Abstract
Purpose:
To evaluate efficacy and safety of venetoclax + azacitidine in treatment-naïve patients with acute myeloid leukemia harboring poor-risk cytogenetics and TP53mut or TP53wt.
Patients and Methods:
We analyzed data from a phase III study (NCT02993523) comparing venetoclax (400 mg orally days 1–28) + azacitidine (75 mg/m2 days 1–7) or placebo + azacitidine, and from a phase Ib study (NCT02203773) of venetoclax + azacitidine. Patients were ineligible for intensive therapy. TP53 status was analyzed centrally; cytogenetic studies were performed locally.
Results:
Patients (n = 127) with poor-risk cytogenetics receiving venetoclax + azacitidine (TP53wt = 50; TP53mut = 54) were compared with patients with poor-risk cytogenetics (n = 56) receiving azacitidine alone (TP53wt = 22; TP53mut = 18).
For poor-risk cytogenetics + TP53wt patients, venetoclax + azacitidine versus azacitidine alone resulted in composite remission rates (CRc) of 70% versus 23%, median duration of remission (DoR) of 18.4 versus 8.5 months, and median overall survival (OS) of 23.4 versus 11.3 months, respectively. Outcomes with venetoclax + azacitidine were comparable with similarly treated patients with intermediate-risk cytogenetics and TP53wt.
For poor-risk cytogenetics + TP53mut patients, venetoclax + azacitidine versus azacitidine alone resulted in CRc of 41% versus 17%, median DoR of 6.5 versus 6.7 months, and median OS of 5.2 versus 4.9 months, respectively.
For poor-risk cytogenetics + TP53mut patients, predominant grade ≥3 adverse events (AE) for venetoclax + azacitidine versus azacitidine were febrile neutropenia (55%/39%), thrombocytopenia (28%/28%), neutropenia (26%/17%), anemia (13%/6%), and pneumonia (28%/33%). AEs were comparable between TP53mut and TP53wt patients.
Conclusions:
In poor-risk cytogenetics + TP53mut patients, venetoclax + azacitidine improved remission rates but not DoR or OS compared with azacitidine alone. However, in poor-risk cytogenetics + TP53wt patients, venetoclax + azacitidine resulted in higher remission rates and longer DoR and OS than azacitidine alone, with outcomes comparable with similarly treated patients with intermediate-risk cytogenetics. Toxicities were similar in TP53mut and TP53wt patients.
Translational Relevance.
Poor-risk cytogenetics are associated with inferior outcomes in patients with acute myeloid leukemia treated with conventional therapies. TP53 mutations (TP53mut) frequently co-occur with poor-risk cytogenetics. The phase III VIALE-A study showed that patients treated with venetoclax + azacitidine had higher remission rates and superior overall survival (OS) than patients treated with azacitidine alone. Herein, we evaluated the efficacy of venetoclax + azacitidine in patients with poor-risk cytogenetics and TP53mut or TP53 wild-type (TP53wt). Patients with poor-risk cytogenetics and TP53mut receiving venetoclax + azacitidine had higher response rates but similar durations of remission (DoR) and OS than treatment with azacitidine alone. However, patients with poor-risk cytogenetics and TP53wt who received venetoclax + azacitidine had remission rates, DoR, and OS that were superior to patients receiving azacitidine alone and comparable with those of similarly treated patients with intermediate-risk cytogenetics. Thus, in the absence of TP53mut, poor-risk cytogenetics may not be an adverse risk factor for venetoclax + azacitidine.
Introduction
The biology of acute myeloid leukemia (AML) changes with age; older adults have a higher incidence of poor-risk cytogenetics, including partial or complete loss of chromosomes 5 or 7 and the presence of complex or monosomal karyotypes (1). Poor-risk cytogenetic profiles are associated with inadequate responses to conventional therapies, a high risk of relapse, and dismal overall survival (OS; refs. 1–6). The TP53 gene plays a pivotal role in activating DNA repair and triggering cell-cycle arrest (2). TP53 is mutated in over half of human cancers, and such mutations frequently occur in AML (2, 7). TP53 mutations (TP53mut) represent an important resistance mechanism to DNA-damaging chemotherapeutic agents (2, 7), resulting in poor treatment outcomes (3, 7–9). In addition, the TP53 mutational burden, as measured by the variant allele frequency (VAF), is also linked to inferior OS (10, 11). Poor-risk cytogenetic profiles and TP53mut are highly correlated (12, 13), but both are considered independent adverse risk factors for AML (5).
Several novel targeted therapies have been approved to treat patients with AML (13). The prognostic significance of poor-risk cytogenetics with TP53mut or TP53wt has been defined in the context of conventional and typically intensive chemotherapeutic approaches, and currently, there is a lack of evidence regarding whether these prognostic classifications also apply to lower-intensity or targeted therapies that have distinct mechanisms of action.
Venetoclax with azacitidine is the current standard of care for patients with treatment-naïve AML who are not eligible for intensive induction chemotherapy (14). Herein, we report the outcomes of treatment-naïve AML patients harboring poor-risk cytogenetics with or without TP53mut, who were unfit for intensive chemotherapy due to age ≥ 75 years and/or comorbidities, and thus were treated with the venetoclax + azacitidine combination or azacitidine alone. We also compared treatment outcomes in this patient population with poor-risk cytogenetics with TP53mut or TP53wt to those of patients with intermediate-risk cytogenetics and TP53mut or TP53wt.
Patients and Methods
Patients and treatment
This pooled analysis included patients from the ongoing randomized double-blind phase III study (NCT02993523, VIALE-A) and a prior non-randomized, single-arm phase Ib study (NCT02203773). The study designs and eligibility criteria have been previously reported (4, 14, 15). Patients received 400-mg venetoclax orally daily on days 3 to 28 (100 and 200 mg given on days 1 and 2 in cycle 1) and 75-mg/m2 azacitidine intravenously or subcutaneously on days 1 to 7 every 28-day cycle, or azacitidine monotherapy. Individuals enrolled in both studies had a confirmed diagnosis of AML by the World Health Organization criteria, received no prior therapy, and were ineligible for standard induction chemotherapy due to age ≥75 years or the presence of comorbidities. A subset of patients in the phase Ib study were also treated with venetoclax 400 mg and decitabine intravenously at 20 mg/m2 on days 1 to 5 every 28-day cycle and are reported separately.
The applicable internal review boards or ethics committees approved study protocols and related documents. The studies were conducted per the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Assessment of outcomes
Response assessments [composite complete remission (CRc) = complete remission (CR) + CR with incomplete hematologic remission (CRi)] were performed at the end of cycle 1 and every three cycles thereafter. They were evaluated per modified International Working Group (IWG) criteria (16). Duration of CRc (DoR) was defined as the number of days from the date of first response (CR or CRi) per the modified IWG criteria to the earliest evidence of confirmed morphologic relapse, disease progression, or death due to disease progression. OS was defined as the time from randomization (phase III study) or the number of days from the first dose of the study drug (phase Ib study) to the date of death from any cause. Rates of transfusion independence to red blood cells (RBCs) and platelets (≥56 consecutive days) were assessed. Measurable residual disease (MRD) was assessed centrally by flow cytometry [Labcorp Drug Development (formerly Covance), Princeton, NJ, USA] in patients who achieved CRc with MRD negativity defined by European Leukemia Network guidelines (14, 17). MRD negativity was defined as one or fewer residual leukemic blasts per 1,000 leukocytes or 10−3. Patients who had one negative sample for MRD value below this cutoff at any time in the study were defined as patients with an MRD-negative response. Samples were collected at baseline from bone marrow aspirates during the clinical assessment end of cycle one and after every three cycles thereafter (17). Adverse events (AE) were graded according to the NCI Common Terminology Criteria for Adverse Events Version 4.0 (18).
Baseline cytogenetic risk was determined locally and was evaluated using National Comprehensive Cancer Network (NCCN) criteria (version 2.2016). DNA was isolated from bone marrow aspirates collected from patients prior to the first dose of the study drug and was analyzed centrally. The MyAML (next-generation sequencing, Invivoscribe, San Diego, CA, USA) assay was used to detect the presence of TP53 variants in both phase Ib and phase III studies. The assay's limit of detection was a variant allelic frequency ≥ 2.5%.
Statistical analysis
Demographics were summarized by descriptive statistics. Remission rates were summarized in counts and proportions. OS and DoR were evaluated by the Kaplan–Meier methodology. The 95% confidence intervals (CI) for time to event endpoints were estimated based on log-log transformation.
Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided following review and approval of a research proposal, Statistical Analysis Plan, and execution of a Data Sharing Agreement. Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.
Results
Patient disposition and baseline characteristics
The clinical data cutoff dates were January 4, 2020, for the phase III study, and July 19, 2019, for the phase Ib study. The pooled analysis included 353 patients treated with venetoclax + azacitidine (phase III, n = 286; phase Ib, n = 67) and 145 patients treated with azacitidine alone.
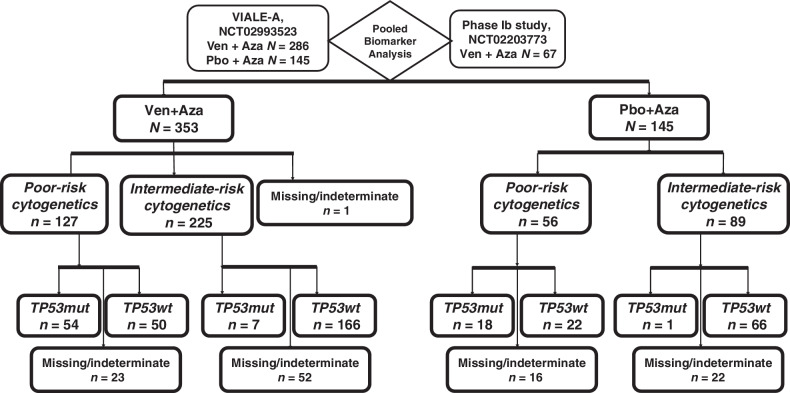
In the biomarker evaluable population, poor-risk cytogenetics were identified in 36% (n = 127/353) of patients in the venetoclax + azacitidine group and 39% (n = 56/145) in the azacitidine alone group. Among those with poor-risk cytogenetics, TP53mut was detected in 52% (n = 54/104) in the venetoclax + azacitidine group and 45% (n = 18/40) in the azacitidine alone group. Among patients with poor-risk cytogenetics, TP53wt was detected in 48% (n = 50/104) in the venetoclax + azacitidine group and 55% (n = 22/40) in the azacitidine alone group. The study design and overview of the molecular categorization of patients are shown in Fig. 1.
Figure 1.
Study design and molecular classification. Abbreviations: Aza, azacitidine; mut, mutated; Pbo, placebo; wt, wild-type; Ven, venetoclax.
The key baseline demographic characteristics for patients with poor-risk and intermediate-risk cytogenetics are shown in Table 1. Patients with poor-risk cytogenetics withTP53mut exhibited del 5 or 7 abnormalities and complex karyotypes more frequently than patients with TP53wt. In addition, FLT3, IDH1/2, and NPM1 mutations were more frequently detected among patients in the intermediate-risk group.
Table 1.
Patient demographics and baseline characteristics.
| Poor-risk cytogenetics | Intermediate-risk cytogenetics | |||||||
|---|---|---|---|---|---|---|---|---|
| Venetoclax + Azacitidine | Azacitidine | Venetoclax + Azacitidine | Azacitidine | |||||
| TP53 mut | TP53 wt | TP53 mut | TP53 wt | TP53 mut | TP53 wt | TP53 mut | TP53 wt | |
| (n = 54) | (n = 50) | (n = 18) | (n = 22) | (n = 7) | (n = 166) | (n = 1) | (n = 66) | |
| Median age, years (range) | 77.0 (53–86) | 74.5 (66–86) | 75.0 (60–86) | 76.5 (62–90) | 76.0(67–84) | 77.0 (49–91) | 75 (75–75) | 76.5 (64–86) |
| Age categories, n (%) | ||||||||
| <75y | 21 (38.9) | 25 (50.0) | 10 (55.6) | 7 (31.8) | 2 (28.6) | 60 (36.1) | 0 (0) | 27 (40.9) |
| ≥75 y | 33 (61.1) | 25 (50.0) | 8 (44.4) | 15 (68.2) | 5 (71.4) | 106 (63.9) | 1 (100) | 39 (59.1) |
| AML types, n (%) | ||||||||
| De novo | 42 (77.8) | 30 (60.0) | 14 (77.8) | 13 (59.1) | 3 (42.9) | 128 (77.1) | 1 (100) | 52 (78.8) |
| Secondary | 12 (22.2) | 20 (40.0) | 4 (22.2) | 9 (40.9) | 4 (57.1) | 38 (22.9) | 0 (0) | 14 (21.2) |
| Blast count, n (%) | ||||||||
| <30% | 24 (44.4) | 14 (28.0) | 8 (44.4) | 6 (27.3) | 4 (57.1) | 38 (22.9) | 0 (0) | 16 (24.2) |
| ≥30–<50% | 12 (22.2) | 12 (24.0) | 5 (27.8) | 6 (27.3) | 1 (14.3) | 36 (21.7) | 0 (0) | 14 (21.2) |
| ≥50% | 18 (33.3) | 24 (48.0) | 5 (27.8) | 10 (45.5) | 2 (28.6) | 92 (55.4) | 1 (100) | 36 (54.5) |
| ECOG score, n (%) | ||||||||
| 1–2 | 32 (59.3) | 29 (58.0) | 10 (55.6) | 16 (72.7) | 3 (42.9) | 98 (59.0) | 1 (100) | 38 (57.6) |
| 3–4 | 22 (40.7) | 21 (42.0) | 8 (44.4) | 6 (27.3) | 4 (57.1) | 68 (41.0) | 0 (0) | 28 (42.4) |
| Molecular mutations, n (%) | ||||||||
| FLT3 detected | 2 (3.7) | 6 (12.0) | 0 (0) | 2 (9.1) | 2 (28.6) | 37 (22.3) | 1 (100) | 25 (37.9) |
| IDH1/2 detected | 2 (3.7) | 15 (30.0) | 0 (0) | 6 (27.3) | 1 (14.3) | 58 (34.9) | 0 (0) | 15 (22.7) |
| NPM1 detected | 0 (0) | 0 (0) | 0 (0) | 1 (4.5) | 1 (14.3) | 42 (25.3) | 0 (0) | 17 (25.8) |
| Cytogeneticsa, n (%) | ||||||||
| t11q23 detected | 5 (9.3) | 2 (4.0) | 1 (5.6) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| t3_3 detected | 1 (1.9) | 5 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| del 5 or 7 detected | 45 (83.3) | 34 (68.0) | 16 (88.9) | 16 (72.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Complex karyotype detected | 49 (90.7) | 25 (50.0) | 17 (94.4) | 7 (31.8) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) |
| del 17 detected | 13 (24.1) | 2 (4.0) | 4 (22.2) | 0 (0) | 0(0) | 0 (0) | 0(0) | 1 (1.5) |
Note: Patients enrolled in the phase III VIALE-A study were stratified by cytogenetic risk (intermediate, poor).
Abbreviations: AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; mut, mutated; wt, wild-type.
aCytogenetics were classified per NCCN (2016) criteria.
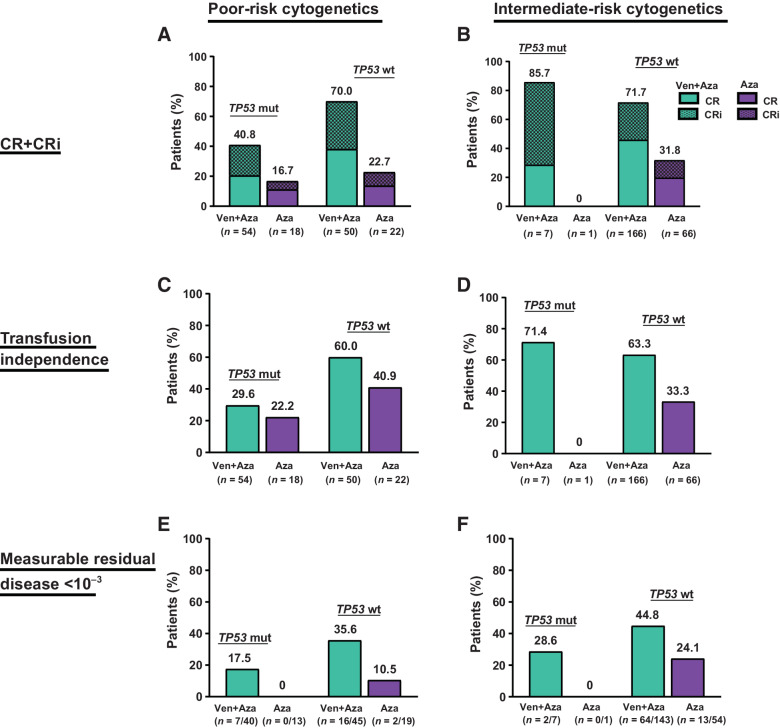
Response to treatment
The response to treatments in the subgroups is shown in Fig. 2A and B. In all treatment groups, higher CRc rates were observed in patients with poor-risk cytogenetics and TP53wt than in those with poor-risk cytogenetics and TP53mut. Among patients with poor-risk cytogenetics and TP53wt, the CRc rate was 70.0% for those treated with venetoclax + azacitidine and 22.7% for those treated with azacitidine alone. In patients with poor-risk cytogenetics and TP53wt, CRc rates with venetoclax + azacitidine were similar to those of intermediate-risk and TP53wt (70.0% vs. 71.7%, respectively). However, in patients with poor-risk cytogenetics and TP53mut, the CRc rates in the venetoclax + azacitidine and azacitidine alone groups were 40.7% and 16.7%, respectively.
Figure 2.
Response rates. A, CR+CRi in patients with poor-risk cytogenetics. B, CR+CRi in patients with intermediate-risk cytogenetics. C, Transfusion independence in patients with poor-risk cytogenetics. D, Transfusion independence in patients with intermediate-risk cytogenetics. E, MRD <10−3 in patients with poor-risk cytogenetics. F, MRD <10−3 in patients with intermediate-risk cytogenetics. Abbreviations: Aza, azacitidine; CR, complete remission; CRi, CR with incomplete hematologic recovery; mut, mutated; wt, wild-type; Ven, venetoclax.
In patients with poor-risk cytogenetics and TP53wt treated with venetoclax + azacitidine, 60% achieved transfusion independence, similar to results observed in patients with intermediate-risk cytogenetics and TP53wt (71.4%; Fig. 2C and D). Among those with poor-risk cytogenetics and TP53wt, the proportion of patients who achieved MRD negativity (MRD <10−3) was also higher in the venetoclax + azacitidine group as compared with the azacitidine alone group (35.6% vs. 10.5%, Fig. 2E). Similarly, in patients with intermediate-risk cytogenetics and TP53wt treated with venetoclax + azacitidine, 44.8% achieved MRD negativity (Fig. 2F). Three (6%) patients with poor-risk cytogenetics and TP53wt treated with venetoclax + azacitidine underwent a transplant. In patients with poor-risk cytogenetics and TP53mut treated with venetoclax + azacitidine, 29.6% achieved transfusion independence, 17.5% achieved MRD negativity, and 22.2% of patients with poor-risk cytogenetics and TP53mut treated with azacitidine achieved transfusion independence, and none achieved MRD negativity.
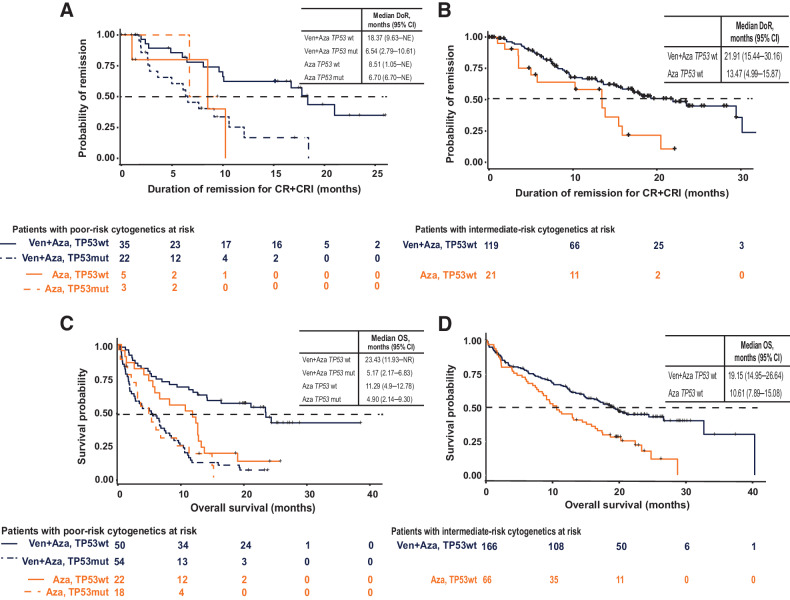
The DoR in patients with poor-risk cytogenetics and TP53wt was also higher in the venetoclax + azacitidine group than in the azacitidine-only group (18.4 vs. 8.5 months; Fig. 3A); however, these conclusions are limited by the very few azacitidine-only patients who achieved a response. Similar to CRc, the DoR for patients with poor-risk cytogenetics and TP53wt who received venetoclax + azacitidine was comparable with that of patients with intermediate-risk cytogenetics and TP53wt who received the same regimen (18.4 vs. 21.9 months, respectively; Fig. 3B). Patients with poor-risk cytogenetics and TP53mut had a shorter DoR, with no appreciable differences seen between the venetoclax + azacitidine (6.5 months) and azacitidine alone (6.7 months) groups.
Figure 3.
Duration of response and OS. A, Duration of response among patients with poor-risk cytogenetics. B, Duration of response among patients with intermediate-risk cytogenetics. C, OS among patients with poor-risk cytogenetics. D, OS among patients with intermediate-risk cytogenetics. Abbreviations: Aza, azacitidine; mut, mutated; wt, wild-type; Ven, venetoclax.
For OS, patients with poor-risk cytogenetics and TP53wt treated with venetoclax + azacitidine had a longer OS than patients treated with azacitidine alone (23.4 vs. 11.3 months; Fig. 3C). Once again, the outcome of patients treated with venetoclax + azacitidine was similar to that of patients with intermediate-risk cytogenetics and TP53wt treated with venetoclax + azacitidine (19.1 months; Fig. 3D). In contrast, patients with poor-risk cytogenetics and TP53mut had an inferior OS, regardless of treatment approach (5.2 months for venetoclax + azacitidine and 4.9 months for azacitidine alone).
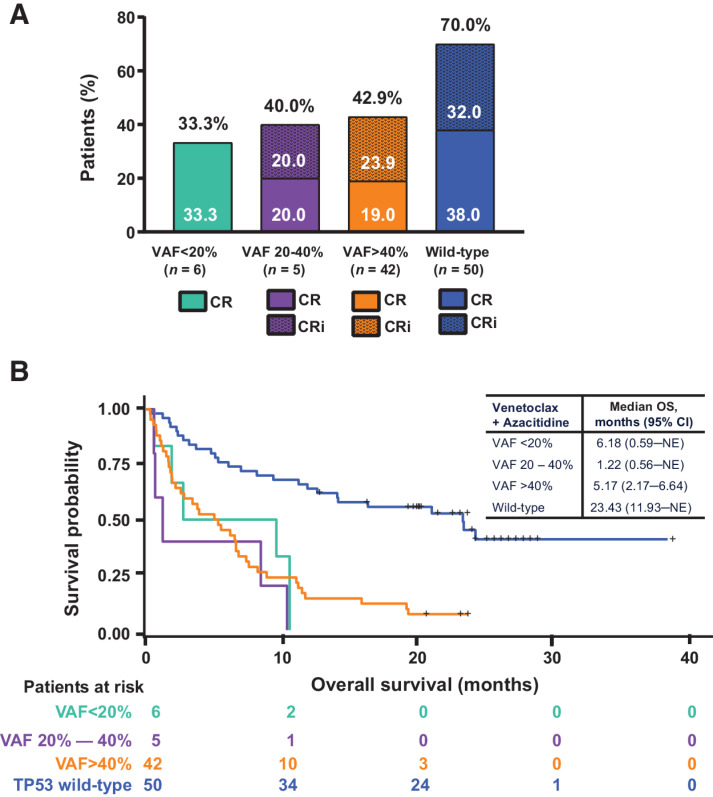
For patients who received venetoclax + azacitidine, CRc rates or OS did not differ on the basis of the VAF of the TP53mut (<20%, 20%–40%, or >40%; Fig. 4A and B). We also compared outcomes of venetoclax + azacitidine treatment in patients with poor-risk cytogenetics and TP53mut overall with those of patients with specific high-risk cytogenetic abnormalities, including abnormalities of t11q23, t3;3, complex karyotype, and del17p. The results are summarized in Supplementary Table S1. Patients with TP53wt consistently had better outcomes than their TP53mut counterparts, regardless of karyotype.
Figure 4.
Response rates and OS by VAF. A, Response rates by VAF among patients with poor-risk cytogenetics and TP53mut treated with venetoclax + azacitidine. B, OS by variant allelic frequency among patients with poor-risk cytogenetics and TP53mut treated with venetoclax + azacitidine. Abbreviations: CR, complete remission; CRi, CR with incomplete hematologic recovery; NE, not evaluable; VAF, variant allele frequency.
Fifteen patients with poor-risk cytogenetics and TP53wt had cooccurring IDH1/2mut (Table 1). It has been demonstrated that patients with an IDH1/2mut have superior responses to venetoclax + azacitidine (19). Therefore, we further evaluated the efficacy among patients with poor-risk cytogenetics and TP53wt who were also IDH1/2wt; 70% (n = 35/50) treated with venetoclax + azacitidine, and 73% (n = 16/22) treated with azacitidine alone, respectively. The patients with poor-risk cytogenetics, TP53wt, and IDH1/2wt, and treated with venetoclax + azacitidine, had longer OS than patients with a similar profile treated with azacitidine alone (21.1 vs. 11.3 months; Supplementary Fig S1). The patients with intermediate-risk cytogenetics, TP53wt, and IDH1/2wt treated with venetoclax + azacitidine had an OS of 18.3 months compared with those with poor-risk cytogenetics, TP53wt, and IDH1/2wt treated with venetoclax + azacitidine.
Safety
All patients with poor-risk cytogenetics and TP53mut experienced at least one treatment-emergent AE with venetoclax + azacitidine or azacitidine alone. All grade ≥ 3 AEs with ≥ 20% occurrence are listed in Supplementary Table S2. Among patients with poor-risk cytogenetics and TP53mut, the predominant grade ≥ 3 hematologic AEs for those treated with venetoclax + azacitidine versus azacitidine alone included febrile neutropenia (55% vs. 39%), thrombocytopenia (28% vs. 28%), neutropenia (26% vs. 17%), pneumonia (28% vs. 33%), and anemia (13% vs. 6%). Similar AE rates were observed in patients with poor-risk cytogenetics and TP53wt.
In patients with poor-risk cytogenetics and TP53mut, 9 (17%) deaths occurred within 30 days of treatment administration in the venetoclax + azacitidine group, and 2 (11%) in the azacitidine alone group.
Discussion
We analyzed a large dataset of newly diagnosed patients with AML unfit for intensive chemotherapy from two multicenter, international studies in which patients were treated uniformly. In patients with poor-risk cytogenetics and TP53wt, treatment with venetoclax + azacitidine conferred better outcomes than patients treated with azacitidine alone. These outcomes were similar to patients with intermediate-risk cytogenetics and TP53wt treated with venetoclax + azacitidine. Historically, studies have shown that poor-risk cytogenetics are an independent poor prognostic factor for poor treatment outcomes (5, 20, 21). However, this association was observed in the context of conventional treatments, typically intensive chemotherapy, which is frequently not a viable option for a disease in which most patients are ineligible for treatment due to advanced age.
As expected, among patients with poor-risk cytogenetics, the occurrence of TP53mut was high; 52% in the venetoclax + azacitidine group and 45% in the azacitidine alone group. While patients with these disease features demonstrated a higher response rate to venetoclax + azacitidine than azacitidine alone (including an 18% MRD < 10−3 negativity rate with venetoclax + azacitidine), the duration of response and OS remained poor and was not different between treatment groups. The OS among patients with TP53mut appeared to be similar to patients treated with intensive chemotherapy in both clinical and real-world studies (22–24). Preclinical studies have shown that TP53mut can result in venetoclax resistance in AML cell lines. The expression levels of antiapoptotic proteins BCL2 and MCL1 are reduced in TP53mut cells, correlating inversely with increased BCLxL expression, resulting in higher ratios of BCLxL to BCL2 (25). Venetoclax binds directly to BCL2; therefore, a decrease in BCL2 expression contributes to the loss of drug sensitivity (26). DiNardo and colleagues have also demonstrated that TP53mut was associated with resistance to venetoclax, hypomethylating agents, and cytarabine, as single agents or in combination (27). TP53mut AML remains a challenging disease entity; novel therapies such as antiCD47 or TP53mut reactivators are being investigated with the hope that these novel approaches may overcome the treatment resistance associated with TP53mut (NCT04214860, NCT05079230, NCT04435691, NCT04778397 NCT04435691, NCT05079230; refs. 28, 29). Similarly, a triple combination of venetoclax + azacitidine with an antiCD47 agent is also under investigation (NCT04912063), intending to improve outcomes in this hard-to-treat subgroup of patients.
We also showed that the OS of patients with venetoclax and azacitidine treatment was not associated with VAF of the TP53mut. This observation, the etiology of which is unclear, is consistent with the data reported by Short and colleagues, in which they showed that the VAF of the TP53mut was significant for patients treated with cytarabine-based regimens but not HMA or venetoclax-based therapies (11).
Our findings suggest that in the absence of a TP53mut, the adverse prognostic implication of poor-risk cytogenetics might not apply to patients treated with venetoclax + azacitidine (Supplementary Fig. S2). The CRc, DoR, and OS for these patients were similar to the outcomes for patients treated with this regimen who had intermediate-risk cytogenetics. We recently reported that patients with poor-risk cytogenetics and IDH1/2wt had inferior outcomes (19). Here, we show that the presence of a TP53mut drives inferior outcomes in the poor-risk cytogenetic group.
The safety and tolerability of venetoclax + azacitidine were similar, irrespective of TP53mut status. Observed toxicities were predominantly hematologic and were consistent with previously reported data (14, 15). No unusual toxicities or significant differences in neutropenia-related AEs were identified between similarly treated patients with TP53mut and TP53wt. However, we observed a higher ≤ 60-day death rate among patients with a TP53mut (36%) as compared with TP53wt (8%) when treated with venetoclax + azacitidine, a reflection of the resistance to treatment associated with this adverse mutation.
There are limitations to this analysis. To increase the number of patients with poor-risk cytogenetics and TP53mut, a pooled analysis of a prior phase III and a phase Ib study was conducted. However, this approach did not increase the number of patients in each subgroup treated with azacitidine alone due to the single-arm design of the phase Ib study. The small sample sizes in the azacitidine alone group limit the interpretation of the key findings between the two treatments.
In conclusion, our results demonstrate that the improved efficacy of the venetoclax + azacitidine combination in treatment-naïve patients with AML unfit for intensive therapy and with poor-risk cytogenetics is restricted to patients without a TP53mut. The status of this gene mutation could inform treatment decisions and would be useful information at diagnosis. This analysis also suggests that conventional prognostic factors in AML, defined mainly in the context of intensive therapeutic approaches, should be reanalyzed in the new setting of novel treatments.
Supplementary Material
Acknowledgments
The authors wish to thank the patients and their families, the study coordinators, and the support staff. The authors would also like to acknowledge all investigators of studies M14-358 and M15-656.
Medical writing support was provided by Dalia Majumdar, PhD, an employee of AbbVie. Editorial support was provided by Angela Hadsell, BA, an employee of AbbVie.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 5231
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
D.A. Pollyea reports grants and other support from AbbVie, Karyopharm, and Bristol-Myers Squibb and other support from Genentech during the conduct of the study as well as other support from Amgen, Novartis, Syndax, Takeda, Syros, Kiadis, Foghorn, Aprea, Gilead, Astellas, Jazz, BeiGene, BerGen Bio, Arcellx, Immunogen, AstraZeneca, Notable Labs, Kura, Ryvu, Magenta, Qihan, Zentalis, and Hibercell and grants from Genentech and Teva outside the submitted work. K.W. Pratz reports grants and personal fees from AbbVie, Astellas, and Agios; personal fees from BMS/Celgene, Servier, Jazz Pharmaceuticals, Novartis, and Boston Biomedical; and grants from Millenium and Daiichi Sankyo during the conduct of the study. A.H. Wei reports other support from WEHI during the conduct of the study. V. Pullarkat reports personal fees from AbbVie outside the submitted work. B.A. Jonas reports grants from AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Aptose, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Forty-Seven, Hanmi, Immune-Onc, Incite, Loxo, Pharmacyclics, Sigma Tau, and Genentech/Roche during the conduct of the study as well as grants and personal fees from AbbVie, BMS, Genentech/Roche, Gilead, GlycoMimetics, Jazz, Pfizer and Treadwell and personal fees from Servier annd Takeda outside the submitted work. C. Recher reports grants, personal fees, and other support from AbbVie during the conduct of the study as well as grants from Amgen, MaatPharma, and Iqvia; grants, personal fees, and other support from BMS, Astellas, and Jazz Pharma; personal fees from Novartis and Takeda; and personal fees and other support from Servier outside the submitted work. S. Babu reports other support from AbbVie, Genentech/Roche, Novartis, TG Therapeutics, and Janssen Oncology; personal fees and other support from Bristol-Myers Squibb and AstraZeneca/MedImmune; and personal fees from Kite and Amgen during the conduct of the study. M. Dail reports other support from Roche-Genentech during the conduct of the study as well as other support from Roche-Genentech outside the submitted work. Y. Sun reports employment with AbbVie. B. Chyla reports other support from AbbVie during the conduct of the study as well as other support from AbbVie outside the submitted work; in addition, B. Chyla is employed by and owns stock in AbbVie, Inc. C.D. DiNardo reports grants and personal fees from AbbVie, Servier, Jazz, and BMS; personal fees from Genentech, GSK, Astellas, and Kura; and other support from Notable Labs outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
D.A. Pollyea: Conceptualization, writing–original draft, writing–review and editing. K.W. Pratz: Conceptualization, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. A.H. Wei: Conceptualization, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. V. Pullarkat: Conceptualization, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. B.A. Jonas: Writing–original draft, writing–review and editing. C. Recher: Writing–original draft, writing–review and editing. S. Babu: Conceptualization, methodology, writing–original draft, writing–review and editing. A.C. Schuh: Conceptualization, data curation, methodology, writing–original draft, writing–review and editing. M. Dail: Conceptualization, data curation, supervision, methodology, writing–original draft, writing–review and editing. Y. Sun: Conceptualization, data curation, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. J. Potluri: Conceptualization, data curation, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. B. Chyla: Conceptualization, data curation, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing. C.D. DiNardo: Conceptualization, data curation, supervision, validation, visualization, methodology, writing–original draft, writing–review and editing.
References
- 1. Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood 2006;107:3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim MP, Zhang Y, Lozano G. Mutant p53: multiple mechanisms define biologic activity in cancer. Frontiers Oncol 2015;5:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Döhner H, Dolnik A, Tang L, Seymour JF, Minden MD, Stone RM, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia 2018;32:2546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012;119:2114–21. [DOI] [PubMed] [Google Scholar]
- 5. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52. [DOI] [PubMed] [Google Scholar]
- 7. Kadia TM, Jain P, Ravandi F, Garcia-Manero G, Andreef M, Takahashi K, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016;122:3484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 2009;23:203–6. [DOI] [PubMed] [Google Scholar]
- 9. Ohgami RS, Ma L, Merker JD, Gotlib JR, Schrijver I, Zehnder JL, et al. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Modern Pathol 2015;28:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goel S, Hall J, Pradhan K, Hirsch C, Przychodzen B, Shastri A, et al. High prevalence and allele burden-independent prognostic importance of p53 mutations in an inner-city MDS/AML cohort. Leukemia 2016;30:1793–5. [DOI] [PubMed] [Google Scholar]
- 11. Short NJ, Montalban-Bravo G, Hwang H, Ning J, Franquiz MJ, Kanagal-Shamanna R, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv 2020;4:5681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schoch C, Kern W, Kohlmann A, Hiddemann W, Schnittger S, Haferlach T. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosomes Cancer 2005;43:227–38. [DOI] [PubMed] [Google Scholar]
- 13. Kayser S, Levis MJ. Updates on targeted therapies for acute myeloid leukemia. Brit J Haematol 2022;196:316–28. [DOI] [PubMed] [Google Scholar]
- 14. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 15. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naïve, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003;21:4642–9. [DOI] [PubMed] [Google Scholar]
- 17. Pratz KW, Jonas BA, Pullarkat V, Recher C, Schuh AC, Thirman MJ, et al. Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol 2021;40:JCO.21.01546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Services US department of health and human. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2018.
- 19. Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res 2022;28:OF1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med 2016;375:900–1. [DOI] [PubMed] [Google Scholar]
- 21. Fröhling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Döhner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood 2006;108:3280–8. [DOI] [PubMed] [Google Scholar]
- 22. Lindsley RC, Gibson CJ, Murdock HM, Stone RM, Cortes JE, Uy GL, et al. Genetic characteristics and outcomes by mutation status in a phase III study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood 2019;134:15–15. [Google Scholar]
- 23. Chiche E, Rahmé R, Bertoli S, Dumas PY, Micol JB, Hicheri Y, et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv 2021;5:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rautenberg C, Stölzel F, Röllig C, Stelljes M, Gaidzik V, Lauseker M, et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J 2021;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thijssen R, Diepstraten ST, Moujalled D, Chew E, Flensburg C, Shi MX, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood 2021;137:2721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nechiporuk T, Kurtz SE, Nikolova O, Liu T, Jones CL, Alessandro A, et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov 2019;9:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020;135:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therapeutics IA. Aprea Therapeutics announces phase I /II trial of eprenetapopt + venetoclax + azacitidine in TP53 mutant AML meets complete remission primary efficacy endpoint. 2021.
- 29. Sallman DA, Asch AS, Malki MMA, Lee DJ, Donnellan WB, Marcucci G, et al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase I b results. Blood 2019;134:569. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided following review and approval of a research proposal, Statistical Analysis Plan, and execution of a Data Sharing Agreement. Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.