Abstract
Objective
Thrombocytosis can occur as a primary event accompanying hematological diseases or as a secondary event. Since the publication of the World Health Organization classification in 2008, thrombocytosis is now generally defined as a platelet count above 450×109/L. Furthermore, the discovery of driver-gene mutations in myeloproliferative neoplasms (MPNs) has simplified the diagnostic approach for thrombocytosis. To identify the causes of thrombocytosis using this new definition, we conducted a retrospective study.
Methods
We identified outpatients and inpatients aged 20 years or older with platelet counts >450×109/L in a half-year period at a single institute and analyzed the causes of thrombocytosis and associated clinical characteristics.
Results
Among 1,202 patients with thrombocytosis, 150 (12.5%) had primary and 999 (83.1%) had secondary thrombocytosis. Of these patients with primary thrombocytosis, 129 (86%) had at least 1 molecular marker indicative of MPNs. The major causes of secondary thrombocytosis were tissue injury (32.2%), infection (17.1%), chronic inflammatory disorders (11.7%) and iron deficiency anemia (11.1%). The median platelet count and the incidence of thrombosis were significantly higher in patients with primary thrombocytosis than in those with secondary thrombocytosis.
Conclusion
Thrombocytosis mainly occurs as a secondary event; however, it is important to determine the cause of and prevent thrombosis, particularly in cases of primary thrombocytosis.
Keywords: thrombocytosis, myeloproliferative neoplasms, essential thrombocythemia, thrombotic events
Introduction
An elevated platelet count, also known as “thrombocytosis,” can occur as a primary event accompanying hematological diseases or as a secondary event. The most frequent hematological diseases that cause thrombocytosis are myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET), polycythemia vera (PV) and chronic myeloid leukemia (CML), myelodysplastic syndromes (MDS) and MDS/MPN. The Polycythemia Vera Study Group proposed a platelet count of >1,000×109/L for the diagnosis of ET in the 1970s (1); however, this was later revised to 600×109/L (2,3). Following this revision, in the 3rd edition of the World Health Organization (WHO) classification system for tumors of hematopoietic and lymphoid tissues (WHO 2001 criteria), the criterion for ET was defined as a platelet count ≥600×109/L (4). Subsequently, in the 4th edition of the WHO classification (WHO 2008 criteria), the threshold platelet level was reduced to 450×109/L (5). This was due to several concerns that the earlier threshold of 600×109/L might not have detected the early phase of this disease (6). In addition, the discovery of the JAK2V617F mutation (7-10) made it possible to lower the threshold. Accompanying these changes, the British Committee for Standards in Haematology also proposed a threshold platelet count of 450×109/L for the diagnosis of ET (11,12). Finally, the revised 4th edition of the WHO classification (WHO 2017 criteria) maintained the platelet threshold at 450×109/L (13); thrombocytosis is therefore now generally defined as a platelet count above 450×109/L.
The major change concerning the diagnostic criteria of ET from the WHO 2008 criteria to the WHO 2017 criteria is the addition of driver-gene mutations associated with ET. As mentioned above, the JAK2V617F mutation was found in the majority of patients with Philadelphia chromosome-negative MPNs in 2005 (7-10), and approximately half of patients with ET harbor the JAK2V617F mutation. The second-most common mutation is in calreticulin (CALR) exon 9, which is detected in 20-30% of patients with ET (14,15), and 3-5% of patients with ET harbor the MPL gene mutations (16). These driver-gene mutations are clearly described in the WHO 2017 criteria as the major diagnostic criteria (13) and have become important molecular markers to diagnose MPNs.
Previously, a large study reported the causes of thrombocytosis (platelet count ≥500×109/L) in 2,000 cases in an adult Turkish population (17). In this study, only 3.3% of the patients had primary thrombocytosis, and approximately half of them were diagnosed with ET. Since the definition of thrombocytosis has now changed, and the driver-gene mutations allow for the clear differentiation between primary and secondary thrombocytosis, a fresh assessment to identify the cause of thrombocytosis using this new definition is warranted.
Therefore, we conducted a retrospective study to investigate the causes of thrombocytosis and the clinical characteristics of patients with thrombocytosis.
Materials and Methods
We retrospectively identified outpatients and inpatients aged 20 years or older with thrombocytosis (platelet count >450×109/L) between July and December 2017 at Juntendo University Hospital, which is a 1,051-bedded hospital providing advanced treatment with approximately 1,250,000 admissions annually. Both new patients and patients regularly receiving medical care at this hospital were included in the current study. If a patient showed elevated platelet counts twice or more during the given period, the highest platelet count was considered for the analysis. The clinical features of each patient were retrieved from the electronic medical records. The patients' age, sex, diagnosis, medical history and laboratory data were recorded. Symptomatic thrombosis included cerebral infarction, transient ischemic attack, myocardial infarction, angina pectoris, deep venous thrombosis, pulmonary embolus, splenic infarction, aortic thrombosis and portal thrombosis. This study was approved by the institutional ethics committee of Juntendo University Hospital (#18-135).
Continuous variables are described as the median and range and were compared between the groups using the Mann-Whitney U test for two groups and the Kruskal-Wallis test for three or more groups. Categorical variables are described as counts and percentages and were compared between groups using the chi-square test. Statistical computations were performed using EZR (18).
Results
During the half-year period, 1,202 patients from 30 departments aged 20 years or older showed elevated platelet counts (>450×109/L). The Department of Hematology saw the largest number of patients (n=169), followed by the Department of Gastroenterology (n=125), Department of Rheumatology (n=92), Department of Gynecology (n=88), Department of General Thoracic Surgery (n=83) and others (Supplementary material 1). Most patients who visited the Department of Hematology were diagnosed with primary thrombocytosis, while only a few patients in other departments were diagnosed with primary thrombocytosis.
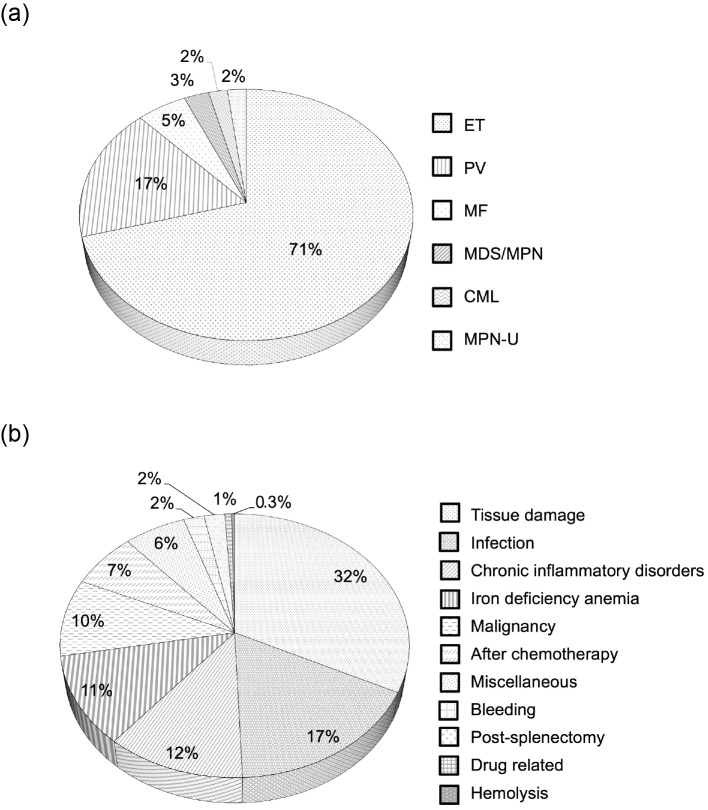
Of the 1,202 patients diagnosed with thrombocytosis, 150 (12.5%) and 999 (83.1%) were diagnosed with primary and secondary thrombocytosis, respectively. The causes of thrombocytosis in 53 patients (4.4%) were not identified. The most common disease leading to primary thrombocytosis was ET, which was seen in 106 patients (70.7%), followed by PV (n=26; 17.3%) and other hematological diseases (n=18; 12%) (Fig. 1a). Of these 150 patients with primary thrombocytosis, 129 (86%) had at least 1 molecular marker among JAK2 gene mutations, CALR gene mutations, MPL gene mutations, or the BCR-ABL1 fusion gene indicative of MPNs. In particularly, among patients with the most common disease of ET, 55 (51.9%) harbored JAK2V617F mutations, 30 (28.3%) harbored CALR gene mutations, and 4 (3.8%) harbored MPL gene mutations.
Figure 1.
(a) Causes of primary thrombocytosis. (b) Causes of secondary thrombocytosis. ET: essential thrombocythemia, PV: polycythemia vera, MF: myelofibrosis, MDS/MPN: myelodysplastic/myeloproliferative neoplasms, CML: chronic myeloid leukemia, MPN-U: myeloproliferative neoplasms, unclassifiable
The major causes of secondary thrombocytosis included tissue damage (n=322; 32.2%), infection (n=171; 17.1%), chronic inflammatory disorders (n=117; 11.7%), iron deficiency anemia (IDA) (n=111; 11.1%) and malignancy (n=95; 9.5%) (Fig. 1b). The median platelet count was 523×109/L (range: 451-1,020×109/L) in patients with tissue damage, 523×109/L (range: 451-1,125×109/L) in those with infection, 517×109/L (range: 451-934×109/L) in those with chronic inflammatory disorders, 491×109/L (range: 451-1,103×109/L) in those with IDA and 560×109/L (range: 455-1,137×109/L) in those with malignancy. Significant differences in the platelet counts were found between patients with these 5 major causes of secondary thrombocytosis (p<0.001) as well as between the patients with IDA and those with tissue damage (p=0.009), those with infection (p=0.013), and those with malignancy (p<0.001). Among infections causing thrombocytosis, respiratory infections (n=51), urinary tract infections (n=23) and soft-tissue infections (n=22) were the most common causes. The most common malignancies causing thrombocytosis were lung cancer (n=15), gastric cancer (n=9), esophageal cancer (n=9), pharyngeal cancer (n=8), malignant lymphoma (n=8) and colorectal cancer (n=7).
The patients' clinical characteristics are shown in Table. Patients with primary thrombocytosis showed significantly higher platelet counts than those with secondary thrombocytosis (median: 786×109/L vs. 518×109/L, p<0.001). In addition, leukocyte counts (median: 9.2×109/L vs. 7.8×109/L, p<0.001), hemoglobin levels (median: 13.6 g/dL vs. 11.0 g/dL, p<0.001), hematocrit counts (median: 41.8% vs. 33.9%, p<0.001) and lactate dehydrogenase levels (median: 253 U/L vs. 191 U/L, p<0.001) were significantly higher in patients with primary thrombocytosis than in those with secondary thrombocytosis. A history of thrombosis was found in 15.3% of patients with primary thrombocytosis. Although 100 patients with secondary thrombocytosis had a history of thrombosis, only 12 cases of thrombosis were linked to thrombocytosis (1.2%), and the most common thrombosis was deep vein thrombosis/pulmonary embolism (Supplementary material 2). The prevalence of thrombosis was significantly higher in patients with primary thrombocytosis than in those with secondary thrombocytosis (15.3% vs. 1.2%, p<0.001). Six cases of thrombosis accompanied with primary thrombocytosis occurred within 1 year prior to the diagnosis among the 23 evaluated cases.
Table.
Clinical Characteristics of Patients with Primary and Secondary Thrombocytosis.
| All patients* | Patients with primary thrombocytosis (n=150) | Patients with secondary thrombocytosis (n=999) | p value (primary thrombocytosis vs. secondary thrombocytosis) | ||||
|---|---|---|---|---|---|---|---|
| Median age, years (range) | 60 (20-97) | 60 (20-88) | 59 (20-94) | 0.227 | |||
| Male, n (%) | 545 (45%) | 75 (50%) | 453 (45%) | 0.293 | |||
| Platelet count, median in 109/L (range) | 530 (451-2,761) | 786 (451-2,761) | 518 (451-1,137) | <0.001 | |||
| Leukocyte count, median in 109/L (range) | 7.9 (2.2-120.5) | 9.2 (3.2-120.5) | 7.8 (2.2-30.7) | <0.001 | |||
| Red blood cell count, median in 1012/L (range) | 3.98 (1.60-7.78) | 4.63 (2.09-7.78) | 3.92 (1.60-6.53) | <0.001 | |||
| Hemoglobin, median in g/dL (range) | 11.3 (4.6-20.2) | 13.6 (8.6-20.2) | 11.0 (4.6-18.0) | <0.001 | |||
| Hematocrit, median in % (range) | 34.9 (15.8-61.1) | 41.8 (27.5-61.1) | 33.9 (15.8-54.1) | <0.001 | |||
| Lactate dehydrogenase, median in U/L (range) | 197 (13-3,795) | 253 (19-1,090) | 191 (13-3,795) | <0.001 | |||
| History of thrombosis related to thrombocytosis | 36/1,201 (3.0%) | 23/150 (15.3%) | 12/999 (1.2%) | <0.001 |
* Patients with primary and secondary thrombocytosis, and patients with unidentified causes of thrombocytosis are included.
We defined a platelet count of 451-700×109/L as mild thrombocytosis, 701-900×109/L as moderate thrombocytosis, 901-1,000×109/L as severe thrombocytosis and >1,000×109/L as extreme thrombocytosis (19). When the patients were classified into these categories, more than 90% of those with secondary thrombocytosis had mild thrombocytosis (Fig. 2). Furthermore, the proportion of patients with extreme thrombocytosis was 30% in the primary thrombocytosis group but only 0.6% in the secondary thrombocytosis group (p<0.001).
Figure 2.
Severity of elevated platelet counts in primary and secondary thrombocytosis. The severity of elevated platelet counts was defined as follows: mild thrombocytosis (451-700×109/L), moderate thrombocytosis (701-900×109/L), severe thrombocytosis (901-1,000×109/L), and extreme thrombocytosis (>1,000×109/L).
Discussion
The causes of thrombocytosis have been reported in several studies. However, to our knowledge, this is the largest study to date analyzing the causes of thrombocytosis defined by a platelet count of >450×109/L in adults. In our study, we identified 150 patients (12.5%) with primary thrombocytosis in the total cohort of 1,202 patients with thrombocytosis, and of these 150 patients with primary thrombocytosis, 129 (86%) had at least 1 molecular marker. These data underscore the importance of analyzing molecular markers to differentiate primary thrombocytosis from secondary thrombocytosis.
The association between malignancies and thrombocytosis has been reported in several studies, and a recent systematic review suggested that thrombocytosis may be an early marker of specific types of malignancies (20). In our study, malignancy was the causative factor in 95 (9.5%) of the 999 patients with secondary thrombocytosis. Given these findings, we believe that it is important to exclude the presence of malignancy from the differential diagnosis of thrombocytosis if a patient shows thrombocytosis of unknown cause.
Rose et al. reported the causes of thrombocytosis (>500×109/L) in 801 cases, of which only 5.2% (n=42) were diagnosed as primary thrombocytosis (21). Among them, approximately half (n=22) were ET, 24% (n=10) were PV, 2.4% (n=1) were CML, and others (n=9) were unspecified myeloproliferative disorders. They particularly emphasized thrombocytosis secondary to infection, which was the largest cause of thrombocytosis, being seen in 47.9% of the patients. Another study, reported by Griesshammer et al. on 732 patients with thrombocytosis (≥500×109/L) revealed that 12% (n=89) had primary thrombocytosis (22). The causes of secondary thrombocytosis were tissue damage, infection, malignancy and chronic inflammation, which were similar to the causes identified in our study. The report also showed that the incidence of thromboembolic complications in patients with primary thrombocytosis (12.4%) was higher than that in patients with secondary thrombocytosis (1.6%). The difference between these two studies and our own is in the definition of thrombocytosis and the driver-gene mutations. The ratio of primary thrombocytosis in our study was similar to that described in the Griesshammer's report, while Rose's report showed a very low rate of primary thrombocytosis. The patients in Rose's study were enrolled between March and December 2005, when the JAK2V617F mutation had just been discovered; thus, most of the patients were likely not tested for the driver-gene mutations. This may be the reason for the low rate of primary thrombocytosis. Another reason might be that the authors emphasized secondary thrombocytosis due to infectious causes but not hematological diseases, and whether or not a hematologist was involved in the study is unclear. Therefore, the diagnosis of primary thrombocytosis might have been missed among patients in whom the causes were unknown, which accounted for 7.4% of the study population. In contrast, although the study by Griesshammer, et al. was also conducted before the discovery of driver-gene mutations, the analyses were conducted by hematologists, which might have affected the results due to an emphasis on the diagnosis of MPNs (22). To account for the difference in the definition of thrombocytosis, i.e. a platelet count of >450×109/L versus >500×109/L, we analyzed the data of patients with a platelet count >500×109/L (n=755) and found that 141 patients (18.7%) had primary and 593 patients (78.5%) had secondary thrombocytosis. Among 447 patients with a platelet count in the range of 450×109/L to 500×109/L, primary and secondary thrombocytosis were seen in 9 (2.0%) and 406 patients (90.8%), respectively. Consequently, raising the threshold of platelet count resulted in an increased rate of patients with primary thrombocytosis. Therefore, the discrepancy in the ratio of primary thrombocytosis with respect to the previous reports, might not be due to the different definitions of thrombocytosis.
As shown in Fig. 2, the ratio of patients with extreme thrombocytosis was significantly higher in patients with primary thrombocytosis than in those with secondary thrombocytosis (p<0.001). Only 8.9% of patients with secondary thrombocytosis had platelet counts >700×109/L, while the ratio was 64.7% in patients with primary thrombocytosis (Fig. 2). In addition, the proportion of patients with platelet counts >900×109/L was only 1.3% in those with secondary thrombocytosis, whereas it was 38.0% in those with primary thrombocytosis. On analyzing these results from a different perspective, among patients who had platelet counts >700×109/L, 97 (50.8%) had primary thrombocytosis, and 89 (46.6%) had secondary thrombocytosis. Furthermore, among the patients who had platelet counts >900×109/L, 57 (80.2%) had primary thrombocytosis, and 13 (18.3%) had secondary thrombocytosis. These results underscore the importance of identifying primary thrombocytosis, especially when patients show platelet counts >700×109/L. In contrast, 0.6% of patients (n=6) with extreme thrombocytosis did not have a primary causative disease. Instead, these patients had tissue damage (n=1), infection (n=1), IDA (n=1), malignancy (n=1) or other conditions that resulted in extreme thrombocytosis. Therefore, although rare, the possibility of secondary thrombocytosis underlying the etiology of patients with extreme thrombocytosis should not be overlooked within the differential diagnosis.
The risk of thrombosis in patients with secondary thrombocytosis as well as secondary erythrocytosis has been frequently discussed. Recently, Nguyen et al. reported the difference in thrombosis rates between patients with PV and secondary erythrocytosis (23). In their report, the occurrence of thrombosis was lower in patients with secondary erythrocytosis than in those with primary erythrocytosis. Similarly, in our study, the incidence of thrombosis was lower in patients with secondary thrombocytosis than in those with primary thrombocytosis, which was consistent with the findings in the study by Griesshammer et al. (22) and Aydogan et al. (17). However, there were a number of thrombotic events that we presumed were unrelated to thrombocytosis because they occurred when the platelet count was in the normal range or due to other reasons. Further studies are therefore necessary to analyze the relationship between thrombotic events and secondary thrombocytosis.
The main limitation of this study is that this was an electronic medical records-based retrospective study, which might not have accurately diagnosed the cause of thrombocytosis. The driver-gene mutations were not analyzed in all patients with thrombocytosis, which may have led to the diagnosis of secondary thrombocytosis instead of primary thrombocytosis. Finally, the Department of Hematology in our hospital follows more MPN patients than other hospitals, and some patients are referred to our hospital under suspicion of MPN; thus, we might have had more patients with primary thrombocytosis than other general hospitals. Nevertheless, this is the first study concerning the causes of thrombocytosis since the latest definition of thrombocytosis (threshold of 450×109/L) came to be widely accepted.
In conclusion, we conducted the largest retrospective study to date from a single institute and showed that, in more than 80% of the patients, thrombocytosis occurred due to secondary events. The severity of thrombocytosis, leukocyte counts, hemoglobin levels, hematocrit counts and lactate dehydrogenase levels can aid in differentiating between primary and secondary thrombocytosis. Furthermore, driver-gene mutations provide useful information for the diagnosis. The risk of thrombotic events is higher in patients with primary thrombocytosis than in those with secondary thrombocytosis; therefore, we emphasize the importance of determining the cause of thrombocytosis, which can ultimately aid in preventing thrombotic events, particularly in cases of primary thrombocytosis.
Author's disclosure of potential Conflicts of Interest (COI).
Marito Araki: Employment, Meiji Seika Pharma. Norio Komatsu: Employment, PharmaEssentia Japan.
Yoko Edahiro and Yasumitsu Kurokawa contributed equally to this work.
Supplementary Materials
* Patients with primary and secondary thrombocytosis, and patients with unidentified causes of thrombocytosis are included.
References
- 1. Laszlo J. Myeloproliferative disorders (MPD): myelofibrosis, myelosclerosis, extramedullary hematopoiesis, undifferentiated MPD, and hemorrhagic thrombocythemia. Semin Hematol 12: 409-432, 1975. [PubMed] [Google Scholar]
- 2. Murphy S, Iland H, Rosenthal D, Laszlo J. Essential thrombocythemia: an interim report from the Polycythemia Vera Study Group. Semin Hematol 23: 177-182, 1986. [PubMed] [Google Scholar]
- 3. Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group. Semin Thromb Hemost 23: 339-347, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, Lyon, 2001. [Google Scholar]
- 5. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, Lyon, 2008. [Google Scholar]
- 6. Lengfelder E, Hochhaus A, Kronawitter U, et al. Should a platelet limit of 600 × 10(9)/l be used as a diagnostic criterion in essential thrombocythaemia? An analysis of the natural course including early stages. Br J Haematol 100: 15-23, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New Engl J Med 352: 1779-1790, 2005. [DOI] [PubMed] [Google Scholar]
- 8. James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144-1148, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7: 387-397, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365: 1054-1061, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Harrison CN, Bareford D, Butt N, et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 149: 352-375, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Harrison CN, Butt N, Campbell P, et al. Modification of British Committee for Standards in Haematology diagnostic criteria for essential thrombocythaemia. Br J Haematol 167: 421-423, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Revised 4th Edition. IARC, Lyon, 2017. [Google Scholar]
- 14. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. New Engl J Med 369: 2379-2390, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. New Engl J Med 369: 2391-2405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 3: e270, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aydogan T, Kanbay M, Alici O, Kosar A. Incidence and etiology of thrombocytosis in an adult Turkish population. Platelets 17: 328-331, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dame C. Pediatric hematology. 3rd edition. Blackwell Publishing, Oxford, 2006. [Google Scholar]
- 20. Bailey SE, Ukoumunne OC, Shephard E, Hamilton W. How useful is thrombocytosis in predicting an underlying cancer in primary care? a systematic review. Fam Pract 34: 4-10, 2017. [DOI] [PubMed] [Google Scholar]
- 21. Rose SR, Petersen NJ, Gardner TJ, Hamill RJ, Trautner BW. Etiology of thrombocytosis in a general medicine population: analysis of 801 cases with emphasis on infectious causes. J Clin Med Res 4: 415-423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med 245: 295-300, 1999. [DOI] [PubMed] [Google Scholar]
- 23. Nguyen E, Harnois M, Busque L, et al. Phenotypical differences and thrombosis rates in secondary erythrocytosis versus polycythemia vera. Blood Cancer J 11: 75, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
* Patients with primary and secondary thrombocytosis, and patients with unidentified causes of thrombocytosis are included.