema was suspected to have been partially caused by post-infectious immune-mediated inflammation.
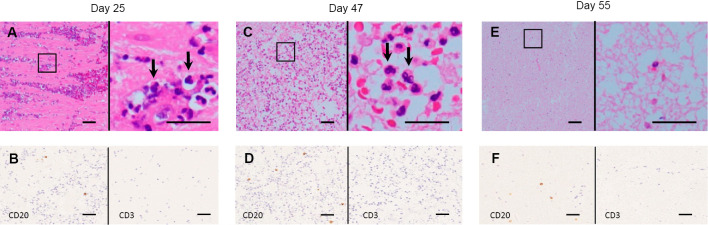
Nevertheless, CT exhibited severely worsened diffuse cerebral edema on day 25. The ventricle was severely compressed, and the sulcus had disappeared. Due to increased intracranial pressure, brain tissue started to leak through the incision made during the drain insertion. Macroscopically there was brain tissue with no pus. We pathologically investigated a leaked specimen and found significant neutrophil infiltration with a few lymphocytes in the degraded brain tissue. No bacteria were detected via Gram staining or culture (Fig. 2A). Immunostaining revealed a few CD20-positive lymphocytes (dyed brown) (Fig. 2B). The patient was considered brain dead based on the findings of neurological examinations, including a flat electroencephalogram. Antibiotics were discontinued, as there was no evidence of systematic infection on day 28. The brain tissue leaked for over a month, which meant that the cerebral edema was persistently progressive. On day 47, a leaked specimen showed marked neutrophil infiltration with a few lymphocytes and macrophages in the necrotic brain cells (Fig. 2C). Immunostaining revealed a few CD20-positive lymphocytes (dyed brown) (Fig. 2D).
Figure 2.
Neuropathological findings. (A) Significant neutrophil infiltration (arrows) with a few lymphocytes in the degenerated parenchyma observed in brain tissue obtained on day 25. (B) Immunostaining for CD3 and CD20 showed a few CD20-positive lymphocytes (dyed brown). (C) Significant neutrophil infiltration (arrows) with a few lymphocytes and macrophages in the necrotic brain cells also observed in brain tissue obtained on day 47. (D) Immunostaining for CD3 and CD20 showed a few CD20-positive lymphocytes (dyed brown). (E) No neutrophils with a few lymphocytes in the severely necrotic brain cells observed in brain tissue obtained on day 55. (F) Immunostaining for CD3 and CD20 showed a few CD20-positive lymphocytes (dyed brown). A, C, and E show Hematoxylin and Eosin staining. The right panels are magnifications of the left panels in A, C, and E. Scale bar=50 μm.
On day 55, the brain tissue stopped leaking, indicating that the cerebral edema had finally subsided. A specimen revealed no neutrophils and a few lymphocytes with severely necrotic brain cells (Fig. 2E). MRI revealed that the cerebral edema had subsequently improved on day 84. The patient was relocated to another hospital on day 90.
Discussion
Compared to other bacteria that cause bacterial meningitis, such as Streptococcus pneumoniae, Neisseria meningitidis,, and Haemophilus influenzae, L. monocytogenes is highly invasive to the brain due to its high affinity for neurons (1). L. monocytogenes easily enters axons and migrates into neurons and glial cells in the brain (1,4). L. monocytogenes is nearly 10-fold more efficient than other Gram-positive bacteria, including S. pneumoniae, at penetrating the central nervous system once an invasive infection has been established (5). Therefore, L. monocytogenes meningitis causes central nervous system infiltration in 47% of infected patients, with a high mortality rate of 14-43% (6,7). Encephalitis, brain abscess, and ventriculitis are often observed in patients with L. monocytogenes meningitis (1,7). In particular, communicating hydrocephalus, which is frequently accompanied by cerebral edema, is a major complication of L. monocytogenes meningitis, increasing the mortality rate to over 50%, and is often refractory to external drainage (2,7-9). In fact, our patient developed hydrocephalus refractory to external drainage, followed by cerebral edema. Ventriculitis was responsible for hydrocephalus in the early disease phase, while parenchymal inflammatory infiltration with neutrophils may have subsequently produced severe cerebral edema in our patient. The absence of bacteria in the CSF culture and leaked specimen and the normalized CSF cell count after antibiotic treatment suggest that post-infectious immune-mediated inflammation was involved in the pathogenesis of the cerebral edema.
Neuropathological case series of L. monocytogenes meningitis autopsies performed 2-21 days after the onset (7,10-12) showed meningeal infiltration in all cases, partial invasion of parenchymal tissue and abscess in some cases, and vascular inflammation of the middle meningeal artery and small parenchymal vessels and thrombosis in some cases. The inflammatory cells present in the meninges were characterized by a mix of monocytes, macrophages, and neutrophils. Gram staining showed rod-shaped bacteria. In the present case, the pathology of the leaked tissue obtained at one and two months after the onset showed significant neutrophil infiltration in the cerebral parenchyma without bacterial detection. Unlike the previously reported cases, our patient showed severe cerebral edema, which may have resulted in the pathological differences between those cases and the present case. In addition, the examination of the leaked specimen was performed a very long time after the disease onset.
The present case exhibited persistent cerebral edema for two months. Previous studies with pigs or mice infected with L. monocytogenes showed that delayed-type hypersensitivity mediated by macrophages and lymphocytes continued for at least two months (13,14), although the dominant inflammatory cells were different from those in the present case (i.e. neutrophils). Chemotactic factors derived from peptides from lysed bacteria and necrotic tissue facilitate continuous neutrophil migration even after bacterial extermination (15), which may have induced post-infectious inflammation leading to persistent cerebral edema with neutrophil infiltration in our patient.
In the present case, despite the use of dexamethasone, cerebral damage gradually progressed even after intrathecal sterility was achieved through antimicrobial therapy. Since early dexamethasone treatment reduces mortality in pneumococcal meningitis (16), dexamethasone is recommended for the initial treatment of bacterial meningitis. In contrast, a recent prospective cohort study revealed that the dexamethasone group had a higher mortality rate than the non-dexamethasone group in L. monocytogenes meningitis (17). Therefore, dexamethasone should be discontinued if L. monocytogenes meningitis is diagnosed. However, we resumed dexamethasone administration on day 14 because post-infectious immune-mediated inflammation was suspected to have caused the severe cerebral edema in the present case. Consequently, the dexamethasone treatment showed no response. Since the time between the onset of symptoms and the initiation of treatment critically influences the prognosis of bacterial meningitis, the pathological process may have irreversibly progressed by the time the patient was admitted, five days after the onset of symptoms. Given these factors, we suspect that the cerebral damage caused by the immune-mediated inflammation worsened even after intrathecal sterility had been achieved through antimicrobial therapy and the dexamethasone treatment had been resumed.
The present case suggests that fatal cerebral edema with significant neutrophil infiltration may develop even after appropriate treatment for L. monocytogenes meningitis. Further investigations are needed to identify effective treatments for preventing post-infectious cerebral edema.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank the staff of the Departments of Neurology and Neurosurgery at the University of Fukui Hospital who were involved in the care of the patient.
References
- 1. Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 53: 151-165, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Kasanmoentalib ES, Brouwer MC, van der Ende A, van de Beek D. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 75: 918-923, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Young N, Thomas M. Meningitis in adults: diagnosis and management. Intern Med J 48: 1294-1307, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Peters M, Hewicker-Trautwein M. Infection of murine fetal brain cell cultures with Listeria monocytogenes. Vet Microbiol 41: 19-28, 1994. [DOI] [PubMed] [Google Scholar]
- 5. Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med 337: 970-976, 1997. [DOI] [PubMed] [Google Scholar]
- 6. de Noordhout CM, Devleesschauwer B, Angulo FJ, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 14: 1073-1082, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engelen-Lee JY, Koopmans MM, Brouwer MC, Aronica E, van de Beek D. Histopathology of Listeria meningitis. J Neuropathol Exp Neurol 77: 950-957, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang KW, Chang WN, Chang HW, Wang HC, Lu CH. Clinical relevance of hydrocephalus in bacterial meningitis in adults. Surg Neurol 64: 61-65, 2005; discussion 66. [DOI] [PubMed] [Google Scholar]
- 9. Bodilsen J, Schønheyder HC, Nielsen H. Hydrocephalus is a rare outcome in community-acquired bacterial meningitis in adults: a retrospective analysis. BMC Infect Dis 13: 321, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antal EA, Løberg EM, Dietrichs E, et al. Neuropathological findings in 9 cases of listeria monocytogenes brain stem encephalitis. Brain Pathol 15: 187-191, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uldry PA, Kuntzer T, Bogousslavsky J, et al. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J Neurol 240: 235-242, 1993. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis 16: 689-702, 1993. [DOI] [PubMed] [Google Scholar]
- 13. Dustoor MM, Blazkovec AA. Acquired cellular resistance, delayed hypersensitivity, and altered macrophage migration in Listeria monocytogenes-infected guinea pigs. Infect Immun 21: 10-16, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koga T, Mitsuyama M, Handa T, Yayama T, Muramori K, Nomoto K. Induction by killed Listeria monocytogenes of effector T cells mediating delayed-type hypersensitivity but not protection in mice. Immunology 62: 241-248, 1987. [PMC free article] [PubMed] [Google Scholar]
- 15. Wittmann S, Fröhlich D, Daniels S. Characterization of the human fMLP receptor in neutrophils and in Xenopus oocytes. Br J Pharmacol 135: 1375-1382, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015: CD004405, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charlier C, Perrodeau É, Leclercq A, et al. ; MONALISA study group. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 17: 510-519, 2017. [DOI] [PubMed] [Google Scholar]