Abstract
Expression of CD40 ligand (CD40L) correlated directly with Mycobacterium tuberculosis-stimulated gamma interferon (IFN-γ) production by peripheral blood mononuclear cells (PBMC) from tuberculosis patients and healthy tuberculin reactors. The CD40L agonist increased M. tuberculosis-induced IFN-γ production by PBMC, and anti-CD40 or anti-CD40L antibodies reduced IFN-γ production. CD40L expression on PBMC was reduced by exposure to B cells and to soluble factors from M. tuberculosis-infected monocytes. These findings suggest that CD40L dysregulation contributes to reduced IFN-γ production in human tuberculosis.
Tuberculosis provides an excellent model to investigate the relationship between the immune response and clinical manifestations of disease from intracellular pathogens. Most persons infected with Mycobacterium tuberculosis are healthy tuberculin reactors with protective immunity against exogenous infection, and their peripheral blood mononuclear cells (PBMC) produce high concentrations of the Th1 cytokine gamma interferon (IFN-γ). In contrast, many patients with tuberculosis have severe disease and ineffective immunity, and their M. tuberculosis-stimulated PBMC produce little IFN-γ (10, 20, 34). Understanding the mechanism for reduced IFN-γ production in tuberculosis will enhance our understanding of susceptibility to intracellular pathogens in which Th1 responses provide protective immunity.
Interactions between CD40 on antigen-presenting cells and CD40 ligand (CD40L) on T cells enhance the development of Th1 responses through inducing production of interleukin-12 (IL-12) by macrophages and dendritic cells (12, 15, 24) and by enhancing expression of costimulatory molecules of the B7 family on antigen-presenting cells (16, 18, 29). CD40L-deficient mice have reduced production of IL-12 and IFN-γ, with markedly reduced resistance to Leishmania species (3, 11). In contrast, CD40L-deficient mice have intact immunity to histoplasmosis and tuberculosis (4, 35). In humans, a defective CD40L gene is a rare abnormality that leads to the hyper-IgM syndrome, with increased susceptibility to some intracellular pathogens (2). However, the contribution of CD40L to immune defenses in the general population is undefined. To evaluate the role of CD40L in the immune response to tuberculosis, we studied the relationship between CD40L expression and IFN-γ production in PBMC from persons with tuberculosis infection and tuberculosis disease.
Subjects.
Blood was obtained from 13 healthy tuberculin reactors who were hospital employees and laboratory personnel and from 16 human immunodeficiency virus-seronegative patients with culture-proven pulmonary tuberculosis who had received less than 2 weeks of antituberculosis therapy. All patients had moderately or far advanced tuberculosis and positive sputum acid-fast stains.
Antibodies.
For cytofluorometric analysis, we used monoclonal antibodies to CD40 (fluorescein isothiocyanate [FITC] conjugated), CD40L (phycoerythrin [PE] conjugated), and CD25 (FITC conjugated), all from Pharmingen, San Diego, Calif.; CD4, CD8, and CD3 (all FITC conjugated; Dako, Carpinteria, Calif.); and IL-12 receptor β1 (2B10 [28]) and IL-12 receptor β2 (2B6 [17]) (both kindly provided by David H. Presky, Hoffmann-La Roche Inc., Nutley, N.J.). Isotype control antibodies were FITC- or PE-conjugated goat anti-mouse immunoglobulin G1 (IgG1; Pharmingen), and secondary antibodies for IL-12 receptor staining were FITC- or PE-conjugated goat anti-rat IgG (Caltag Laboratories, Burlingame, Calif.). Antibodies to CD40 (M2, mouse IgG1) and to CD40L (M91, mouse IgG1) were used, as well as a soluble stimulatory trimeric CD40L (all from Immunex Corporation, Seattle, Wash.).
Cell culture.
PBMC (2 × 106/ml) were plated in RPMI (GIBCO, Grand Island, N.Y.) with 10% heat-inactivated human serum, in the presence or absence of heat-killed M. tuberculosis Erdman strain (1 μg/ml), provided by Patrick Brennan, Colorado State University, Fort Collins. In some experiments, PBMC were depleted of B cells by immunomagnetic depletion, using magnetic beads conjugated to anti-CD22 (Dynal, Lake Success, N.Y.).
Flow cytometry.
After culture for 1 to 7 days, PBMC or B-cell-depleted PBMC were centrifuged over Ficoll-Paque to remove dead cells, and single and double immunolabeling was performed, by standard methods (32). In some experiments, flow cytometry was performed on freshly isolated PBMC. Data were analyzed on an EPICS C fluorescence-activated cell sorter (Coulter Corporation, Hialeah, Fla.). Staining with FITC- or PE-conjugated isotype control antibodies alone yielded 0.1 to 0.6% (mean, 0.4%) positively stained cells. These low values were not subtracted from values obtained with specific antibodies.
Coculture of macrophages and lymphocytes.
Adherent cells (90 to 95% monocytes) were obtained by standard techniques (31). Adherent cells were plated at the bottom of 12-well plates containing Transwell inserts (Costar, Cambridge, Mass.) at 5 × 105 cells/well in 2 ml of RPMI 1640 containing 10% heat-inactivated human serum. In some wells, adherent cells were infected with live M. tuberculosis strain H37Ra at a ratio of 5 bacilli per cell, as previously described (33).
While adherent cells were cultured in the 12-well plates, 2 × 105 PBMC from a healthy tuberculin reactor were cultured in the Transwell insert in RPMI 1640 with 10% heat-inactivated human serum and 10 μg of heat-killed M. tuberculosis Erdman per ml. The Transwell insert contains 0.4-μm-diameter pores that allow diffusion but not cell-to-cell contact. Adherent cells and PBMC were cocultured for 5 days, and the percentages of PBMC expressing CD40L were measured by flow cytometry.
Cytokine production.
M. tuberculosis-stimulated PBMC produce maximal concentrations of IL-10, IL-12, and IFN-γ after 1, 2, and 4 days of culture, respectively (31, 34). Supernatants were collected at these time points and stored at −70°C, and cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA), using coating antibodies (Pharmingen).
Statistical analysis.
Data for different groups were expressed as the mean ± standard error of the mean and were compared by the paired or unpaired Student t test, as appropriate. Data that were not normally distributed were compared by the Wilcoxon rank sum test.
Expression of CD40L and CD40 in tuberculosis patients and healthy tuberculin reactors.
In preliminary experiments, CD40L+ cells were not detectable in freshly isolated PBMC. When PBMC were cultured with heat-killed M. tuberculosis, expression of CD40L was maximal at day 5. The percentage of CD40+ cells was highest in freshly isolated PBMC, decreasing throughout the culture period (data not shown).
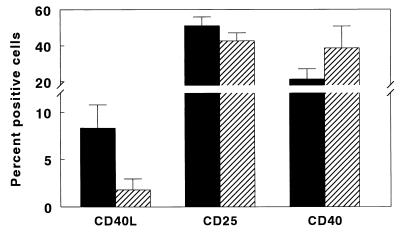
We cultured PBMC from 11 healthy tuberculin reactors and 16 tuberculosis patients with heat-killed M. tuberculosis for 5 days and measured expression of CD40L (Fig. 1). CD40L expression was significantly reduced in tuberculosis patients (1.8% ± 0.3% versus 8.3% ± 0.8%, P < 0.001), whereas CD25 expression was unchanged, indicating that there was no generalized defect in T-cell activation in tuberculosis patients. Double immunolabeling showed that the CD40L+ cells were all CD3+ and >80% CD4+ (data not shown), confirming prior reports that CD40L is expressed predominantly by CD4+ T cells (14, 19). Reduced CD40L expression in tuberculosis patients was not due to a reduced percentage of CD4+ cells, which was 57% ± 3% in tuberculosis patients, compared to 55% ± 4% in healthy tuberculin reactors. The percentage of CD40+ cells in freshly isolated PBMC was significantly increased in 16 tuberculosis patients, compared to 13 healthy tuberculin reactors (39% ± 3% versus 21% ± 2%, P < 0.001 [Fig. 1]). Therefore, reduced expression of CD40L in tuberculosis patients was not due to reduced expression of CD40.
FIG. 1.
Expression of CD40L, CD25, and CD40 in tuberculosis patients (▨) and healthy tuberculin reactors (■). PBMC from 16 tuberculosis patients and 11 healthy tuberculin reactors were cultured with heat-killed M. tuberculosis for 5 days, and the percentages of CD40L+ and CD25+ cells were measured by flow cytometry. The percentages of CD40+ cells in freshly isolated PBMC from 16 tuberculosis patients and 13 healthy tuberculin reactors were determined by flow cytometry. Mean values and standard errors are shown.
CD40-CD40L interactions and M. tuberculosis-induced IFN-γ production.
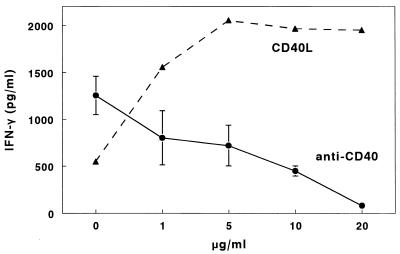
Mean IFN-γ concentrations were reduced in supernatants of M. tuberculosis-stimulated PBMC from 16 tuberculosis patients, compared to those from 11 healthy tuberculin reactors (766 ± 277 pg/ml versus 2,474 ± 275 pg/ml, P = 0.002), confirming previous studies (10, 20, 34). To determine if reduced CD40L expression contributed to this reduction in IFN-γ production, we studied the effects of soluble CD40L, an agonist that binds to CD40 and enhances production of IL-12 and IFN-γ in other experimental systems (8). In a dose-dependent manner, CD40L enhanced IFN-γ production by M. tuberculosis-stimulated PBMC from a tuberculosis patient (Fig. 2). In five tuberculosis patients, addition of 10 μg of CD40L per ml increased IFN-γ concentrations from 465 ± 106 pg/ml to 1,021 ± 284 pg/ml (P = 0.05). In contrast, in three healthy tuberculin reactors, CD40L did not increase IFN-γ production by M. tuberculosis-stimulated PBMC (2,997 ± 197 pg/ml versus 2,955 ± 330 pg/ml, P = 0.89).
FIG. 2.
Effects of soluble CD40L and anti-CD40 on M. tuberculosis-induced IFN-γ production by PBMC. PBMC from a tuberculosis patient was cultured with heat-killed M. tuberculosis and increasing concentrations of CD40L for 4 days, and IFN-γ concentrations were measured by ELISA. PBMC from four healthy tuberculin reactors were cultured for 4 days with heat-killed M. tuberculosis and increasing concentrations of anti-CD40 antibodies. IFN-γ concentrations were measured by ELISA. For the experiments with anti-CD40, mean values and standard errors are shown.
To determine if blocking the interaction between CD40 and CD40L would reduce IFN-γ production, we added increasing concentrations of the anti-CD40 antibody M2 to M. tuberculosis-stimulated PBMC from four healthy tuberculin reactors. Anti-CD40 caused a dose-dependent reduction in IFN-γ production (Fig. 2). In contrast, addition of up to 10 μg of an isotype control antibody per ml had no effect on IFN-γ production (data not shown).
To confirm these results, we added the neutralizing anti-CD40L antibody M91 to M. tuberculosis-stimulated PBMC from four healthy tuberculin reactors. When this antibody was added at the initiation of culture, there was no effect on IFN-γ production, presumably because CD40L is not expressed on freshly isolated cells. However, when the antibody was added after PBMC had been cultured with M. tuberculosis for 2 to 3 days, IFN-γ production was reduced by 51 to 87% (P = 0.001). Isotype control antibodies had no effect (data not shown).
Effects of CD40-CD40L interactions on IL-12 receptor expression and IL-12 production.
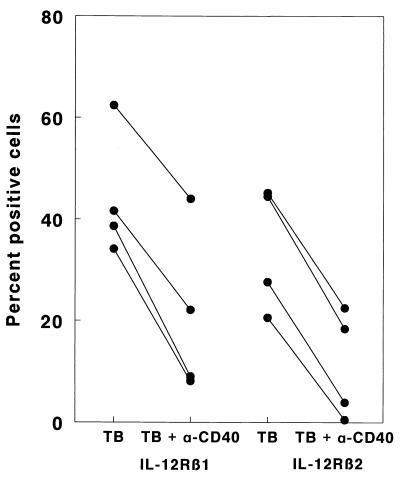
Prior studies have shown that reduced IFN-γ production by M. tuberculosis-stimulated PBMC of tuberculosis patients is associated with reduced expression of IL-12 receptors on T cells (32). To explore the relationship of CD40L expression to IL-12 receptor expression in tuberculosis, we added anti-CD40 antibodies to M. tuberculosis-stimulated PBMC from healthy tuberculin reactors (Fig. 3). In four persons, expression of IL-12 receptor β1 was reduced by 30 to 71% (P = 0.003), and expression of IL-12 receptor β2 was reduced by 50 to 98% (P < 0.001), whereas addition of isotype control antibodies had no effect (data not shown). Addition of anti-CD40 antibodies increased mean IL-12 p70 levels from 163 ± 155 pg/ml to 570 ± 323 pg/ml (P = nonsignificant) and reduced IL-10 concentrations slightly from 498 ± 187 pg/ml to 239 ± 118 pg/ml (P = nonsignificant). Therefore, reduction in IL-12 receptor expression was not accompanied by reduced production of IL-12 or enhanced production of IL-10.
FIG. 3.
Effect of anti-CD40 on M. tuberculosis-induced expression of IL-12 receptor β1 and β2 by PBMC from four healthy tuberculin reactors. PBMC were cultured for 5 days with heat-killed M. tuberculosis in the presence or absence of anti-CD40 antibodies. The percentages of cells expressing IL-12 receptor β1 and β2 were determined by flow cytometry.
Effect of M. tuberculosis infection on CD40L expression.
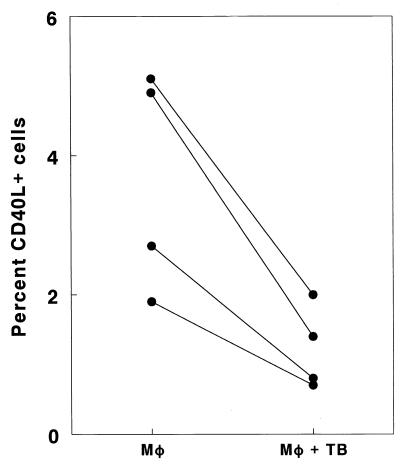
To determine if macrophages infected with M. tuberculosis contributed to reduced CD40L expression, PBMC from healthy reactors were cultured in Transwells in 12-well plates containing macrophages, either infected or uninfected with M. tuberculosis. In four experiments, coculture of PBMC with infected macrophages reduced the percentage of cells expressing CD40L by 58 to 71%, compared to cells cocultured with uninfected macrophages (P = 0.02 [Fig. 4]). Staining with isotype control antibodies yielded 0.1 to 0.3% positive cells, and subtraction of these values did not affect the results.
FIG. 4.
Effect of M. tuberculosis (TB)-infected monocytes (Mφ) on CD40L expression by T cells. Adherent cells from four healthy tuberculin reactors were cultured in 12-well plates, either infected or uninfected with live M. tuberculosis. Autologous PBMC were cultured with heat-killed M. tuberculosis in Transwell inserts. After 5 days of coculture, CD40L expression in PBMC was determined by flow cytometry.
Effect of B-cell depletion on CD40L expression and IFN-γ production.
In other experimental systems, B cells can reduce CD40L expression by T cells, reducing IL-12 production and IFN-γ production (14, 21, 30). To investigate this possibility in tuberculosis patients, we depleted PBMC of B cells by immunomagnetic depletion with anti-CD22, cultured B-cell-depleted PBMC with heat-killed M. tuberculosis, and measured CD40L expression after 5 days. Depletion of B cells substantially increased expression of CD40L from 2.2% ± 0.2% to 6.8% ± 1.4% in seven tuberculosis patients (P = 0.03). Depletion of B cells also enhanced production of IFN-γ by PBMC from five tuberculosis patients from 779 ± 327 pg/ml to 1,651 ± 437 pg/ml (P = 0.004). These effects were not due to a significant change in the percentage of T cells, which increased marginally from 68% ± 3% to 71% ± 2%. The percentage of CD19+ cells in PBMC was 6.5% ± 1.2% and was 1.1% ± 0.2% after depletion. Depletion of B cells in healthy tuberculin reactors had no effect on IFN-γ production by M. tuberculosis-stimulated PBMC from four healthy tuberculin reactors (data not shown).
Changes in expression of CD40L during antituberculosis therapy.
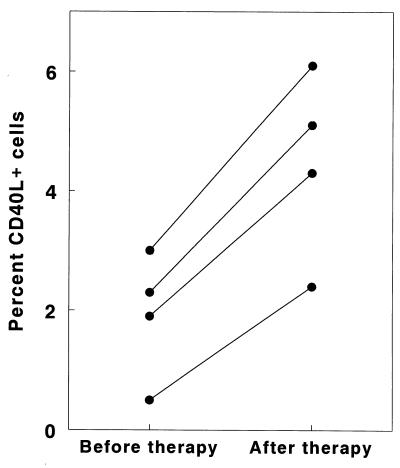
We have previously shown that M. tuberculosis-induced IFN-γ production increases in tuberculosis patients when their clinical condition has improved after antituberculosis therapy. In four tuberculosis patients, the percentage of PBMC expressing CD40L increased two- to fivefold after 6 months of antituberculosis therapy (P = 0.002 [Fig. 5]), paralleling changes in IFN-γ production (525 ± 144 pg/ml versus 1,624 ± 268 pg/ml).
FIG. 5.
Changes in CD40L expression in M. tuberculosis-stimulated T cells before and after therapy for tuberculosis. In four tuberculosis patients, PBMC were obtained initially after they had received <2 weeks of antituberculosis therapy and again after they had been treated for 6 months. PBMC were cultured with heat-killed M. tuberculosis, and CD40L expression was measured by flow cytometry after 5 days.
Summary of results.
Our findings demonstrate that dysregulated interactions between CD40 and CD40L correlate strongly with reduced antigen-stimulated IFN-γ production in patients with ineffective immunity to M. tuberculosis. Stimulation of PBMC from tuberculosis patients resulted in minimal expression of CD40L despite normal expression of the IL-2 receptor. The soluble agonist CD40L increased IFN-γ production by PBMC from tuberculosis patients but did not increase IFN-γ production by PBMC from healthy tuberculin reactors, suggesting that defective CD40L expression in tuberculosis patients contributed to reduced IFN-γ production. Addition of anti-CD40 or anti-CD40L antibodies to M. tuberculosis-stimulated PBMC from healthy tuberculin reactors reduced IL-12 receptor expression and IFN-γ production. CD40L expression was reduced by exposure to soluble factors produced by monocytes infected with M. tuberculosis. In addition, B cells may contribute to reduced CD40L expression in tuberculosis patients, as removal of B cells enhanced both CD40L expression and IFN-γ production.
In patients with the hyper-IgM syndrome due to a rare defective CD40L gene, susceptibility to disease from intracellular pathogens is enhanced (2). In these patients, production of IL-12 and IFN-γ in response to Toxoplasma gondii is markedly reduced (25). Our findings extend the potential role of CD40-CD40L interactions to a much larger population of patients with tuberculosis, of which there are more than 8 million new cases each year (6). Compared to PBMC from healthy tuberculin reactors, PBMC from tuberculosis patients have reduced M. tuberculosis-stimulated production of IFN-γ (20, 34), and our present findings demonstrate a close correlation between CD40L expression and IFN-γ production.
CD40L interactions and IL-12.
In other experimental systems, binding of CD40 on antigen-presenting cells to CD40L on T cells stimulates IL-12 production, which in turn enhances IFN-γ production by NK cells and T cells (12, 24). This mechanism is unlikely to explain the relationship we found between CD40L expression and M. tuberculosis-induced IFN-γ production. First, although CD40L expression was fivefold higher in healthy tuberculin reactors than in tuberculosis patients, IL-12 production by monocytes is similar in both groups (31). Second, blocking interactions between CD40 and CD40L in M. tuberculosis-stimulated PBMC reduced production of IFN-γ but not IL-12. M. tuberculosis is a potent stimulus for IL-12 secretion by monocytes (7, 13, 31), and this may be independent of the CD40/CD40L pathway, as is the case for bacterial products such as lipopolysaccharide (5). CD40-CD40L interactions can enhance IFN-γ production through mechanisms independent of IL-12, such as upregulation of the costimulatory B7 and class II major histocompatibility complex molecules on antigen-presenting cells (15, 23) or through direct stimulatory effects on T-cell proliferation (1). Our present findings suggest that binding of CD40 to CD40L upregulates IL-12 receptor expression, which increases T-cell responsiveness to IL-12 and enhances M. tuberculosis-induced IFN-γ production. Reduced IL-12 receptor expression is characteristic of M. tuberculosis-stimulated PBMC from tuberculosis patients (32) and is likely to play a central role in decreased IFN-γ production.
CD40L and mycobacteria.
Studies in animals have yielded conflicting results regarding the contribution of CD40-CD40L interactions to immunity against mycobacteria. Mice with a disrupted CD40L gene do not show increased susceptibility to intravenous infection with M. tuberculosis (4). The relevance of these findings to human tuberculosis is uncertain because the immune responses to pulmonary infection and intravenous infection may differ significantly. In an animal model of M. avium infection, anti-CD40L antibodies enhanced mycobacterial growth, and in human macrophages, CD40L-expressing transfectants and soluble CD40L inhibited intracellular growth of M. avium (9). Our data for humans suggest that CD40-CD40L interactions contribute to production of IFN-γ and to immunity against tuberculosis.
Mechanisms of depressed CD40L expression in tuberculosis.
Our findings suggest two potential mechanisms by which CD40L expression is diminished in tuberculosis patients. First, macrophages infected with M. tuberculosis produced soluble factors that reduced CD40L expression on T cells. Although M. tuberculosis-infected monocytes do not circulate in blood of immunocompetent tuberculosis patients, macrophages at the site of disease may reduce the capacity of local T cells to express CD40L, and these T cells may subsequently enter the circulation. Alternatively, monocytes in blood may be exposed to mycobacterial antigens that induce production of soluble factors that inhibit CD40L expression. Potential mediators of this effect include transforming growth factor β and prostaglandin E2 (22, 26, 27). A second potential mechanism for reduction of CD40L expression is through B cells, as depletion of B cells from PBMC of tuberculosis patients reduced CD40L expression and IFN-γ production. In other experimental systems, CD40-expressing B cells downregulate CD40L expression by receptor-mediated endocytosis (30) or by competing with macrophages for binding to CD40L-expressing T cells. Whereas CD40-CD40L interactions induce macrophages to produce IL-12, B cells fail to produce IL-12 (14) and can produce IL-10 (21), resulting in reduced IFN-γ production.
Conclusion.
Reduced expression of CD40L correlates strongly with decreased antigen-stimulated IFN-γ production in tuberculosis patients, and these abnormalities are probably mediated by B cells and by soluble factors secreted by M. tuberculosis-infected macrophages. Binding of CD40 to CD40L enhances M. tuberculosis-induced IFN-γ production through upregulation of IL-12 receptor expression, rather than through increased production of IL-12. Further studies are needed to delineate the roles of macrophages and B cells in reducing CD40L expression in tuberculosis.
Acknowledgments
We thank David Presky for provision of antibodies for measurement of IL-12 receptors and Patrick Brennan for provision of M. tuberculosis Erdman.
This work was supported by the National Institutes of Health (AI27285), Center for Pulmonary and Infectious Disease Control, and Cain Foundation Endowment for Infectious Disease Research. Peter F. Barnes holds the Margaret E. Byers Cain Chair for Tuberculosis Research. Mycobacterial products were provided through contract AI05074 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Armitage R J, Tough T W, Macduff B M, Fanslow W C, Spriggs M K, Ramsdell F, Alderson M R. CD40 ligand is a T cell growth factor. Eur J Immunol. 1993;23:2326–2331. doi: 10.1002/eji.1830230941. [DOI] [PubMed] [Google Scholar]
- 2.Callard R E, Armitage R J, Fanslow W C, Spriggs M K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 3.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 4.Campos-Neto A, Ovendale P, Bement T, Koppi T A, Fanslow W C, Rossi M A, Alderson M R. Cutting edge: CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 5.DeKruyff R H, Gieni R S, Umetsu D T. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158:359–366. [PubMed] [Google Scholar]
- 6.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 7.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurunathan S, Irvine K R, Wu C Y, Cohen J I, Thomas E, Prussin C, Restifo N P, Seder R A. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi T, Rao S P, Meylan P R, Kornbluth R S, Catanzaro A. Role of CD40 ligand in Mycobacterium avium infection. Infect Immun. 1999;67:3558–3565. doi: 10.1128/iai.67.7.3558-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon y production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Hakamada R, Yamene H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J Immunol. 1996;156:3932–3938. [PubMed] [Google Scholar]
- 13.Ladel C H, Szalay G, Riedel D, Kaufmann S H E. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infect Immun. 1997;65:1936–1938. doi: 10.1128/iai.65.5.1936-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruo S, Oh-hora M, Ahn H-J, Ono S, Wysocka M, Kaneko Y, Yagita H, Olumura K, Kikutani H, Kishimoto T, Kobayashi M, Hamaoka T, Trinchieri G, Fujiwara H. B cells regulate CD40 ligand-induced IL-12 production in antigen-presenting cells (APC) during T cell/APC interactions. J Immunol. 1997;158:120–126. [PubMed] [Google Scholar]
- 15.McDyer J F, Goletz T J, Thomas E, June C H, Seder R A. CD40 ligand/CD40 stimulation regulates the production of IFN-γ from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J Immunol. 1998;160:1701–1707. [PubMed] [Google Scholar]
- 16.Morse M A, Lyerly H K, Gilboa E, Thomas E, Nair S K. Optimization of the sequence of antigen loading and CD40-ligand-induced maturation of dendritic cells. Cancer Res. 1998;58:2965–2968. [PubMed] [Google Scholar]
- 17.Presky D H, Yang H, Minetti L J, Chua A O, Nabavi N, Wu C Y, Gately M K, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranheim E A, Kipps T J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy M, Waldschmidt T, Aruffo A, Ledbetter J A, Noelle R J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 20.Sanchez F O, Rodriguez J I, Agudelo G, Garcia L F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song F, Matsuzaki G, Mitsuyama M, Nomoto K. The role of B cells in in vitro induction of IFN-γ-producing CD4+ T cells specific for Listeria monocytogenes: positive and IL-10-mediated negative regulation. Cell Immunol. 1994;157:403–414. doi: 10.1006/cimm.1994.1237. [DOI] [PubMed] [Google Scholar]
- 22.Splawski J B, Nishioka J, Nishioka Y, Lipsky P E. CD40 ligand is expressed and functional on activated neonatal T cells. J Immunol. 1996;156:119–127. [PubMed] [Google Scholar]
- 23.Stout R D, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–491. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 24.Stüber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subauste C S, Wessendarp M, Sorensen R U, Leiva L E. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–6700. [PubMed] [Google Scholar]
- 26.Takeuchi M, Alard P, Streilein J W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- 27.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 28.Wu C-Y, Warrier R R, Carvajal D M, Chua A O, Minetti L J, Chizzonite R, Mongini P K A, Stern A S, Gubler U, Presky D H, Gately M K. Biological function and distribution of human interleukin-12 receptor β chain. Eur J Immunol. 1996;26:345–350. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 30.Yellin M J, Sippel K, Inghirami G, Covey L R, Lee J J, Sinning J, Clark E A, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell-B cell activating molecule/CD40-L. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 31.Zhang M, Gately M K, Wang E, Gong J, Wolf S F, Lu S, Modlin R L, Barnes P F. Interleukin-12 at the site of disease in tuberculosis. J Clin Investig. 1994;93:1733–1739. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Gong J-H, Presky D H, Xue W, Barnes P F. Expression of the IL-12 receptor β1 and β2 subunits in human tuberculosis. J Immunol. 1999;162:2441–2447. [PubMed] [Google Scholar]
- 33.Zhang M, Gong J, Lin Y, Barnes P. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun. 1998;66:794–799. doi: 10.1128/iai.66.2.794-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou P, Seder R A. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]