Abstract
Catalase is widely used as a pharmacological probe to evaluate the role of hydrogen peroxide in antimicrobial activities of phagocytic cells. This report demonstrates that the ability of a commercial preparation of catalase to inhibit concomitantly macrophage antimycobacterial activity and production of reactive nitrogen intermediates can be attributed, at least in part, to the depletion of l-arginine by contaminating arginase. In experimental systems that employ pharmacological probes, the existence of nonspecific effects should be considered in data interpretation.
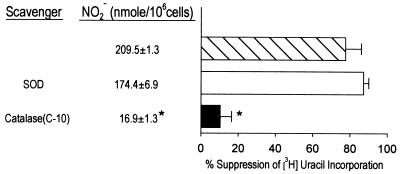
The l-arginine-dependent cytotoxic mechanism confers effective antimycobacterial function on cytokine-activated murine macrophages via the generation of toxic reactive nitrogen intermediates (RNI) (reviewed in reference 2). The role of reactive oxygen intermediates (ROI) in resistance to the tubercle bacillus, however, remains controversial. In a series of studies designed to examine the relative contribution of ROI and nitrogen oxides to host defense against Mycobacterium tuberculosis, we have previously demonstrated that catalase (Sigma, St. Louis, Mo.; catalogue no. C-3155), a scavenger for hydrogen peroxide (H2O2), has no significant effects on the antimycobacterial function of murine D9 and J774.16 macrophages stimulated to produce nitric oxide by gamma interferon (IFN-γ) and tumor necrosis factor alpha or Escherichia coli lipopolysaccharide (LPS) (3). The ability of these activated phagocytes to inhibit M. tuberculosis Erdman was shown to correlate with RNI production (3). Subsequently, we observed that a commercial preparation of catalase (Sigma; catalogue no. C-10) had the ability to reverse the inhibitory effects of IFN-γ- and LPS-stimulated macrophages against M. tuberculosis Erdman, as assessed by metabolic labeling, using incorporation of [5,6-3H]uracil (specific activity, 34 Ci/mmol; New England Nuclear, Boston, Mass.) as an index of mycobacterial nucleic acid synthesis (3) (Fig. 1; compare closed bar to hatched bar, P < 0.05). Investigation into the mechanism underlying the ability of catalase C-10 to reverse the antimycobacterial activity of immunologically activated macrophages revealed that this preparation of the enzyme markedly decreased the production of RNI by these phagocytes, as measured by quantitation of nitrite (NO2−) content in culture supernatants using the Griess reagent (11) (Fig. 1; NO2− production by cultures with and without catalase C-10: 16.9 ± 1.3 and 209.5 ± 1.3 nmol/106 cells, respectively; P < 0.05). The goal of the present report is to characterize the mechanism by which catalase C-10 inhibits RNI production by IFN-γ- and LPS-activated murine macrophages in our in vitro system. D9 and J774.16 macrophages, as well as BALB/c peritoneal macrophages (3), were employed in this study.
FIG. 1.
Ability of catalase C-10 to inhibit antimycobacterial effects of IFN-γ- and LPS-activated J774.16 macrophages is associated with suppression of RNI production. Catalase C-10 (2,600 U/ml) was added to macrophage cultures 4 h prior to infection with M. tuberculosis Erdman. Macrophages (1.5 × 105 cells per well in 96-well tissue culture plates) were primed with IFN-γ (250 U/ml) for 12 to 16 h. Supernatants were then removed and replaced with culture medium containing LPS (1 μg/ml) and M. tuberculosis Erdman (multiplicity of infection of 5 to 10:1) with or without catalase. Cultures were pulsed with [5,6-3H]uracil (specific activity, 34 Ci/mmol; New England Nuclear) at 24 h postinfection. After 16 to 24 h, cells and supernatants were assayed for [3H]uracil incorporation and NO2− content, respectively. Uninfected macrophages incorporated 1,500 to 4,000 cpm of [3H]uracil. Incorporation of label by nonactivated infected macrophages was in the range of 8,000 to 10,000 cpm. Nucleic acid synthesis by mycobacteria was measured as [3H]uracil incorporation by cultures with organisms minus that by control cultures (dcpm). The inhibitory effect of activated macrophages on mycobacteria was measured as percent suppression of [3H]uracil incorporation and expressed as follows: 100 × [1 − (dcpm for stimulated macrophages/dcpm for unstimulated macrophages)]. Data shown represent those of two independent experiments. SOD, superoxide dismutase. Error bars indicate standard errors. Asterisks indicate a P value of <0.05 (one-way analysis of variance; controls were samples without addition of scavenger).
We examined the effects of different preparations of catalase on RNI production by IFN-γ- and LPS-activated murine macrophages (3). The various catalases (Sigma) used in these studies were C-10 (specific activity, 1,600 U/mg [solid]; 2,600 U/mg of protein), C-3155 (specific activity, 48,700 U/mg of protein; 20.7 mg of protein/ml), C-30 (18,600 U/mg of protein; 75.2 mg of protein/ml), and C-100 (58,000 U/mg of protein; 105 mg of protein/ml). Results of these studies indicate that the ability of catalase to markedly inhibit RNI production by activated macrophages is restricted to C-10, the preparation with the lowest specific activity (Table 1). A corollary to this observation might be that a factor other than catalase is responsible for the RNI production-inhibitory effect. This inhibitory effect of catalase on RNI production can be observed in J774.16, D9, and primary murine peritoneal macrophages.
TABLE 1.
Ability of catalase to inhibit production of RNI by activated D9 macrophages is restricted to C-10, the preparation with the lowest specific activitya
| Catalase | Sp act (U/mg of protein) | % Reduction in NO2− production |
|---|---|---|
| C-3155 | 48,700 | 11.4 |
| C-100 | 58,000 | 13.4 |
| C-30 | 18,600 | 8.1 |
| C-10 | 2,600 | 92.4 |
Macrophages (1.5 × 105 cells per well in 96-well tissue culture plates) were primed with IFN-γ (250 U/ml) for 12 h. The various preparations of catalase were added to cultures at a final concentration of approximately 2,600 U/ml. Four hours later, supernatants were removed and replaced with culture medium containing LPS (1 μg/ml) with or without catalase. Culture supernatants were assayed for NO2− content 24 h later. Experiments involving catalase preparations C-10 and C-3155 were carried out three times with similar results. Preparations C-100 and C-30 were tested only once. Similar results were obtained using J774.16 and primary peritoneal macrophages.
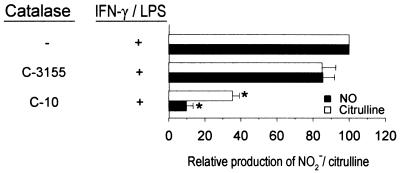
As an initial step to confirm the inhibitory activity of catalase C-10 on RNI production, the amounts of l-citrulline (the l-arginine-derived product of the enzymatic activity of nitric oxide synthase 2 [NOS2] besides nitric oxide) in culture supernatants of IFN-γ- and LPS-activated macrophages were determined as described previously (12). Results in Fig. 2 demonstrate that the amounts of NO2− and l-citrulline produced by IFN-γ- and LPS-activated macrophages are concomitantly reduced in the presence of C-10 catalase. These results suggest that catalase C-10 inhibits RNI production by targeting either the substrate l-arginine or the enzyme NOS2 rather than the products (reactive nitrogen species).
FIG. 2.
Catalase C-10 inhibits production of both RNI and citrulline by IFN-γ- and LPS-activated J774.16 macrophages. Experiments were carried out as described in footnote a of Table 1, except that, at the end point of the studies, both RNI and citrulline contents were assayed as previously described (11, 12). Catalase C-10 and C-3155 were used at a final concentration of 2,600 U/ml. The ability of C-10 to inhibit production of RNI is apparent at concentrations as low as 250 U/ml (data not shown). Data derived from three independent experiments are shown. Error bars indicate standard errors. Asterisks indicate a P value of <0.05 (one-way analysis of variance; controls were samples without catalase).
To begin characterizing this inhibitory activity, catalase C-10 was subjected to trypsinization prior to addition to IFN-γ- and LPS-stimulated macrophages. These studies reveal that the ability of catalase C-10 to suppress NO2− production by IFN-γ- and LPS-stimulated macrophages is completely abrogated by trypsinization (data not shown), suggesting that the inhibitory activity is mediated by a protein. The amount of trypsin introduced into the cultures as a result of this trypsinization procedure did not affect the production of RNI by macrophages in response to stimulation with IFN-γ and LPS. Consequently, we explored the possibility that arginase, an enzyme abundant in the liver (the organ from which catalase C-10 was generated) and having the ability to deplete the NOS2 substrate arginine, was responsible for the inhibitory activity of catalase C-10 for macrophage RNI production. We evaluated the levels of l-ornithine, a product resulting from the catalytic action of arginase on l-arginine, in culture supernatants of IFN-γ- and LPS-stimulated macrophages in the presence of C-10 (6, 10). Results of these studies demonstrate that the inhibition of RNI production by catalase C-10 correlates well with the amounts of l-ornithine in the culture supernatants (Table 2). Quantitation of the amount of urea (another l-arginine-derived product of arginase activity) using the Sigma Diagnostics urea nitrogen kit (5, 9) revealed that this compound was also increased in the supernatants of activated macrophage cultures treated with C-10 (data not shown). These results strongly suggest that the ability of C-10 to inhibit RNI production by immunologically activated macrophages is mediated by arginase present as a contaminant in this particular preparation.
TABLE 2.
Inhibition of D9 macrophage RNI production by catalase C-10 correlates with the production of ornithinea
| Catalase (U/ml) | % Increase in ornithine production | NO2− (nmol/106 cells) |
|---|---|---|
| No addition | NDb | 113.7 |
| C-10 (2,600) | 111.6 | 5.2 |
| C-10 (260) | 78.6 | 31.9 |
| C-10 (26) | 6.0 | 77.7 |
Experiments were carried out as described in footnote a of Table 1. Ornithine contents were quantitated as described previously (6, 10). Percent increase in ornithine production = [(ornithine level in activated macrophage culture with catalase/ornithine level in activated macrophage culture without catalase) − 1] × 100. Data shown are representative of two experiments.
ND, not done.
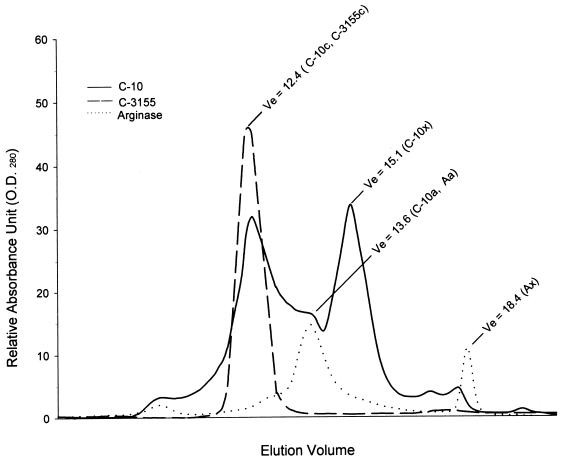
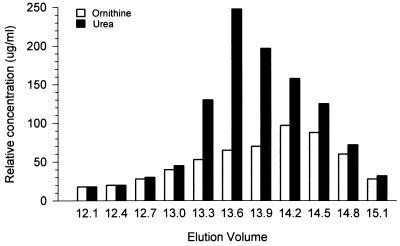
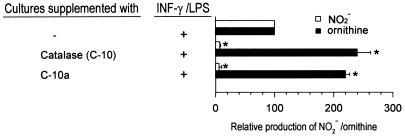
To investigate the role of arginase in the ability of C-10 to inhibit RNI production by IFN-γ- and LPS-activated murine macrophages, this catalase preparation, together with C-3155, was subjected to chromatographic analysis by the Pharmacia fast protein liquid chromatography (FPLC) system using the Superose gel filtration column. Analysis of C-10 demonstrated a complex profile composed of two distinct peaks, designated C-10c and C-10x, as well as a shoulder, C-10a (Fig. 3). The elution volumes (Ve) of C-10c, C-10x, and C-10a are 12.4, 15.1, and 13.6, respectively (Fig. 3). Similar analysis of C-3155 demonstrated a single peak (C-3155c) with a Ve of 12.4 (Fig. 3), indistinguishable from that of the Cc fraction of C-10. For comparison, l-arginase (Sigma; catalogue no. A-8013) was also subjected to chromatographic analysis, which revealed two major peaks, Aa and Ax, with Ve of 13.6 and 18.4, respectively (Fig. 3). Based on these results, catalase activity was assigned to C-10c and C-3155c and arginase activity was assigned to C-10a and Aa. To confirm that peak C-10a (Ve of 13.6) contains arginase, FPLC fractions of C-10 were allowed to react with l-arginine and the reaction mixture was analyzed for urea and ornithine (6, 10). Results of these studies show that arginase activity peaked at a Ve of 13.6, coinciding with peak C-10a (Fig. 4). Evaluation of enzymatic activity of peak C-10c affirmed the correct assignment of catalase activity to this fraction (reference 4 and data not shown). Finally, addition of C-10a to IFN-γ- and LPS-stimulated macrophages markedly inhibited RNI production, with a concomitant increase in the amounts of ornithine, a product of arginase activity, in culture supernatants (Fig. 5). In sum, these data provide strong evidence that the ability of the catalase preparation C-10 to inhibit RNI production by immunologically activated macrophages in vitro is, at least in part, due to the presence of arginase as a contaminant.
FIG. 3.
Chromatographic analysis of catalase C-10, catalase C-3155, and arginase. Arginase and two preparations of catalase (C-10 and C-3155) were subjected to FPLC analysis. Phosphate buffer (10 mM; pH 7.4) was used in these studies. The flow rate was 0.15 ml/min, and 0.3-ml fractions were collected. Data depicted are representative of five experiments. O.D.280, optical density at 280 nm.
FIG. 4.
Fractions of catalase C-10 at a Ve of 13.6 (peak C-10a) contain arginase activity. Catalase C-10 was subjected to FPLC fractionation. Individual fractions (50 μl) were allowed to react with l-arginine, and arginase activity was assessed by measurement of the production of urea and ornithine. The experiment was carried out twice with similar results.
FIG. 5.
Fractions of catalase C-10 at a Ve of 13.6 (peak C-10a) inhibit IFN-γ- and LPS-activated J774.16 macrophage production of RNI with concomitant enhancement of ornithine generation. Eluents were collected as 0.3-ml fractions. Fractions were added at a volume of 20 μl. Experiments were set up as described in the legend to Fig. 2. The phosphate buffer used did not attenuate RNI production or increase generation of ornithine. Data shown represent those of three independent experiments. Error bars indicate standard errors. Asterisks indicate a P value of <0.05 (one-way analysis of variance; controls were cultures without supplementation of C-10 or C-10a).
Catalase has been used extensively as a pharmacological probe to study the effects of hydrogen peroxide in various biological systems. The present study provides evidence that experimental outcome can be totally dependent on the purity of the particular preparation of catalase employed. Specifically, this study demonstrates that the reversal of antimycobacterial effects of IFN-γ- and LPS-activated macrophages by treatment with the relatively crude preparation of catalase C-10 is due to, at least partially, the presence of arginase. The latter enzyme depletes arginine, from which antimycobacterial RNI are generated via the expression of NOS2. Recently, arginase has been identified as the agent responsible for the antiapoptotic activity of catalase in an in vitro neuronal cell system (8). In this latter study, however, the observed antiapoptotic effects of arginase are unrelated to NOS2.
Catalase has previously been reported to inhibit RNI production by activated macrophages (15). This inhibitory effect was reported to be reversible, to some degree, by the addition of tetrahydrobiopterin (BH4), a cofactor critical for the conformational and functional integrity of NOS2 (7, 18). It is possible that BH4 enhances the enzymatic activity of NOS2 and, as a result, allows generation of RNI even in the presence of arginase. It is curious, however, that there is no correlation between the degree of inhibition of RNI production and the various preparations of catalase tested (15). The discrepancy between this latter observation and that of the present study is unclear. Finally, it has been reported that H2O2 can substitute for NADPH and O2 as an oxygen donor in the NOS2-catalyzed oxidation of NG-hydroxy-l-arginine (17, 18). Thus, the catalase-related attenuation of NOS2 activity in the present study may theoretically be accounted for by the enzymatic depletion of H2O2 produced by activated macrophages. While the relative contribution of NADPH-O2 and H2O2 to the overall function of NOS2 in macrophages remains to be determined, the observation that, in our experimental system, the catalytic activity of this enzyme is attenuated substantially only by the arginase-containing catalase preparation of a low degree of purity argues against a major role of this reactive oxygen species in the production of NO and citrulline.
That immunologically activated macrophages express arginase (reviewed in reference 20) suggests that the interaction between this enzyme and NOS2 may go beyond mere competition in vitro for the substrate l-arginine. It has been shown that macrophages stimulated with LPS express both NOS2 and arginase (reviewed in reference 20). In vivo, increased arginase activity in sera of cattle infected with Fasciola gigantica has been reported previously (13). Accumulating evidence suggests that the interaction of NOS2 and arginase may play an important role in modulating l-arginine metabolism in various disease states (reviewed in reference 20). Clearly, the biological significance of the interaction of NOS2 and arginase deserves further investigation.
RNI have been well established as effective antimycobacterial effectors in the murine system; emerging evidence also supports a role of these toxic nitrogen oxides in host defense against the tubercle bacillus in humans (16, 19). For example, alveolar macrophages lavaged from patients with active tuberculosis have been shown to highly express NOS2, as determined by immunohistochemical studies (16). More recently, the expression of immunoreactive NOS2 protein in tuberculous patients has been shown to correlate with production of nitric oxide (19). Despite the significance of RNI in host defense against M. tuberculosis, the protective role of ROI in tuberculosis cannot be completely excluded (1, 14). Scavengers of various oxygen species are commonly used as a tool to evaluate the biological roles of ROI. Results of the present study should serve as a reminder of the limitations and caveats for the use of pharmacological probes, particularly those that may contain contaminants, in biological experimentation in vitro.
Acknowledgments
This work was supported in part by a grant from the Heiser Foundation and the Foundation of the University of Medicine and Dentistry of New Jersey.
REFERENCES
- 1.Adams L B, Dinauer M C, Morgenstern D E, Krahenbuhl J L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 1997;78:237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 2.Chan J, Flynn J L. Nitric oxide in Mycobacterium tuberculosis infection. In: Fang F, editor. Nitric oxide and infection. New York, N.Y: Plenum Publishing Corporation; 1999. pp. 281–310. [Google Scholar]
- 3.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chance B, Herbert D. The enzyme-substrate compounds of bacterial catalase and peroxides. Biochem J. 1950;46:402–414. doi: 10.1042/bj0460402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaney A L, Marback E P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–132. [PubMed] [Google Scholar]
- 6.Colombo J P, Konarska L. Arginase. In: Bergmeyer H V, editor. Methods of enzymatic analysis II. Amsterdam, The Netherlands: Elsevier; 1985. pp. 285–294. [Google Scholar]
- 7.Crane B R, Arvai A S, Ghosh D K, Wu C, Getzoff E D, Stuehr D J, Tainer J A. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 8.Esch F, Lin K I, Hills A, Zaman K, Baraban J M, Chatterjee S, Rubin L, Ash D E, Ratan R R. Purification of a multipotent antideath activity from bovine liver and its identification as arginase: nitric oxide-independent inhibition of neuronal apoptosis. J Neurosci. 1998;18:4083–4095. doi: 10.1523/JNEUROSCI.18-11-04083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawcett J K, Scott J E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geyer J W, Dabich D. Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem. 1971;39:412–417. doi: 10.1016/0003-2697(71)90431-3. [DOI] [PubMed] [Google Scholar]
- 11.Green L C, Wagner D A, Glogowski J A, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrite, nitrate, and [15N]-nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Guthohrlein G, Knappe J. Modified determination of citrulline. Anal Biochem. 1968;261:188–191. doi: 10.1016/0003-2697(68)90045-6. [DOI] [PubMed] [Google Scholar]
- 13.Haroun E M, Hussein M F. Clinico-pathological studies on naturally-occurring bovine fasciolosis in the Sudan. J Helminthol. 1975;49:143–152. doi: 10.1017/s0022149x00023567. [DOI] [PubMed] [Google Scholar]
- 14.Lau Y L, Chan G C, Ha S Y, Hui Y F, Yuen K Y. The role of phagocytic respiratory burst in host defense against Mycobacterium tuberculosis. Clin Infect Dis. 1998;26:226–227. doi: 10.1086/517036. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Severn A, Rogers M V, Palmer R M J, Moncada S, Liew F Y. Catalase inhibits nitric oxide synthesis and the killing of intracellular Leishmania major in murine macrophages. Eur J Immunol. 1992;22:441–446. doi: 10.1002/eji.1830220223. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson S, Monecini-Almeida M D G, Lapa e Silva J R, Nathan C, Xie Q W, Mumford R, Weidner J R, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J L. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pufahl R A, Wishnok J S, Marletta M A. Hydrogen peroxide-supported oxidation of NG-hydroxy-L-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 18.Rusche K M, Spiering M M, Marletta M A. Reactions catalyzed by tetrahydrobiopterin-free nitric oxide synthase. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- 19.Wang C H, Liu C Y, Lin H C, Yu C T, Chung K F, Kuo H P. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11:809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Morris S M., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]