Abstract
Merozoite surface protein 1 (MSP-119) is a leading malaria vaccine candidate. Specific antibodies contribute to immunity; binding to macrophages is believed to represent the main action of malaria antibodies. We show that an MSP-119-specific immunoglobulin G3 (IgG3) monoclonal antibody can passively transfer protection to mice deficient in the α chain of Fc-γRI whose macrophages cannot bind IgG3.
Malaria parasites are the cause of significant morbidity and mortality throughout the world. The disease is caused by protozoan parasites of the genus Plasmodium. The increased incidence of drug-resistant strains of the parasite has raised the importance of producing an effective vaccine against this disease. Since the asexual blood stage of the parasite is the cause of symptoms and pathology, much vaccine research has been directed at antigens from this stage.
The 19-kDa carboxyl-terminal fragment of merozoite surface protein 1 (MSP-119) is a leading vaccine candidate and has been shown to induce a protective immune response in rodents (3, 8, 11) and monkeys (10). Data from studies in murine models point to high-titer antibodies present at the time of challenge as being critical to protection. The mechanism(s) of action of these antibodies is unknown, although antibody-dependent cellular inhibition, which requires Fc receptor (FcR) function, has been demonstrated to be a major mechanism of action for malaria parasite-specific antibodies (5). A recent report showed that polyclonal anti-MSP-119 antibodies (containing multiple isotypes) could passively protect mice deficient in the FcR γ chain (15). Although the γ chain is an important component of Fc-γRIII, the low affinity receptor for immunoglobulin G (IgG), and is associated with the α chain of the high-affinity receptor (Fc-γRI) (17), it appears that IgG3 isotypes can bind Fc receptors in γ chain-deficient mice (19). In the MSP-119 study of these mice (15), the authors attempted to minimize the amount of IgG3 in their polyclonal sera and also tested diluted MSP-119-specific IgG3 monoclonal antibody (MAb), which contained levels of IgG3 equivalent to those found in polyclonal sera. The diluted IgG3 antibody did not transfer protection. However, their data did suggest that anti-MSP-119-specific antibodies of IgG1 and IgG2a isotypes (the main isotypes present in the antisera) protect against malaria via an Fc-independent mechanism. Transfection and knockout (KO) studies have shown that Fc-γRI is both sufficient and necessary for binding of IgG3 (7). We have therefore tested Fc-γRI-deficient (α chain KO) mice to determine whether the IgG3 MAb 302—the most potent anti-MSP-119-specific antibody described to date—can protect via an Fc-independent mechanism. The goal of this study was thus to enhance understanding of the role and mechanism of action of MSP-119-specific antibodies in malaria immunity.
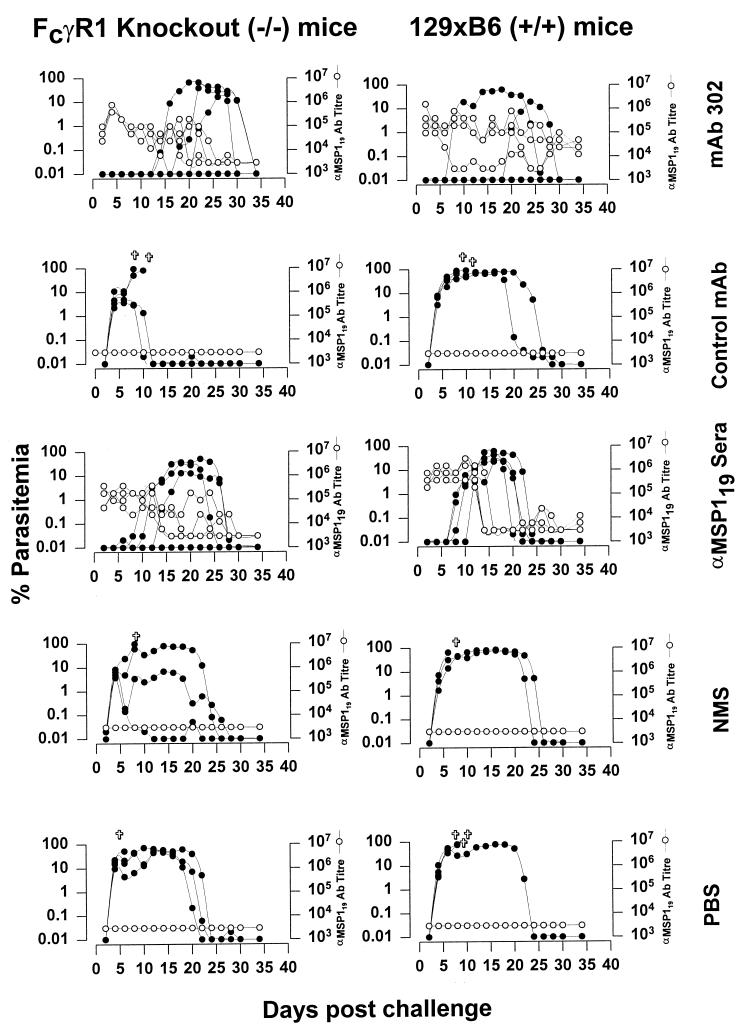
Groups of four naive 129 × C57BL/6 Fc-γRI KO mice (−/−) (7) and identical groups of 129 × C57BL/6 (+/+) mice were given via the intraperitoneal (i.p.) route 0.5-ml doses of phosphate-buffered saline (PBS), MAb 302, control IgG3 MAb (ASH2121, an antibody with allelic specificity to Ly2.1—an allele not found in the mice in this study), polyclonal anti-yeast MSP-119 antisera (8), or normal mouse serum (NMS) on days −1, 0, and +1 relative to challenge with 104 P. yoelii YM parasites i.p. The MAbs were prepared by two 45% ammonium sulfate cuts of ascites fluid produced in BALB/c nude mice and dialyzed against PBS. Polyclonal immune sera were generated in native BALB/c mice after immunization and boosting with yeast-expressed MSP-119 as described by Hirunpetcharat et al. (8). These sera predominantly contained IgG1 and IgG2b antibodies with negligible amounts of IgG3 antibodies. Figure 1 illustrates the parasitemias of the mice in all groups. All mice within the control groups (NMS, PBS, and control MAb) developed a rapidly ascending parasitemia, and many succumbed to infection. This is similar to the parasite density curves in normal, untreated mice infected with P. yoelii YM. Mice that recovered from infection were able to clear the infection by day 30. Enzyme-linked immunosorbent assays (ELISAs) of serum from control mice showed no detectable anti-MSP-119 antibody throughout the course of the experiment.
FIG. 1.
Parasitemias (filled circles) and MSP-119-specific-antibody titers (open symbols) in mice that received different antibody preparations as indicated. Mice received the antibodies via the i.p. route at days −1, 0, and +1 relative to the day of challenge with 104 P. yoelii YM-infected RBCs i.p. IgG-specific reagents were used to determine ELISA titers in recipients of antibodies. ELISAs were performed as previously described (8). Titertek immunoassay plates (ICN Biomedical, Inc., Aurora, Ohio) were coated overnight with 0.5 μg of recombinant P. yoelii MSP-119 per ml in bicarbonate coating buffer (50 μl per well) (pH 9.6). Plates were blocked with 3% nonfat skim milk–PBS for 1 h at 37°C. Sera and secondary antibodies were diluted in 0.5% skim milk–PBS with incubation times of 1 h at 37°C each. Serum dilutions began at 1/3,200 and were continued serially 1:2 across the plate. The substrate used was ABTS [2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid]. Plates were read at 405 nm after a half-hour incubation at room temperature. Titers were calculated by end point dilution. Symbol: ✞, death of a mouse.
Mice (both +/+ and −/−) that were administered anti-MSP-119 antisera showed a marked delay (6 to 8 days) in the onset of parasitemia compared to mice that received NMS or PBS. The delay in patency is the most defining feature of the efficacy of passively transferred MSP-119-specific antibodies (9). The similarity of efficacies of MSP-119-specific sera in +/+ and −/− mice was expected since even though these mice have intact Fc receptors for the isotypes present in these sera (IgG1 and IgG2b), Fc receptors are not required for the expression of immunity mediated by MSP-119-specific antibodies of these isotypes (15). However, these receptors are not functional for binding IgG3 (7). The most potent MSP-119-specific antibody defined to date, MAb 302, is an IgG3 antibody; the receptor for this antibody is Fc-γRI. The data relevant to this antibody and receptor are also shown in Fig. 1. However, +/+ and −/− recipients of MAb 302 had very similar parasitemia curves, and for mice both having and lacking Fc-γRI, there were significant delays in patency compared to recipients of the control IgG3 MAb. The entire experiment was repeated, and the data again showed that the course of parasitemia was similar in control and Fc-γRI KO (−/−) mice. A Mann-Whitney test showed no significant difference between peak parasitemias for the MAb-treated normal and KO mice in either experiment.
In order to determine whether mice which received specific antibodies and resolved their patent parasitemia were able to sterilize (completely eliminate) their infection, we transferred blood (0.2 ml) from each mouse into a naive mouse. In all cases the recipient mice failed to develop infection, indicating that the donor mice had cleared their infection. Figure 1 also displays the anti-MSP-119 antibody titers as shown by ELISA over the course of the experiment. IgG-specific reagents were used to determine MSP-119-specific titers in recipients of MAb 302 or MSP-119-specific sera. Antibody levels in these mice were high just after the transfer of the sera or MAb (105 to 106), but as the parasitemia began to increase, the titers decreased.
That transfer of immune sera can protect against and even treat malaria infections has been observed repeatedly. The exact mechanism of action of antibodies, however, remains incompletely explained. Quinn and Wyler (13) reported that antibody from hyperimmune sera is protective and appears to interfere with the interaction of the merozoite and the erythrocyte during invasion. Clarification of the exact mechanism of this protection has become the subject of renewed investigation with the possibilities that antibody acts through receptors on the surface of macrophages (Fc receptors) to induce antibody-dependent cell-mediated cytotoxicity (14), that antibody causes agglutination of the parasite or prevents binding to red blood cells (RBCs) (6), and that antibody binding to precursor molecules on the surface of the merozoite interferes with the secondary processing of the protein, which may in turn prevent invasion of fresh RBCs (1).
In this study we have examined the role of MSP-119-specific IgG3 antibody-mediated immunity to blood stage malaria using mice deficient in receptors for IgG3 antibody Fc (Fc-γRI KO mice). The results indicate that antibody-dependent cell-mediate cytotoxicity and Fc-mediated phagocytosis are not necessary for malarial parasite clearance by this antibody. Our findings, together with recent results obtained with FcR γ chain KO mice (15), suggest that MSP-119-specific antibodies, independent of isotypes, do not require Fc function for protection.
Although Rotman et al. tested MAb 302 in their γ chain KO mice and observed protection, this result was not surprising since IgG3 isotypes may still bind to specific FcRs even in γ chain KO mice. However, FcRs in the γ chain KO mice, while able to bind IgG3, would not be able to signal. Binding of anti-MSP-119-specific antibodies, without signaling, may have had an antiparasite effect at the surface of the FcR-bearing cell. This issue was not resolved in the study by Rotman et al. (7). Our data are therefore the first to show that MSP-119-specific IgG3 antibodies do not require Fc function at all (binding or signaling). If other IgG3 binding receptors exist, the present experiments could be alternatively interpreted.
A critical unanswered question is whether this also applies to P. falciparum MSP-119-specific antibodies in humans. It is also unknown whether the requirement for high-titer MSP-119-specific antibodies is related to the Fc-independent function of these antibodies. If cells are involved in the effector function of MSP-119-specific antibodies, it is possible that some amplification of effector signals might take place, arguing that the requirement for a high titer might result from lack of Fc involvement. If high titers are required for efficacy of a P. falciparum vaccine in humans, then adjuvants that assist in the generation of such titers will need to be defined, although there are candidate adjuvants that could be considered (16) and which have shown efficacy in murine model systems (12).
A further implication of the lack of requirement for Fc function relates to the use of MSP-119-specific antibodies in passive immunity. In animals, such antibodies can passively transfer resistance to malaria (9), while in humans immune sera (antibody specificity unknown) can passively transfer resistance to children (2). If Fc function is not required for MSP-119-specific immunity in humans, it may be possible to generate effective therapeutics using engineered single-chain antibodies.
Acknowledgments
This study was supported in part by grants from The National Health and Medical Research Council (Australia) and the Cooperative Research Centre for Vaccine Technology.
We thank Carole Long for providing MAb 302 and Louis H. Miller and Colleen Olive for reviewing the manuscript.
REFERENCES
- 1.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S, McGregor I A, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 3.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond B, Yelton D E. A new Fc receptor on mouse macrophages binding IgG3. J Exp Med. 1981;153:514–519. doi: 10.1084/jem.153.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druilhe P, Perignon J L. Mechanisms of defence against P. falciparum asexual blood stages in humans. Immunol Lett. 1994;41:115–120. doi: 10.1016/0165-2478(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 6.Epstein N, Miller L H, Kaushel D C, Udeinya I J, Rener J, Howard R J, Asofsky R, Aikawa M, Hess R L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981;127:212–217. [PubMed] [Google Scholar]
- 7.Gavin A L, Barnes N, Dijstelbloem H M, Hogarth P M. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 8.Hirunpetcharat C, Tian J, Kaslow D C, van Rooijen N, Kumar S, Berzofsky J A, Miller L H, Good M F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 9.Hirunpetcharat C, Vukovic P, Liu X Q, Kaslow D C, Miller L H, Good M F. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J Immunol. 1999;162:7309–7314. [PubMed] [Google Scholar]
- 10.Kumar S, Yadavam A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 11.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 12.Ling I T, Ogun S A, Momin P, Richards R L, Garcon N, Cohen J, Ballou W R, Holder A A. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 13.Quinn T C, Wyler D J. Mechanisms of action of hyperimmune serum in mediating protective immunity to rodent malaria (Plasmodium berghei) J Immunol. 1979;123:2245–2249. [PubMed] [Google Scholar]
- 14.Ravetch J V, Clynes R A. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 15.Rotman H L, Daly T M, Clynes R, Long C A. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J Immunol. 1998;161:1908–1912. [PubMed] [Google Scholar]
- 16.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 17.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. FcRγ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 18.Tian J H, Miller L H, Kaslow D C, Ahlers J, Good M F, Alling D W, Berzofsky J A, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 19.Yuan R, Clynes R, Oh J, Ravetch J V, Scharff M D. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J Exp Med. 1998;187:641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]