Abstract
Objective
The current coronavirus pandemic caused a significant decrease in cancer-related encounters resulting in a delay in treatment of cancer patients. The objective of this study was to examine the survival effect of delay in starting concurrent chemo-radiotherapy (CCRT) in women with locally-advanced cervical cancer.
Methods
This is a retrospective observational study querying the National Cancer Database from 2004 to 2016. Women with stage IB2-IVA squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix who received definitive CCRT with known wait-time for CCRT initiation after cancer diagnosis were eligible (N=13,617). Cox proportional hazard regression model with restricted cubic spline transformation was fitted to assess the association between CCRT wait-time and all-cause mortality in multivariable analysis.
Results
The median wait-time to start CCRT was 6 (IQR 4–8) weeks. In a multivariable analysis, older age, non-Hispanic black and Hispanic ethnicity, recent year of diagnosis, Medicaid and uninsured status, medical comorbidities, and absence of nodal metastasis were associated with longer CCRT wait-time (P<.05). Women with aggressive tumor factors (poorer differentiation, large tumor size, nodal metastasis, and higher cancer stage) were more likely to have a short CCRT wait-time (P<.05). After controlling for the measured covariates, CCRT wait-time of 6.1–9.8 weeks was not associated with increased risk of all-cause mortality compared to a wait-time of 6 weeks. Similar association was observed when the cohort was stratified by histology, cancer stage, tumor size, or brachytherapy use.
Conclusion
An implication of this study for the current coronavirus pandemic is that in the absence of aggressive tumor factors, a short period of wait-time to start definitive CCRT may not be associated with increased risk of mortality in women with locally-advanced cervical cancer.
Keywords: Cervical cancer, Concurrent chemo-radiotherapy, Wait time, Coronavirus pandemic, Survival
1. Introduction
In 2021 the world continues to face the impact of a global pandemic crisis caused by a novel coronavirus (COVID-19) that has created unprecedented stress on health service systems creating unique challenges to providing timely care for cancer patients [[1], [2], [3], [4], [5]]. Multiple global studies found that the current COVID-19 pandemic may cause a significant decrease in cancer-related encounters for a variety of malignancies [6,7]. An important concern is a delay in treatment and care for cancer patients [6,7]. A recent high-quality meta-analysis showed that the wait-time for treatment initiation is a critical component for patient prognosis in various malignancies [8].
In women with locally-advanced cervical cancer, the use of radiation remains an essential component in management. The American Society for Radiation Oncology (ASTRO) Clinical Practice Guidelines conclude that concurrent chemo-radiotherapy (CCRT) offers curative intent for women with this disease [9]. To date, evidence examining the effect of wait-time prior to initiation of CCRT on survival for locally-advanced cervical cancer is scarce and has reported mixed results [10,11]. Available evidence is also limited in interpretation due to limited sample size [11], restricted histology (squamous alone), inclusion of non-standard treatment approaches (omission of concurrent chemotherapy), and the inclusion of early-stage disease (stage IA-IB1) [10].
Given the constraints on delivering timely oncologic care due to the current COVID-19 pandemic [6,7], it is of paramount importance to assess the effects of treatment wait-time on survival in oncologic care. The objective of our study was to examine the impact of delay in starting CCRT on survival in women with locally-advanced cervical cancer.
2. Patients and methods
2.1. Data source
This is a retrospective observational cohort study using the National Cancer Database (NCDB). NCDB is a nationwide tumor registry that collects data from Commission on Cancer (CoC)-accredited facilities in the United States [12]. National Cancer Database collects >1 million invasive cancer cases per year, representing ~70% of all new invasive cancers in the U.S. Over 1500 CoC-affiliated institutions participate in the database through a joint mechanism of the CoC of the American College of Surgeons (ACoS) and the American Cancer Society (ACS) Society. The study was determined to not be human subjects research by the Columbia University Institutional Review Board.
2.2. Study eligibility
Women with the American Joint Committee on Cancer 6th and 7th version and the 2009 International Federation of Gynecology and Obstetrics stage of IB2-IVA squamous carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix diagnosed from 2004 to 2016 who received definitive radiotherapy with chemotherapy were examined. All patients had known information for CCRT wait-time, defined as the time interval between the diagnosis of cervical cancer and the time to initiation of CCRT. Exclusion criteria included histologic types and cancer stage other than above, lack of chemotherapy, primary surgical or chemotherapy treatment, and absence of CCRT wait-time information.
2.3. Clinical information
Among cases that met the eligibility criteria, the following information was abstracted from the database: Patient demographics included age (<40, 40–49, 50–59, 60–69, 70–79, ≥80 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and non-Hispanic others, year of diagnosis (2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015, and 2016), medical comorbidity (0, 1, and 2), insurance status (Medicare, Medicaid, private, uninsured, and others), neighborhood average household income (<$40,227, $40,227–$50,353, $50,354–$63,332, and ≥$63,333) and education level (≥17.6%, 10.9–17.5%, 6.3–10.8%, and <6.3%), and residential status (metropolitan, urban, and rural). Facility information included registered location (Eastern, South, Midwest, and West) and facility type (academic / research program, integrated network cancer program, comprehensive community cancer program, and community cancer program).
Tumor characteristics included histologic type (squamous cell, adenocarcinoma, and adenosquamous), cancer stage (IB2, IIA, IIB, IIIA, IIIB, and IVA), tumor differentiation (well, moderate, and poor), tumor size (≤20, 21–40, 41–60, 61–80, 81–100, and >100 mm), lympho-vascular space invasion (yes versus no), and regional nodal metastasis (positive versus negative). Radiotherapy type include external beam radiation with brachytherapy versus external beam radiation alone. Survival data included follow-up time after diagnosis of diagnosis and vital status (dead or alive). Overall survival was defined as time interval between the date of radiation initiation and death from all-causes. Women who were alive at last follow-up were censored.
2.4. Statistical analysis
We first examined the association between the clinico-pathological characteristics and CCRT wait-time. A generalized linear regression model using a generalized estimating equation with normal distribution and identity link was fitted. All the measured covariates were entered in the model. The residuals were plotted visually to check the violation of linear assumption. The interpretation for the parameters (betas) is that compared to the referent group what is the increased (positive) or decreased (negative) value for the average of continuous outcome.
The second step of analysis was to examine the association between CCRT wait-time and all-cause mortality. Cox proportional hazards models with restricted cubic spline transformation of CCRT wait-time were fitted to assess the non-linear associations between CCRT wait-time and survival while adjusting for the measured characteristics [13]. Clinically relevant cut points were applied as 4, 8, 12, and 16 weeks for CCRT wait-time. Six weeks from diagnosis to initiation of therapy was chosen as a reference point as prior work has shown that the median wait-time for initiation of therapy for locally-advanced cervical cancer is approximately 6 weeks [10,11,14]. Effect size for all-cause mortality at each tested week relative to week 6 was expressed with adjusted-hazard ratio and 95% confident interval.
Various sensitivity analyses were undertaken to assess the robustness of the study findings. Subcohorts were created as stratified by histology subtypes, cancer stage, and tumor size. CCRT wait-time of week 4 was also tested. The study cohort was stratified based on the use of brachytherapy. The rationale of this analysis is that brachytherapy plays a critical role in definitive radiotherapy for locally-advanced cervical cancer [15]. All statistical analyses were based on two-sided hypothesis, and results were deemed statistically significant at a P<.05. The STROBE guidelines were consulted to display the observational study [16].
3. Results
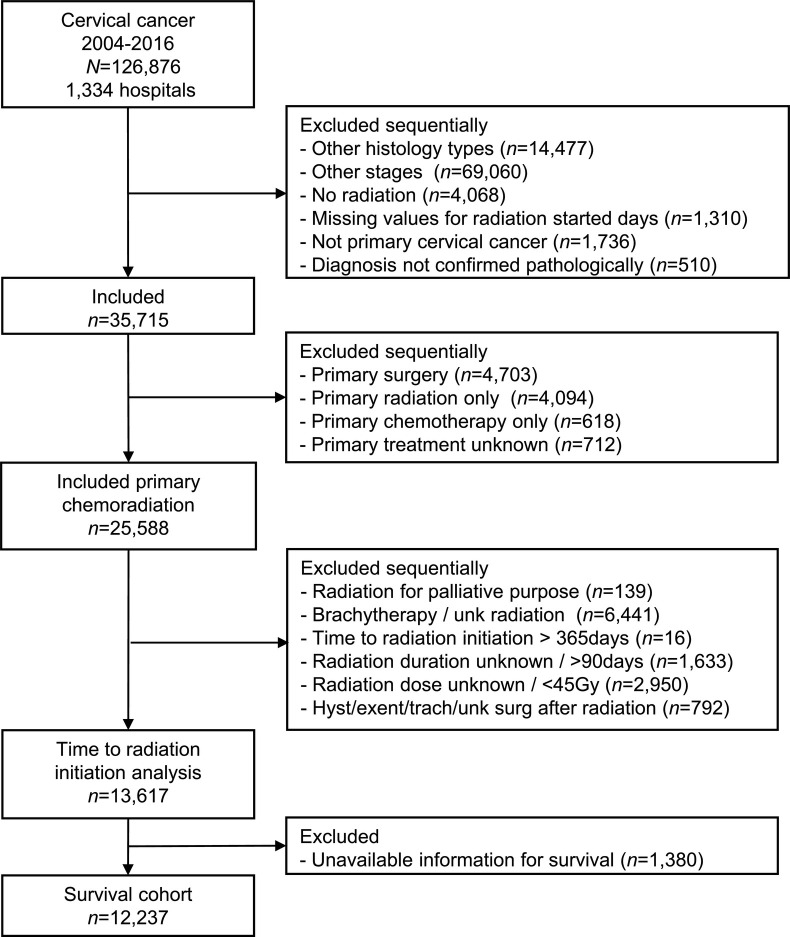
A total of 13,617 women were identified (Table 1 , Fig. 1 ). The median age was 51 (interquartile range 42–61). The majority of patients were non-Hispanic white (59.3%), had squamous tumors (86.1%), and received brachytherapy (64.6%). The most frequent cancer stage was IIIB (43.3%), and nearly a quarter of tumors were >6 cm in diameter (25.1%). The median CCRT wait-time was 6 (interquartile range 4–8) weeks. The median duration of definitive radiotherapy was 7.6 (interquartile range 6.3–9.0) weeks. The median of total radiation dose for external beam treatment was 48.6 (interquartile range 45–54) Gy.
Table 1.
Patient demographics and factors associated with CCRT wait-time.
| N (%) | Mean (SD) | Estimated parameters (beta) (95%CI) § |
|
|---|---|---|---|
| Overall | 13,617 (100.0) | 6.6 (4.3) | |
| Age (y) | |||
| <40 | 2535 (18.6) | 6.7 (4.5) | Referent |
| 40–49 | 3737 (27.4) | 6.4 (4.3) | −0.04 (−0.25,0.17) |
| 50–59 | 3569 (26.2) | 6.6 (4.1) | 0.19 (−0.03,0.40) |
| 60–69 | 2266 (16.6) | 6.8 (4.3) | 0.51 (0.25,0.76)** |
| 70–79 | 1138 (8.4) | 6.8 (4.3) | 0.53 (0.19,0.87)* |
| ≥80 | 372 (2.7) | 6.4 (4.7) | 0.29 (−0.20,0.79) |
| Race/ethnicity | |||
| Non-Hispanic: White | 8071 (59.3) | 6.2 (3.9) | Referent |
| Non-Hispanic: Black | 2240 (16.5) | 7.1 (4.8) | 0.89 (0.69,1.10)** |
| Hispanic | 1860 (13.7) | 7.9 (5.1) | 1.07 (0.84,1.29)** |
| Non-Hispanic: Other | 746 (5.5) | 6.9 (4.2) | 0.10 (−0.21,0.42) |
| Unknown | 700 (5.1) | 5.8 (3.9) | −0.01 (−0.33,0.32) |
| Year of diagnosis | |||
| 2004 | 728 (5.3) | 5.7 (4.3) | Referent |
| 2005 | 745 (5.5) | 5.8 (4.4) | 0.00 (−0.42,0.42) |
| 2006 | 793 (5.8) | 6.0 (4.3) | 0.29 (−0.13,0.70) |
| 2007 | 885 (6.5) | 6.4 (4.9) | 0.81 (0.41,1.22)** |
| 2008 | 951 (7.0) | 6.1 (4.0) | 0.36 (−0.04,0.75) |
| 2009 | 948 (7.0) | 6.5 (4.4) | 0.72 (0.32,1.12)* |
| 2010 | 1019 (7.5) | 6.6 (4.4) | 0.83 (0.43,1.23)** |
| 2011 | 1093 (8.0) | 6.7 (4.1) | 0.93 (0.54,1.32)** |
| 2012 | 1168 (8.6) | 6.6 (4.5) | 0.84 (0.45,1.23)** |
| 2013 | 1197 (8.8) | 6.7 (4.3) | 0.87 (0.49,1.26)** |
| 2014 | 1303 (9.6) | 6.9 (4.0) | 1.16 (0.78,1.54)** |
| 2015 | 1407 (10.3) | 6.9 (4.1) | 1.19 (0.82,1.57)** |
| 2016 | 1380 (10.1) | 7.3 (4.4) | 1.64 (1.26,2.01)** |
| Insurance Status | |||
| Not Insured | 1769 (13.0) | 6.9 (4.8) | 0.56 (0.33,0.79)** |
| Private | 5326 (39.1) | 6.2 (3.9) | Referent |
| Medicaid | 3574 (26.2) | 7.0 (4.6) | 0.55 (0.37,0.73)** |
| Medicare | 2539 (18.6) | 6.5 (4.2) | 0.08 (−0.16,0.33) |
| Other Government | 155 (1.1) | 7.6 (5.7) | 1.50 (0.84,2.16)** |
| Unknown | 254 (1.9) | 6.7 (4.0) | 0.40 (−0.12,0.92) |
| Household income¶ | |||
| <$40,227 | 3682 (27.0) | 6.8 (4.5) | Referent |
| $40,227 - $50,353 | 3479 (25.5) | 6.5 (4.2) | 0.13 (−0.07,0.34) |
| $50,354 - $63,332 | 3066 (22.5) | 6.5 (4.4) | 0.15 (−0.08,0.38) |
| ≥$63,333 | 3200 (23.5) | 6.5 (4.2) | 0.21 (−0.06,0.48) |
| Not Available | 190 (1.4) | 6.1 (3.8) | −0.74 (−2.27,0.79) |
| Neighborhood average education† | |||
| ≥17.6% | 4393 (32.3) | 7.1 (4.7) | Referent |
| 10.9% - 17.5% | 3922 (28.8) | 6.5 (4.2) | −0.27 (−0.46,-0.08)* |
| 6.3% - 10.8% | 3270 (24.0) | 6.2 (4.0) | −0.56 (−0.79,-0.33)** |
| <6.3% | 1870 (13.7) | 6.0 (4.0) | −0.88 (−1.18,-0.59)** |
| Not Available | 162 (1.2) | 6.2 (4.0) | 0.25 (−1.41,1.91) |
| Urban/Rural | |||
| Metropolitan | 11,021 (80.9) | 6.7 (4.4) | Referent |
| Urban | 2030 (14.9) | 6.1 (3.9) | −0.25 (−0.45,-0.04)* |
| Rural | 269 (2.0) | 5.8 (3.9) | −0.35 (−0.86,0.15) |
| Unknown | 297 (2.2) | 6.7 (4.1) | 0.28 (−0.20,0.76) |
| Charlson/Deyo comorbidity | |||
| 0 | 11,729 (86.1) | 6.6 (4.3) | Referent |
| 1 | 1474 (10.8) | 6.6 (4.5) | −0.03 (−0.25,0.20) |
| 2 | 414 (3.0) | 7.1 (4.8) | 0.46 (0.05,0.86)* |
| Facility location | |||
| Eastern | 2566 (18.8) | 6.9 (4.4) | Referent |
| South | 3892 (28.6) | 6.1 (4.1) | −0.80 (−1.01,-0.58)** |
| Midwest | 4901 (36.0) | 6.4 (4.1) | −0.52 (−0.72,-0.31)** |
| West | 2253 (16.5) | 7.5 (4.8) | 0.33 (0.09,0.57)* |
| Unknown | – | 6.6 (1.9) | −1.05 (−4.67,2.58) |
| Facility type | |||
| Community Cancer Program | 734 (5.4) | 6.7 (4.6) | Referent |
| Comp Community Cancer Program | 4320 (31.7) | 6.1 (4.2) | −0.52 (−0.84,-0.19)* |
| Academic/Research Program | 6769 (49.7) | 7.0 (4.4) | 0.00 (−0.31,0.32) |
| Integrated Network Cancer Program | 1789 (13.1) | 6.1 (3.9) | −0.70 (−1.06,-0.34)* |
| Other / unknown | – | 6.6 (1.9) | ‡ |
| Histology | |||
| Squamous cell | 11,722 (86.1) | 6.5 (4.3) | Referent |
| Adenocarcinoma | 1529 (11.2) | 6.9 (4.4) | 0.21 (−0.01,0.44) |
| Adenosquamous | 366 (2.7) | 6.6 (4.5) | 0.01 (−0.42,0.44) |
| Stage | |||
| IB2 | 1339 (9.8) | 7.2 (4.9) | Referent |
| II NOS | 172 (1.3) | 6.2 (3.5) | −0.74 (−1.40,-0.08)* |
| IIA NOS | 586 (4.3) | 7.1 (4.6) | 0.13 (−0.28,0.54) |
| IIA1 | 132 (1.0) | 7.8 (4.2) | −0.27 (−1.02,0.49) |
| IIA2 | 266 (2.0) | 6.9 (3.5) | −0.37 (−0.91,0.17) |
| IIB | 3884 (28.5) | 6.8 (4.2) | −0.36 (−0.63,-0.10)* |
| III NOS | 157 (1.2) | 6.3 (4.7) | −0.79 (−1.47,-0.10)* |
| IIIA | 341 (2.5) | 6.1 (3.9) | −0.99 (−1.49,-0.49)** |
| IIIB | 5897 (43.3) | 6.4 (4.3) | −0.83 (−1.08,-0.58)** |
| IVA | 843 (6.2) | 5.6 (4.1) | −1.30 (−1.67,-0.93)** |
| Tumor differentiation | |||
| Well | 575 (4.2) | 7.1 (4.6) | Referent |
| Moderate | 4508 (33.1) | 6.5 (4.2) | −0.61 (−0.97,-0.25)* |
| Poorly | 4489 (33.0) | 6.5 (4.3) | −0.59 (−0.95,-0.23)* |
| Unknown | 4045 (29.7) | 6.7 (4.4) | −0.36 (−0.72,0.00) |
| Tumor Size | |||
| ≤20 mm | 285 (2.1) | 6.9 (3.8) | Referent |
| 21–40 mm | 1495 (11.0) | 7.6 (4.6) | 0.57 (0.05,1.09)* |
| 41-60 mm | 4044 (29.7) | 6.8 (4.1) | −0.29 (−0.79,0.21) |
| 61-80 mm | 2579 (18.9) | 6.1 (4.2) | −0.81 (−1.32,-0.31)* |
| 81-100 mm | 634 (4.7) | 5.5 (4.1) | −1.31 (−1.89,-0.73)** |
| >100 mm | 202 (1.5) | 5.2 (3.8) | −1.37 (−2.11,-0.62)* |
| Unknown | 4378 (32.2) | 6.5 (4.5) | −0.18 (−0.67,0.32) |
| LVSI | |||
| No | 1320 (9.7) | 7.0 (4.5) | Referent |
| Yes | 437 (3.2) | 7.2 (5.0) | 0.35 (−0.09,0.80) |
| Unknown | 11,860 (87.1) | 6.5 (4.3) | −0.16 (−0.40,0.08) |
| Regional lymph nodes | |||
| Negative | 473 (3.5) | 7.7 (4.4) | 1.33 (0.83,1.84)** |
| Positive | 599 (4.4) | 8.9 (4.7) | −1.26 (−1.64,-0.88)** |
| Unknown | 12,545 (92.1) | 6.4 (4.3) | Referent |
| Radiation type | |||
| External bean / brachytherapy | 8803 (64.6) | 6.6 (4.2) | 0.03 (−0.12,0.18) |
| External beam | 4814 (35.4) | 6.4 (4.5) | Referent |
Mean (standard deviation) wait-time (weeks) from cervical cancer diagnosis to CCRT initiation is shown. § Estimated parameters (beta) from generalized linear regression model. *P<.05, **P<.001. – Number suppressed per the NCDB instruction. † % of not graduating from high school. ‡ Con-linearity with unknown in facility location. ¶ Neighborhood average. Abbreviations: y, year; N, number; SD, standard deviation; CI, confidence interval; CCRT, concurrent chemo-radiotherapy; LVSI, lympho-vascular space invasion; comp, comprehensive; and NOS, not otherwise specified.
Fig. 1.
Study cohort selection criteria.
Abbreviations: N, number; unk, unknown; hyst, hysterectomy; exent, exenteration; and trach, trachelectomy.
There were 13 factors independently associated with CCRT wait-time on multivariable analysis (Table 1). Older age, non-Hispanic black and Hispanic race/ethnicity, more recent year of diagnosis, Medicaid and uninsured status, greater medical comorbidity, and absence of nodal metastasis were associated with longer CCRT wait-time (all, P<.05). In contrast, women living in urban regions, residents of the Midwest and South, residents in neighborhoods with higher educational attainments (lower percentage of not graduating from high school), those who received care at a comprehensive community cancer program or integrated network cancer program, and those with aggressive tumor factors (higher grade, large tumor size, nodal metastasis, and higher stage) were more likely to have shorter CCRT wait-time (all, P < .05).
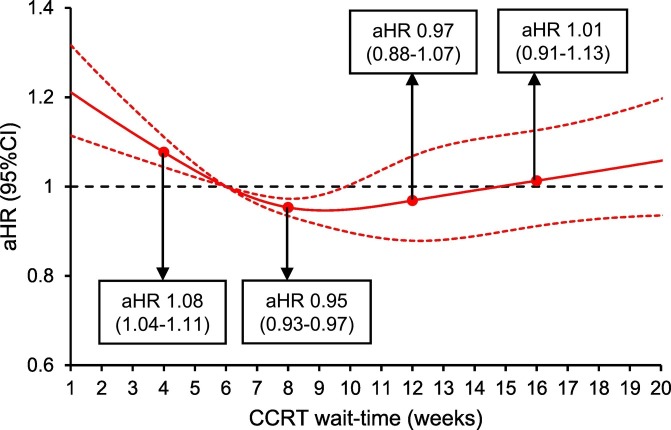
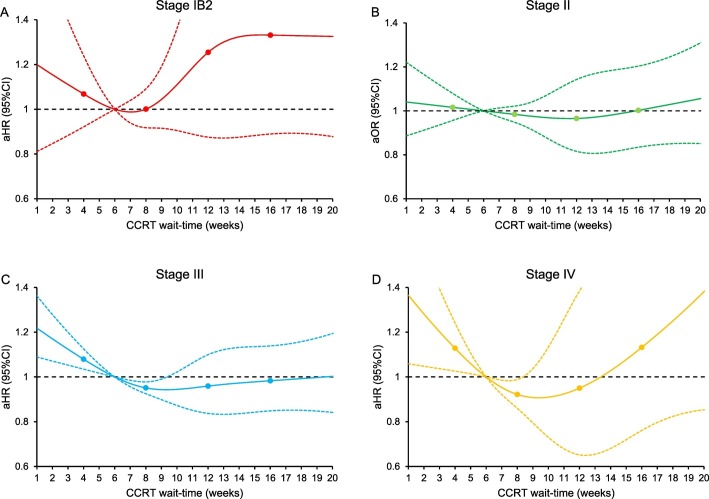
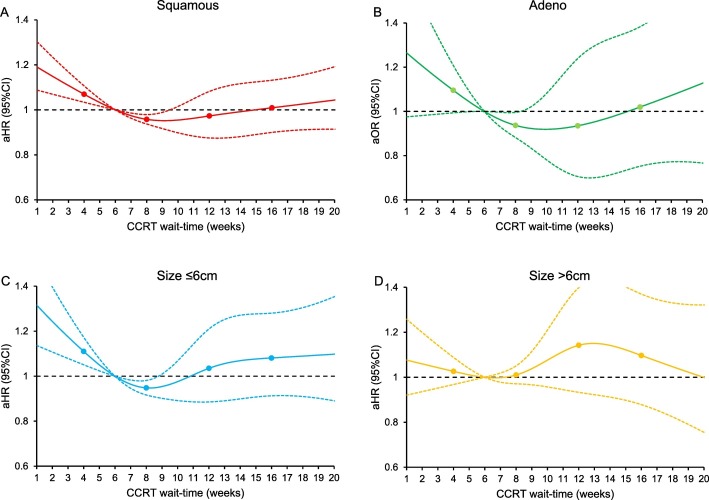
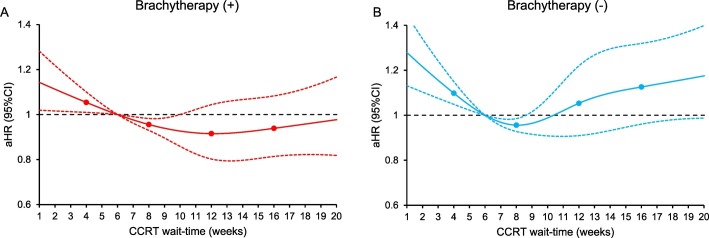
There were 12,237 women who had survival information. The median follow-up time was 30.6 (interquartile range 14.2–63.1) months, and there were 5019 (41.0%) women who died of any cause. After controlling for patient factors (age, race/ethnicity, year of diagnosis, insurance status, household income, educational status, residential status), facility factors (location and type), tumor factors (histology, cancer stage, tumor differentiation, tumor size, LVSI, and nodal status), and treatment factors (radiotherapy type and radiotherapy duration), women who had CCRT wait-time of 6.1–9.8 weeks were not at increased risk for all-cause mortality compared those who had a 6-week wait-time (Fig. 2 ). In contrast, women with a short CCRT wait-time had increased all-cause mortality risk versus those with 6-week wait-time (Fig. 2). Similar trends were observed when the cohort was stratified by cancer stage (Fig. 3A-D), histologic type (Fig. 4A-B), tumor size (Fig. 4C-D), and brachytherapy use (Fig. 5A-B and Supplemental Figs. S1-4). Lastly, similar results were observed when the referent group was set as 4-week CCRT wait-time (data not shown).
Fig. 2.
Adjusted associations between CCRT wait-time and all-cause mortality.
Adjusted-hazard ratio for all-cause mortality controlling for patient factors (age, race/ethnicity, year of diagnosis, insurance status, household income, educational status, residential status), facility factors (location and type), tumor factors (histology, cancer stage, tumor differentiation, tumor size, LVSI, and nodal status), and treatment factor (radiotherapy type and radiotherapy duration) is shown by week of CCRT wait-time for the whole cohort. CCRT wait-time was coded using restricted cubic spline transformation with four knots located at 4, 8, 12, and 16 weeks (shown as knots). At CCRT wait-time of 9.8-week, the upper-boundary of confidence interval was <1.00 (aHR 0.95, 95%CI 0.90–0.99) that the value crossed 1.00 afterwards. The Y-axis represents the effect size for all-cause mortality. The X-axis represents the wait-time (week) from cervical cancer diagnosis to CCRT initiation. Week 6 is set as the referent group. The solid line represents the estimate as effect size. The dashed lines are 95% confidence interval. Abbreviations: CCRT, concurrent chemo-radiotherapy; aHR-adjusted-hazard ratio; and CI, confidence interval.
Fig. 3.
Adjusted associations between CCRT wait-time and all-cause mortality (cancer stage stratification).
Adjusted-hazard ratios for all-cause mortality are shown by week of CCRT wait-time for (A) stage IB2, (B) stage II, (C) stage III, and (D) stage IVA. CCRT wait-time was coded using restricted cubic spline transformation with four knots located at 4, 8, 12, and 16 weeks (shown as knots). The Y-axis represents the effect size for all-cause mortality. The X-axis represents wait-time (week) from cervical cancer diagnosis to CCRT initiation. Week 6 is set as the referent group. The solid line represents the estimate as effect size. The dashed lines are 95% confidence interval. Abbreviations: CCRT, concurrent chemo-radiotherapy; aHR-adjusted-hazard ratio; and CI, confidence interval.
Fig. 4.
Adjusted associations between CCRT wait-time and all-cause mortality (histology stratification and tumor size stratification).
Adjusted-hazard ratios for all-cause mortality are shown by week of CCRT wait-time for (A) squamous carcinoma, (B) adenocarcinoma, (C) tumor size ≤6 cm, and (D) tumor size >6 cm. Wait time was coded using restricted cubic spline transformation with four knots located at 4, 8, 12, and 16 weeks (shown as knots). The Y-axis represents the effect size for all-cause mortality. The X-axis represents wait-time (week) from cervical cancer diagnosis to CCRT initiation. Week 6 is set as the referent group. The solid line represents the estimate as effect size. The dashed lines are 95% confidence interval. Abbreviations: squamous, squamous cell carcinoma; adeno, adenocarcinoma; CCRT, concurrent chemo-radiotherapy; aHR-adjusted-hazard ratio; and CI, confidence interval.
Fig. 5.
Adjusted associations between CCRT wait-time and all-cause mortality (brachytherapy stratification).
Adjusted-hazard ratios for all-cause mortality are shown by week of CCRT wait-time for (A) external beam and brachytherapy and (B) external beam without brachytherapy. CCRT wait-time was coded using restricted cubic spline transformation with four knots located at 4, 8, 12, and 16 weeks (shown as knots). The Y-axis represents the effect size for all-cause mortality. The X-axis represents the wait-time (week) from cervical cancer diagnosis to CCRT initiation. Week 6 is set as the referent group. The solid line represents the estimate as effect size. The dashed lines are 95% confidence interval. Abbreviation: CCRT, concurrent chemo-radiotherapy; aHR-adjusted-hazard ratio; and CI, confidence interval.
4. Discussion
Our study highlights that the effect of time to initiation of CCRT on survival for locally-advanced cervical cancer is complex, reflecting various underlying patient, facility, and tumor factors. Women whose tumors had poor prognostic factors were more likely to initiate therapy more rapidly. Thus, increased mortality in the short wait-time cases may reflect pre-existing advanced tumor factors. In contrast, the trend towards gradually increasing mortality with longer time to initiation of therapy may reflect the effect of delay in treatment initiation.
Data from a multi-continent study from the COVID and Cancer Research Network (CCRN) raise concern for cancer patients [6]. The results of this study imply not only that there will be a significant delay in starting treatment for cancer patients but also that there will be a stage shift as a consequence of the current global pandemic [6]. These concerns are particularly relevant to radiation oncologists because cervical cancer is more common in minority populations and socioeconomically disadvantaged groups, such groups have been particularly affected by the pandemic [[17], [18], [19]]. Moreover, the Society of Gynecologic Oncology (SGO) suggests non-surgical therapy with definitive radiotherapy as an alternative treatment approach for women with early-stage disease if treatment delay for radical hysterectomy will be prolonged due to the COVID-19 pandemic [20]. Studies examining survival effect of radical hysterectomy wait-time for early-stage cervical cancer are also limited in the current literature and warrant further investigation [21,22]. Our recent analysis of stage IB-IIA cervical cancer showed that longer hysterectomy wait-time was associated with increased risk of pathological parametrial invasion and all-cause mortality [22].
To date, limited data has been reported describing the impact of time to initiation of radiation and survival for locally-advanced cervical cancer. One study showed that longer wait-time to initiation of CCRT was associated with decreased survival although the sample size was limited (n=195) [11]. Another study concluded that longer wait-time to CCRT was not associated with increased mortality [10]. As wait-time was grouped by four week increments in this study, our data illustrate the association between time to initiation of therapy by week.
One major concern observed in this study was that nearly one-third of study population did not receive brachytherapy for the definitive radiotherapy in women with locally-advanced cervical cancer. Notable significance is that this was observed in the CoC-affiliated centers in the recent years (2004–2016). In 2019, American Brachytherapy Society (ABS) and SGO endorsed the importance of brachytherapy as a critical component of definitive radiotherapy for cervical cancer [15]. While this was not the primary objective of current study, the importance of brachytherapy needs to be emphasized.
Strengths of this study include rigorous selection criteria, large sample size and measured covariates, use of a modern analytic approach, and multiple sensitivity analyses. However, there are several limitations in this study. First, unmeasured bias is inherent to the retrospective nature of this study. For example, the data on the underlying cause of treatment delay is not available. It may be possible that urgent initiation of CCRT may have been indicated for patients who had vaginal bleeding in women with large tumor or higher cancer stage, but the database does not have this information. Second, there were few women with long periods of treatment delay thus limiting our statistical power to detect changes in survival. The restricted cubic spline curves suggest that mortality risk may be increased after a certain wait-time.
The ASTRO has compiled COVID-19 Clinical Guidance for a number of malignancies [23]. This includes treatment prioritization, approaches, and practice recommendations during the COVID-19 pandemic. Given the possible survival effects of CCRT wait-time as well as possible pandemic-related demographic change in cervical cancer, establishing clinical guidance for cervical cancer treatment in the current situation would be of utmost use. Recent international expert consensus recommendations for radiotherapy during the COVID-19 pandemic categorize that women with locally-advanced cervical cancer has the highest priority for radiotherapy [24]. Recommendation on wait-time for CCRT initiation was not addressed.
In conclusion, our study suggests that in the absence of aggressive tumor factors, a short period of wait-time to start definitive CCRT (approximately 3 additional weeks from the cohort median wait-time) may not be associated with increased risk of mortality in women with locally-advanced cervical cancer. While treatment delay at COVID-19 burdened hospitals may be necessary for patient safety purposes, when feasible, attempts should be made to avoid prolonged delays to start CCRT for women with locally-advanced cervical cancer. This study also observed variable differences in CCRT wait-time per patient and facility factors. This inequity of cancer treatment merits further investigation.
Contributors
K.M. designed the study, initiated the collaborations, cleaned and analyzed the data, created the figures and tables, interpreted the results, and drafted and revised the manuscript with others. Y.H. accessed the data, contributed to the analysis, interpreted the results and revised the manuscript. S.M. contributed to the literature overview, intellectual inputs, interpreted the results, and edited the manuscript. L.D.R. supervised the study, and revised manuscript. P.H. contributed to the intellectual aspect of the study, reviewed the results, and revised manuscript. J.D.W. contributed to the study concept and design, instructed the analytic approach, interpreted the results, and revised the manuscript. He is the corresponding author of the study.
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Role of the funding source
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability statement
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.
Transparency
The manuscript's corresponding author (J.D.W.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. National Cancer Database is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The program is the source of the de-identified data used; and the program has not verified and is not responsible for the statistical validity of the data analysis or the conclusions derived by the study team.
Declaration of Competing Interest
Consultant, Clovis Oncology, and research funding, Merck (J.W.); consultant, Quantgene (L.D.R.); honorarium, Chugai, textbook editorial, Springer, and meeting expense, VBL therapeutics (K.M.); research grant, MSD (S.M.); none for others.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2021.02.026.
Appendix A. Supplementary data
Supplementary material
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Coronavirus Disease (COVID-19) Dashboard World Health Organization. https://covid19.who.int/ (accessed 2/6//2021)
- 6.London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jazieh A.R., Akbulut H., Curigliano G., et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna T.P., King W.D., Thibodeau S., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;m4087:371. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chino J., Annunziata C.M., Beriwal S., et al. Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2020;10:220–234. doi: 10.1016/j.prro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramey S.J., Asher D., Kwon D., et al. Delays in definitive cervical cancer treatment: an analysis of disparities and overall survival impact. Gynecol. Oncol. 2018;149:53–62. doi: 10.1016/j.ygyno.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 11.E C., Dahrouge S., Samant R., Mirzaei A., Price J. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:1071–1077. doi: 10.1016/j.ijrobp.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Database American College of Surgeons. https://www.facs.org/quality-programs/cancer/ncdb (accessed 8/9/2020)
- 13.Croxford R. Restricted Cubic Spline Regression: A Brief Introduction. https://support.sas.com/resources/papers/proceedings16/5621-2016.pdf
- 14.Ramirez P.T., Chiva L., Eriksson A.G.Z., et al. COVID-19 global pandemic: options for Management of Gynecologic Cancers. Int. J. Gynecol. Cancer. 2020;30:561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- 15.Holschneider C.H., Petereit D.G., Chu C., et al. Brachytherapy: a critical component of primary radiation therapy for cervical cancer: from the Society of Gynecologic Oncology (SGO) and the American brachytherapy society (ABS) Gynecol. Oncol. 2019;152(3):540–547. doi: 10.1016/j.ygyno.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E., Altman D.G., Egger M., et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coronavirus Disease 2019 (COVID-19) Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html (accessed 8/9/2020)
- 18.United States Cancer Statistics Data visualizations. Center for Disease Control and Prevention. https://gis.cdc.gov/Cancer/USCS/DataViz.html (accessed 8/9/2020)
- 19.COVID-19 in Racial and Ethnic Minority Groups Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html (accessed 8/9/2020)
- 20.Pothuri B., Alvarez Secord A., Armstrong D.K., et al. Anti-cancer therapy and clinical trial considerations for gynecologic oncology patients during the COVID-19 pandemic crisis. Gynecol. Oncol. 2020;158:16–24. doi: 10.1016/j.ygyno.2020.04.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuo K., Novatt H., Matsuzaki S., Hom M.S., Castaneda A.V., Licon E., Nusbaum D.J. Roman LD. Wait-time for hysterectomy and survival of women with early-stage cervical cancer: a clinical implication during the coronavirus pandemic. Gynecol. Oncol. 2020;158:37–43. doi: 10.1016/j.ygyno.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K., Huang Y., Matsuzaki S., Klar M., Wright J.D. Effect of delay in surgical therapy for early-stage cervical cancer: an implication in the coronavirus pandemic. Eur. J. Cancer. 2020;139:173–176. doi: 10.1016/j.ejca.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 Clinical Guidance American Society of Radiation Oncology. https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Clinical-Guidance (accessed 8/9/2020)
- 24.Elledge C.R., Beriwal S., Chargari C., Chopra S., Erickson B.A., Gaffney D.K., Jhingran A., Klopp A.H., Small W., Jr., Yashar C.M., Viswanathan A.N. Radiation therapy for gynecologic malignancies during the COVID-19 pandemic: international expert consensus recommendations. Gynecol. Oncol. 2020;158(2):244–253. doi: 10.1016/j.ygyno.2020.06.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.