Abstract
Background
Hidradenitis suppurativa were associated with comorbidities in various organ systems. Inflammatory dermatological diseases such as pyoderma gangrenosum were reported to be associated with hidradenitis suppurativa. Nevertheless, as for the association between hidradenitis suppurativa and psoriasis, evidences were insufficient. In many studies, the association between psoriasis and hidradenitis suppurativa has been reported. However, some evidence seems to be controversial. The purpose of the systematic review and meta-analysis was to assess whether there was significant association between HS and psoriasis.
Methods
On June 01, 2022, we appraised 2,795 articles from databases including PubMed, Web of Science and Embase. Search syntaxes were based on ‘hidradenitis suppurativa’ or ‘acne inversa’ with “psoriasis”, “comorbidities” or ‘epidemiology’. Synonyms were determined based on MeSH terms and Emtree. Observational results that evaluated the odds ratio for people with hidradenitis suppurativa who had psoriasis were extracted for qualitative synthesis.
Results
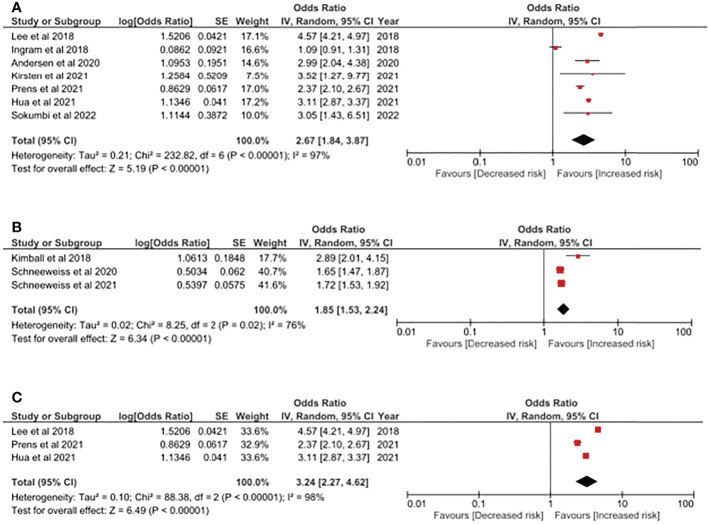
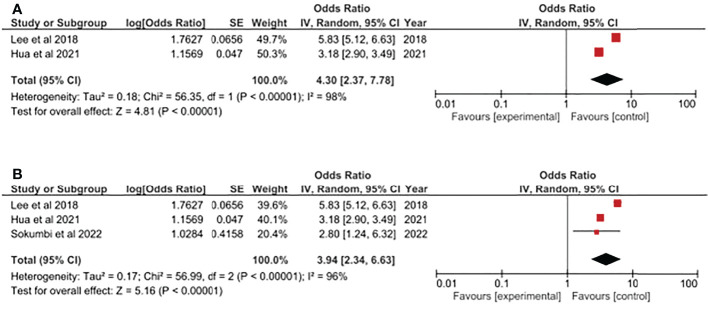
After the selection process of the initial 2,795 studies, ten observational studies, including 3 cohort studies, 1 case-control study, and 6 cross-sectional studies, were extracted for critical appraisal. Based on the integration of 7 studies (with more than 560,000 participants included), people with hidradenitis suppurativa had a higher risk of having psoriasis, with a 2.67-fold risk (95% CI, 1.84, 3.87). The association remained in the sensitivity analyses utilizing strict adjustment models. In the analysis that only included studies with a similar study design and adjustments in obesity-related factors, the risk of people with hidradenitis suppurativa having psoriasis was 3.24 (95% CI, 2.27, 4.62). In male patients with HS, the risk of having psoriasis was 4.30-fold higher than male patients without HS (95% CI, 2.37, 7.78). Likewise, in an analysis including 3 cross-sectional studies, the risk of female HS patients having psoriasis was 3.94-fold higher than female HS-free patients (95% CI, 2.34, 6.63).
Conclusions
The co-occurrence of hidradenitis suppurativa and psoriasis can greatly increase the burden of the disease. Psoriasis could be one of the critical comorbidities of hidradenitis suppurativa and should be recommended for future screening and follow up. The association between the two diseases should be kept in mind in managing hidradenitis suppurativa patients. More prospective studies are needed to establish the true magnitude of the association between psoriasis and hidradenitis suppurativa.
Keywords: hidradenitis suppurativa, psoriasis, meta-analysis, epidemiology, immunology
Highlights
Identifying comorbidities in hidradenitis suppurativa (HS) is critical to give patients recommendations for the screening for comorbidities. Recently, HS has been reported to have a strong association with psoriasis. However, some evidence seemed to be controversial and the current guidelines for comorbidity screening recommendation have insufficient information regarding the HS-psoriasis association. In the current systematic review and meta-analysis, we report that, based on the integration of 7 studies with more than 560,000 participants included, people with HS had a more than 2.6-fold risk of having psoriasis. The significance of the association remained in the sensitivity analyses using a strict adjustment model and different study design. In the analysis that only included studies with similar study design and adjusted for obesity-related factors, the risk of people with HS having psoriasis was 3.24 (95% CI, 2.27, 4.62). The coexistence of HS and psoriasis can greatly increase the burden of the disease that may require aggressive treatment. Further prospective studies are needed to establish the magnitude of the association between psoriasis and HS.
Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder that affects apocrine units. It is characterized by nodules and cysts that form sinus tracts, which is seen prominently in intertriginous areas (1). Psoriasis is a chronic inflammatory disorder that prominently affects skin and joints. Erythematous papules and plaques topped with thick micaceous scales are typical and are seen more commonly on the extensor surfaces of the extremities (2). Despite their differences in skin manifestations, both chronic disorders share common risk factors, such as obesity and cigarette smoking (3), heavy burden on quality of life (4, 5) and common inflammatory mediators, such as the interleukin (IL) 12-IL 23 pathway, IL-17 interactions, and tumor necrosis factor (TNF) alpha (1, 6, 7).
Several reports documented associations with different autoimmune diseases and lifestyle diseases in both HS and psoriasis. Diseases including metabolic syndrome, diabetes mellitus, inflammatory bowel disease, and spondyloarthropathies, were reported to be associated with both HS and psoriasis (8, 9). Even though sharing similar comorbidities, the association between HS and psoriasis was not yet confirmed. Recently, there were studies reporting potential association between psoriasis and HS (10). However, evidences from different studies appeared to be controversial (11, 12). Despite the evidence on the association between the two diseases, studies were still limited to determine its magnitude of association. Furthermore, in the current guidelines for screening for HS comorbidities, the evidences regarding HS-psoriasis association remained insufficient (13). The purpose of the study is to assess whether there is significant association between HS and psoriasis. The authors performed a systematic review and meta-analysis of cases compared to controls to determine the association between HS and psoriasis.
Methods
Literature searching, process of screening and eligibility criteria for studies
In the current meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was utilized to ensure the screening process being precise and objective (14). Real-world observational studies that evaluated the odds ratio of psoriasis in people with HS, including studies in cohort, case-control and cross-sectional study design, were targeted for data extraction in systematic review and meta-analysis.
On June 01, 2022, we appraised 2,795 articles from databases including PubMed, Web of Science and Embase. Search syntaxes were based on ‘hidradenitis suppurativa’ or ‘acne inversa’ with “psoriasis”, “comorbidities” or ‘epidemiology’. Synonyms were determined based on MeSH terms and Emtree. The detailed study protocol on the search strategy and inclusion criteria were provided in the Supplementary Information.
In this study, our aim was to provide integrated real-world evidence based on observational results. The following are the exclusion criteria: (1) studies that had no relationship with HS, (2) studies related to HS but not focusing on HS related comorbidities related to HS, (3) studies focused on genetic roles or pathophysiologic mechanisms in HS, and (4) studies reporting coexistence but not containing proper comparative groups. In the screening process, the study language and ethnicity of the participants were not established as specific exclusion criteria. Conference abstracts were also included in the selection process to address potential publication bias.
Data extraction and assessment of risk of bias
Study characteristics were reported in Table 1 . Baseline information from the extracted studies, including the definition of HS diagnosis, the number of participants, the gender ratio, the mean age of overall participants, was recorded. Regarding the evaluation of the risk of bias and study quality, the Newcastle-Ottawa scale was used (22).
Table 1.
Baseline characteristic of included observational studies.
| Author | Study design | Year | Location | Definition of HS patients | Definition of outcome event | Female percentage case/control (%) | Mean age (yo) case/control | Adjustment | No. of participants | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Ingram et al. (11) | case-control (proxy cases) | 2018 | UK | CPRD Read code algorithms identifying clinical features. Validated by subsequent questionnaire | Read codes in CPRD | NA | NA | age, sex and registration at the same primary care practice | 163711 | 7 |
| Ingram et al. (11) | case-control (physician-diagnosed cases) | 2018 | UK | Read codes in CPRD and ICD-10-based diagnosis | Read codes in CPRD | NA | NA | age, sex and registration at the same primary care practice | 117896 | 7 |
| Lee et al. (12) | cross sectional | 2018 | Korea | at least two documented physician contacts based on ICD-10 diagnosis | at least 2 physician validated ICD-10-based diagnosis | 38.7/38.7 | 33.6/33.6 | age, sex, socioeconomic status and the presence of diabetes, hypertension and dyslipidemia. | 171096 | 6 |
| Kimball et al. (15) | cohort (moderate HS) | 2018 | US | at least two ICD-9-based diagnoses | medical claims recorded | 74/74 | 42.19/42.19 | age, sex, region of residence, and healthcare plan | 4584 | 7 |
| Kimball et al. (15) | cohort (severe HS) |
2018 | US | at least two ICD-9/ICD-10-based diagnosis diagnoses and experienced at least one of the disease severity indicators | medical claims recorded | 71/71 | 42.19/42.19 | age, sex, region of residence, and healthcare plan | 6130 | 7 |
| Andersen et al. (10) | cross sectional | 2020 | Denmark | ICD-10-based diagnosis HS | ICD-10-based diagnosis | 87.5/NA | NA | NA | 440 HS group/NA for control | 5 |
| Schneeweiss et al. (16) | cohort | 2020 | US | at least three ICD-9/ICD-10-based diagnosis or more than one HS diagnosis by a dermatologist | NA | 75.5/76.7 | 36.6/37.6 | age, sex, region, number of outpatient visits, number of unique medications, use of systemic biologic or nonbiologic immunomodulatory agents, comorbidities, combined comorbidity score | 121744 | 6 |
| Schneeweiss et al. (17) | cohort | 2021 | US | at least three ICD-9/ICD-10-based diagnosis on or more than one HS diagnosis by a dermatologist | NA | 75.5/76.6 | 36.48/37.93 | age, sex, health care utilization patterns, comorbidities, comedications, Gagne combined comorbidity score | 132896 | 6 |
| Hua et al. (18) | cross sectional | 2021 | US | at least two ICD-9/ICD-10-based diagnosis | ICD9/10 diagnosis record | 73.6/73.6 | 40.7/40.7 | age, sex, race, index date, obesity, tobacco use | 150337 | 7 |
| Kirsten et al. (19) | cross sectional | 2021 | German | Assessed by dermatologist based on clinical exams and findings | Assessed by dermatologists | 38.6/47.8 | (pooled)43.6 | NA | 20112 | 5 |
| Prens et al. (20) | cross sectional | 2021 | Netherlands | Self-reported by validated questionnaire | Self-reported by validated questionnaire | 73.5/60.1 | 52.1/56.0 | age, sex, BMI, smoking status and socioeconomic status | 56084 | 7 |
| Sokumbi et al. (21) | cross sectional | 2022 | US | ICD-10 diagnosis and validation of dermatologists | ICD-10 diagnosis | 73.0/73.0 | 35.4/35.5 | Age, sex, index date | 2320 | 6 |
HS, Hidradenitis suppurativa; ICD-9, International Classification of Diseases, 9th Revision; ICD-10, International Classification of Diseases, 10th Revision;
y/o, years old; NA, not available; NOS, Newcastle-Ottawa Scale.
Statistical analysis
In the current study, Review Manager 5.4 (Cochrane, London, UK) was used to perform statistical analyses. The results of the observational studies evaluating HS-psoriasis relationship were extracted and analyzed to present the pooled odds ratio of the association. Given that the clinical heterogeneity could possibly exist, we utilized the random-effects model in all of the qualitative synthesis. The odds ratio (OR) was evaluated based on 95% confidence interval (95% CI). For the pooled studies in each analysis, heterogeneity was determined by applying the value of I (2). If the presented I (2) value was greater than 75%, there could be high heterogeneity within pooled studies (23).
Results
Baseline characteristics and the quality evaluation of included observational studies
The PRISMA flow diagram was reported in Figure 1 . Initially, there were 2,795 studies identified from the databases. After the screening process excluding ineligible studies, we included 10 observational studies for critical appraisal. This includes 3 cohort studies (15–17), 1 case-control study (11) and 6 cross-sectional studies (10, 12, 18–21). Within the included studies, only one of them were originated from Asia, whereas most of the studies were originated from Europe (4out of 10 studies) and the United States (5 out of 10 studies). In most of the studies, the definition of HS was based on ICD-9/10 diagnostic codes or based on the evaluation of a dermatologist. Given that some of the studies did not provide sufficient information on the criteria for psoriasis diagnosis, detection bias may be present in the included studies.
Figure 1.
PRISMA study flowchart.
Qualitative synthesis: Odds ratio of HS-psoriasis
In Figure 2A , the odds ratio of having psoriasis in people with HS was reported. Based on the integration of 7 studies with more than 560,000 participants included, people with HS had a higher risk of having psoriasis, with a 2.67-fold risk (95% CI, 1.84, 3.87). The heterogeneity should not be neglected since the I (2) value in the current analysis was 97%. Populations included in this analysis included patients from Asia, Europe and the United States. We have performed additional sensitivity analyses including other study design (using cohort studies only) to evaluate the association of HS and psoriasis. As reported in Figure 2B , the HS-psoriasis association remained after including cohort studies, with a 1.85-fold risk (95% CI, 1.53, 2.24). The I (2) value in the analysis was reported to be 76%, which could be moderate to massive heterogeneity between included studies. Furthermore, given that obesity-related factors could serve as a critical confounder in the pathogenesis of HS and psoriasis, we also conducted an additional analysis to address this bias. Therefore, in the analysis performed in Figure 2C , only studies with similar study design and adjustment in obesity-related factors were included. In this analysis, the risk of HS patients having psoriasis was also statistically significant, with a 3.24-fold risk (95% CI, 2.27, 4.62).
Figure 2.
(A) Odds ratio of psoriasis in people with Hidradenitis Suppurativa. (B) Sensitivity analysis: Odds ratio of psoriasis in people with Hidradenitis Suppurativa (evidences based on cohort study design). (C) Sensitivity analysis: Odds ratio of psoriasis in people with Hidradenitis Suppurativa in obesity-adjusted models. Legends: Obesity-related factors could serve as critical confounding factors in the pathogenesis in both HS and psoriasis. Thereby, in this model, only studies performing adjustment in obesity-related factors (i.e: BMI, hyperlipidemia, etc.) were included.
Qualitative synthesis: Stratification analysis based on gender
Additionally, based on the current evidence, we have evaluated the HS-psoriasis association based on different genders. For HS patients, both genders presented approximately 4-fold risk of having psoriasis. In male patients with HS, the risk of having psoriasis was 4.30-fold higher (95% CI, 2.37, 7.78) ( Figure 3A ). In an analysis including 3 cross-sectional studies, the risk of female HS patients having psoriasis was 3.94-fold higher (95% CI, 2.34, 6.63) ( Figure 3B ). In both analyses, the results should be cautiously interpreted since both analyses were based on the integrated analysis of small amount of studies. Moreover, the heterogeneity might be great within included studies due to high I (2) values.
Figure 3.
(A) Odds ratio of psoriasis in male population with Hidradenitis Suppurativa. (B) Odds ratio of psoriasis in female population with Hidradenitis Suppurativa.
Discussion
HS is a chronic, inflammatory disorder of the apocrine units affecting the intertriginous areas. It occurs in 6 out of 100,000 people with the highest incidence among young women aged 20 to 29 years old. Obesity and smoking history are associated with the occurrence of HS, while smoking and female sex are significantly correlated with more severe disease (24). Several disease correlations have been found including autoimmune diseases such as type 1 diabetes mellitus, rheumatoid arthritis, ankylosing spondylitis and ulcerative colitis; renal diseases; dermatological diseases such as vitiligo and alopecia areata; and metabolic diseases such as thyroid diseases, hypertension and hyperlipidemia (25–27). The present study demonstrated that patients with HS have a 2-3 times the risk of being affected with psoriasis. Among the extracted articles, the strongest correlation was observed in a study of 13,667 HS patients where there is three times the risk of developing psoriasis compared to controls (18). Moreover, in the stratification of gender, both male and female patients with HS presented the trend of higher psoriasis risk. The integrated evidence provided in the current study could possibly serve as useful reference for clinicians for giving recommendations of comorbidity screening in people with HS.
Prevalence and incidence of psoriasis were found to be high in previous studies. One Asian study reported a higher prevalence of psoriasis in patients with HS (prevalence rate=38.6) than those without HS (prevalence rate=8.9) (12). In Western populations, the trend was also observed. According to a American single-center study recruiting 1,160 HS patients and the same amount of controls, odds ratio for HS patients having psoriasis was significantly higher than controls, with an observed more than 3-fold higher risk (21). Another study has identified that the incidence of psoriasis in HS patients was approximately 6% with a greater psychological burden compared to healthy controls and without disease co-occurrence (28). In a study by Pinter et al. (29), there was a slight male predominance (1.15:1) with the first symptoms of psoriasis occurring earlier than symptoms of HS. However, for those with HS disease as its first symptoms, the severity of the disease is greater than those patients who started with symptoms of psoriasis. Then, the onset of the second disease occurs around 14.3 years after the first disease. Furthermore, the most frequent comorbidity in the cohort was obesity, psychiatric complaints and psoriatic arthritis.
In many diseases, the inflammatory state could play potential roles in the association with autoimmune or endocrinological comorbidities (30–32). The association between HS and psoriasis may be related to their common inflammatory pathways. For a long time, psoriasis was believed to be initiated by the release of TNF alpha, interferon (IFN)-alpha and IFN-beta from plasmacytoid dendritic cells (DCs). These soluble factors further enhance inflammation by activation of inflammatory DCs that secrete more TNF alpha and IL-23. The key cytokine, IL-23, is the main mediator in activating and maintaining the inflammatory cascade brought about by T helper (Th) 17 and Th22 cells (33). Parallel with this, recent studies have shown that there was also an overexpression of IL-23 on lesional and perilesional skin, and serum levels of patients with HS (34). This was further studied by Navrazhina et al. (6) in which detailed biomarkers were investigated in both HS and psoriasis. It was found that there is a significant increase in IL-17A and HGF in HS comparatively similar to psoriasis. HS was thought to be highly involved in the pathway of Th17 mechanism. As for the concentration of IL17 in blood, previous studies also reported that the mean level for HS patients was 3.68 ± 2.08 pg/mL, which was significantly higher when comparing with healthy controls, which presented mean level of serum IL17 of 2.5 ± 1.11 pg/mL (35). Moreover, in different area of HS patients, including lesioned or normal cutaneous areas, Th17-related cytokines such as IL17 and IL23 were observed to be elevated (34, 36). The similarity in the cytokines involved may explain the usefulness of biologic therapies in HS. Among biologic therapies, TNF alpha inhibitors such as adalimumab and IL-17 inhibitors such as brodalumab were found to have a positive effect in patients with HS and psoriasis (29, 37, 38).
The present study has limitations that need to be addressed. First, the meta-analysis included a small number of studies. However, the studies included have generally observed the positive association between HS and psoriasis. Second, the included literature was retrospective observational studies that may not determine the true association between HS and psoriasis. Future prospective studies are recommended to establish the association between the two. Furthermore, there were some studies at high risk of bias in outcome assessment due to the application of administrative codes for disease definition. Third, confounders could cause potential bias. In observational studies, some residual confounders might not be adjusted as covariates (39–41). Therefore, although we have tried our best to perform sensitivity analyses in integrating evidence with critical adjustment models, confounders could still exist in observational studies and the meta-analysis based on these studies. Fourth, given that most of the retrieved studies utilized administrative codes to identify psoriasis, in most of the retrieved studies for data extraction, subcategories of psoriasis were not available. In this case, we might not be able to identify which specific psoriasis phenotype is associated with higher co-occurrence of HS. However, this was described in another study where the psoriasis phenotype observed with co-occurrence of HS was guttate psoriasis and palmoplantar pustulosis (10) Future studies with larger scale were warranted to perform detailed classification of psoriasis while determining their association with HS. Fifth, among the studies retrieved, the burden of disease in patients with psoriasis and HS have not been discussed. It’s difficult to evaluate the high prevalence and odds ratio of having psoriasis could influence the patients’ quality of life. Sixth, the information of the disease activity of psoriasis was limited in the in most extracted studies of the current meta-analysis. Mean age of enrolled HS patients might be able to represent the disease activity of psoriasis. In this case, we were not able to exactly know the psoriasis onset time of patients. it’s difficult to determine whether or not the psoriasis status was early or late onset types. Finally, publication bias may be probable. Therefore, the results should be interpreted with caution.
In conclusion, the meta-analysis confirmed the strong association between HS and psoriasis. This supports the advantageous use of biologic therapies with or without HS patients due to its common inflammatory pathway. The co-occurrence of HS and psoriasis can greatly increase the burden of the disease that may require aggressive treatment. Further prospective studies are needed to establish the true magnitude of the association between psoriasis and HS.
Data availability statement
The original contributions presented in the study are included in the article and Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
All the authors involved in drafting or revising the article and approved of the submitted version. Study conception and design: S-YG, Y-PH, KM, C-YL, Y-HK, S-EJ, and JW. Data analysis and demonstration: S-YG. Original draft preparation: S-YG and IP. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1033844/full#supplementary-material
References
- 1. Jemec GB. Clinical practice. hidradenitis suppurativa. N Engl J Med (2012) 366(2):158–64. doi: 10.1056/NEJMcp1014163 [DOI] [PubMed] [Google Scholar]
- 2. Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci (2019) 20(6). doi: 10.3390/ijms20061475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers (2020) 6(1):18. doi: 10.1038/s41572-020-0149-1 [DOI] [PubMed] [Google Scholar]
- 4. Hamzavi IH, Sundaram M, Nicholson C, Zivkovic M, Parks-Miller A, Lee J, et al. Uncovering burden disparity: A comparative analysis of the impact of moderate-to-severe psoriasis and hidradenitis suppurativa. J Am Acad Dermatol (2017) 77(6):1038–46. doi: 10.1016/j.jaad.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 5. Storer MA, Danesh MJ, Sandhu ME, Pascoe V, Kimball AB. An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int J Womens Dermatol (2018) 4(4):198–202. doi: 10.1016/j.ijwd.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Navrazhina K, Garcet S, Gonzalez J, Grand D, Frew JW, Krueger JG. In-depth analysis of the hidradenitis suppurativa serum proteome identifies distinct inflammatory subtypes. J Invest Dermatol (2021) 141(9):2197–207. doi: 10.1016/j.jid.2021.02.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gau SY, Huang KH, Lee CH, Kuan YH, Tsai TH, Lee CY. Bidirectional association between psoriasis and nonalcoholic fatty liver disease: Real-world evidence from two longitudinal cohort studies. Front Immunol (2022) 13:840106. doi: 10.3389/fimmu.2022.840106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohorst JJ, Kimball AB, Davis MD. Systemic associations of hidradenitis suppurativa. J Am Acad Dermatol (2015) 73(5 Suppl 1):S27–35. doi: 10.1016/j.jaad.2015.07.055 [DOI] [PubMed] [Google Scholar]
- 9. Wu JJ, Nguyen TU, Poon KY, Herrinton LJ. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol (2012) 67(5):924–30. doi: 10.1016/j.jaad.2012.04.039 [DOI] [PubMed] [Google Scholar]
- 10. Andersen RK, Saunte SK, Jemec GBE, Saunte DM. Psoriasis as a comorbidity of hidradenitis suppurativa. Int J Dermatol (2020) 59(2):216–20. doi: 10.1111/ijd.14651 [DOI] [PubMed] [Google Scholar]
- 11. Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol (2018) 178(4):917–24. doi: 10.1111/bjd.16101 [DOI] [PubMed] [Google Scholar]
- 12. Lee JH, Kwon HS, Jung HM, Kim GM, Bae JM. Prevalence and comorbidities associated with hidradenitis suppurativa in korea: A nationwide population-based study. J Eur Acad Dermatol Venereology (2018) 32(10):1784–90. doi: 10.1111/jdv.15071 [DOI] [PubMed] [Google Scholar]
- 13. Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the us and canadian hidradenitis suppurativa foundations. J Am Acad Dermatol (2022) 86(5):1092–101. doi: 10.1016/j.jaad.2021.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimball AB, Sundaram M, Gauthier G, Guérin A, Pivneva I, Singh R, et al. The comorbidity burden of hidradenitis suppurativa in the united states: A claims data analysis. Dermatol Ther (2018) 8(4):557–69. doi: 10.1007/s13555-018-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneeweiss MC, Kim SC, Schneeweiss S, Rosmarin D, Merola JF. Risk of inflammatory arthritis after a new diagnosis of hidradenitis suppurativa. JAMA Dermatol (2020) 156(3):342–45. doi: 10.1001/jamadermatol.2019.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneeweiss MC, Merola JF, Schneeweiss S, Rosmarin D, Merola JF. Risk of connective tissue disease, morphea and systemic vasculitis in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereology (2020) 156(3):342–5. doi: 10.1111/jdv.16728 [DOI] [PubMed] [Google Scholar]
- 18. Hua VJ, Kilgour JM, Cho HG, Li S, Sarin KY. Characterization of comorbidity heterogeneity among 13,667 patients with hidradenitis suppurativa. JCI Insight (2021) 6(21). doi: 10.1172/jci.insight.151872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirsten N, Zander N, Augustin M. Prevalence and cutaneous comorbidities of hidradenitis suppurativa in the german working population. Arch Dermatol Res (2021) 313(2):95–9. doi: 10.1007/s00403-020-02065-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prens LM, Bouwman K, Troelstra LD, Prens EP, Alizadeh BZ, Horváth B. New insights in hidradenitis suppurativa from a population-based dutch cohort: Prevalence, smoking behaviour, socioeconomic status and comorbidities. Br J Dermatol (2021) 186(5):814–22. doi: 10.1111/bjd.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sokumbi O, Hodge DO, Ederaine SA, Alavi A, Alikhan A. Comorbid diseases of hidradenitis suppurativa: A 15-year population-based study in olmsted county, minnesota, USA. Int J Dermatol (2022) 61(11):1372–9. doi: 10.1111/ijd.16228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wells G SB, O’Connell D, Robertson J. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analysis (2018). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 21 March 2022).
- 23. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions (2022). Available at: https://training.cochrane.org/handbook/current (Accessed 22 March 2022).
- 24. Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: A population-based study of olmsted county, minnesota. J Invest Dermatol (2013) 133(1):97–103. doi: 10.1038/jid.2012.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phan K, Huo YR, Charlton O, Smith SD. Hidradenitis suppurativa and thyroid disease: Systematic review and meta-analysis. J Cutan Med Surg (2020) 24(1):23–7. doi: 10.1177/1203475419874411 [DOI] [PubMed] [Google Scholar]
- 26. Gau SY. Increased risk of renal diseases in people with hidradenitis suppurativa: A systematic review and meta-analysis. Int J Dermatol (2022). doi: 10.1111/ijd.16423 [DOI] [PubMed] [Google Scholar]
- 27. Gau SY, Lee CY, Kuan YH, Ma KS, Wei JC. Hyperthyroidism and hypothyroidism in patients with hidradenitis suppurativa: A systematic review and meta-analysis. Int J Dermatol (2022). doi: 10.1111/ijd.16484 [DOI] [PubMed] [Google Scholar]
- 28. Pinter A, Kokolakis G, Rech J, Biermann MHC, Haberle BM, Multmeier J, et al. Hidradenitis suppurativa and concurrent psoriasis: Comparison of epidemiology, comorbidity profiles, and risk factors. Dermatol Ther (Heidelb) (2020) 10(4):721–34. doi: 10.1007/s13555-020-00401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinter A, Sarlak M, Zeiner KN, Malisiewicz B, Kaufmann R, Romanelli M, et al. Coprevalence of hidradenitis suppurativa and psoriasis: Detailed demographic, disease severity and comorbidity pattern. Dermatol (Basel Switzerland) (2021) 237(5):759–68. doi: 10.1159/000511868 [DOI] [PubMed] [Google Scholar]
- 30. Gau SY, Huang JY, Yong SB, Wei JC. Higher risk of hyperthyroidism in people with asthma: Evidence from a nationwide, population-based cohort study. J Allergy Clin Immunol Pract (2022) 10(3):751–58.e1. doi: 10.1016/j.jaip.2021.09.021 [DOI] [PubMed] [Google Scholar]
- 31. Gau SY, Lai JN, Yip HT, Wu MC, Wei JC. Higher dementia risk in people with gastroesophageal reflux disease: A real-world evidence. Front Aging Neurosci (2022) 14:830729. doi: 10.3389/fnagi.2022.830729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang SC, Gau SY, Huang JY, Wu WJ, Wei JC. Increased risk of hypothyroidism in people with asthma: Evidence from a real-world population-based study. J Clin Med (2022) 11(10). doi: 10.3390/jcm11102776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furue K, Ito T, Furue M. Differential efficacy of biologic treatments targeting the tnf-alpha/il-23/il-17 axis in psoriasis and psoriatic arthritis. Cytokine (2018) 111:182–88. doi: 10.1016/j.cyto.2018.08.025 [DOI] [PubMed] [Google Scholar]
- 34. Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the il-23/th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol (2011) 65(4):790–98. doi: 10.1016/j.jaad.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 35. Matusiak L, Szczech J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (il)-17 serum levels in patients with hidradenitis suppurativa: Implications for treatment with anti-il-17 agents. J Am Acad Dermatol (2017) 76(4):670–75. doi: 10.1016/j.jaad.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 36. Liu T, Li S, Ying S, Tang S, Ding Y, Li Y, et al. The il-23/il-17 pathway in inflammatory skin diseases: From bench to bedside. Front Immunol (2020) 11:594735. doi: 10.3389/fimmu.2020.594735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gkanti V, Dalamaga M, Papadavid E. Drug survival of brodalumab is greater in patients with psoriasis and psoriatic arthritis in a real-world setting. Int J Dermatol (2022). doi: 10.1111/ijd.16408 [DOI] [PubMed] [Google Scholar]
- 38. Kashetsky N, Mufti A, Alabdulrazzaq S, Lytvyn Y, Sachdeva M, Rahat A, et al. Treatment outcomes of il-17 inhibitors in hidradenitis suppurativa: A systematic review. J Cutan Med Surg (2022) 26(1):79–86. doi: 10.1177/12034754211035667 [DOI] [PubMed] [Google Scholar]
- 39. Lee YH, Tsou HK, Kao SL, Gau SY, Bai YC, Lin MC, et al. Patients with rheumatoid arthritis increased risk of developing osteoarthritis: A nationwide population-based cohort study in taiwan. Front Med (Lausanne) (2020) 7:392. doi: 10.3389/fmed.2020.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gau SY, Huang JY, Wei JC. Tramadol use increases mortality and risk of major adverse cardiovascular events in rheumatoid arthritis patients: Evidence from a population-based cohort study. Eur J Prev Cardiol (2022) 29(6):e237–e38. doi: 10.1093/eurjpc/zwab176 [DOI] [PubMed] [Google Scholar]
- 41. Huang KH, Chang YL, Gau SY, Tsai TH, Lee CY. Dose-response association of metformin with parkinson's disease odds in type 2 diabetes mellitus. Pharmaceutics (2022) 14(5). doi: 10.3390/pharmaceutics14050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material . Further inquiries can be directed to the corresponding authors.