Summary paragraph/Abstract
Immunologic responses during sepsis vary significantly among patients and evolve over the course of illness. Sepsis has a direct impact on the immune system due to adverse alteration of the production, maturation, function, and apoptosis of immune cells. Dysregulation in both the innate and adaptive immune responses during sepsis leads to a range of phenotypes consisting of both hyperinflammation and immunosuppression that can result in immunoparalysis. In this review, we discuss components of immune dysregulation in sepsis, biomarkers and functional immune assays to aid in immunophenotyping patients, and evolving immunomodulatory therapies. Important research gaps for the future include: 1) Defining how age, host factors including prior exposures, and genetics impact the trajectory of sepsis in children, 2) Developing tools for rapid assessment of immune function in sepsis, and 3) Assessing how evolving pediatric sepsis endotypes respond differently to immunomodulation. Although multiple promising immunomodulatory agents exist or are in development, access to rapid immunophenotyping will be needed to identify which children are most likely to benefit from which therapy. Advancements in the ability to perform multidimensional endotyping will be key to developing a personalized approach to children with sepsis.
Introduction
According to the international consensus guidelines, sepsis is defined as a life-threatening state of organ dysfunction that is caused by a dysregulated host response to a pathogen1. Globally, sepsis contributes to more than 20% of all deaths in the pediatric age group, with children <5 years of age being particularly vulnerable2. Mortality in severe sepsis varies widely by geographical region, and can be as high as: 21% in North America, 29% in Europe, 32% in Australia/New Zealand, 40% in Asia, 11% in South America, and 40% in Africa3. The majority of pediatric patients (67%) present with multi-organ dysfunction syndrome (MODS) at the onset of sepsis, with a further 30% also developing MODS in the first 7 days of sepsis. Among survivors across all age groups, up to 28% may have mild disability, and 17% may have moderate disability at the time of hospital discharge3.
Immunologic responses during sepsis vary significantly among patients and evolve over the course of illness. Immune cell activation and inflammatory responses can result in a spectrum of immune dysregulation characterized by excess inflammation, compensatory immune suppression, and immune-mediated organ damage. Although the immune system matures significantly throughout childhood4,5, our understanding of how this underlying immunologic maturation impacts the features and outcomes of sepsis in children is evolving6. In this review we discuss the key components of immune dysregulation in sepsis, biomarkers to aid in immunophenotyping patients during acute illness and recovery, and evolving immunomodulatory therapies.
Immune dysregulation in sepsis
Sepsis has a direct impact on the immune system due to adverse alteration of the production, maturation, function, and apoptosis of immune cells7 (Figure 1). The initial stages of sepsis are marked by a pro-inflammatory state with activation of the innate immune system7. Invading pathogens are recognized by the host immune system through pattern recognition receptors (PRRs) on innate immune cells (monocytes and neutrophils) and somatic tissues8. Invading microbes, both commensal and pathogenic, express pathogen-associated molecular patterns (PAMPs), which are recognized by PRRs, including toll-like receptors (TLRs), nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene (RIG)-I-like receptors (RLRs). Similarly, host tissue components that are released after cell damage and breakdown are recognized as damage-associated molecular patterns (DAMPs)7,9. Overall, activation of innate immune cells through these mechanisms results in release of pro-inflammatory cytokines including IL-1, IL-2, IL-6, IFN-gamma, and TNF-α8. There is additional activation of the complement system that together with the cytokine storm can result in further damage to host tissue and worsening multi-organ failure10. If this initial phase of sepsis does not resolve promptly, overactivation of the immune system can be accompanied by an excessive anti-inflammatory and potentially immunosuppressive response that can result in an increased risk of secondary infections11.
Figure 1: Pathways of immune dysfunction associated with sepsis.
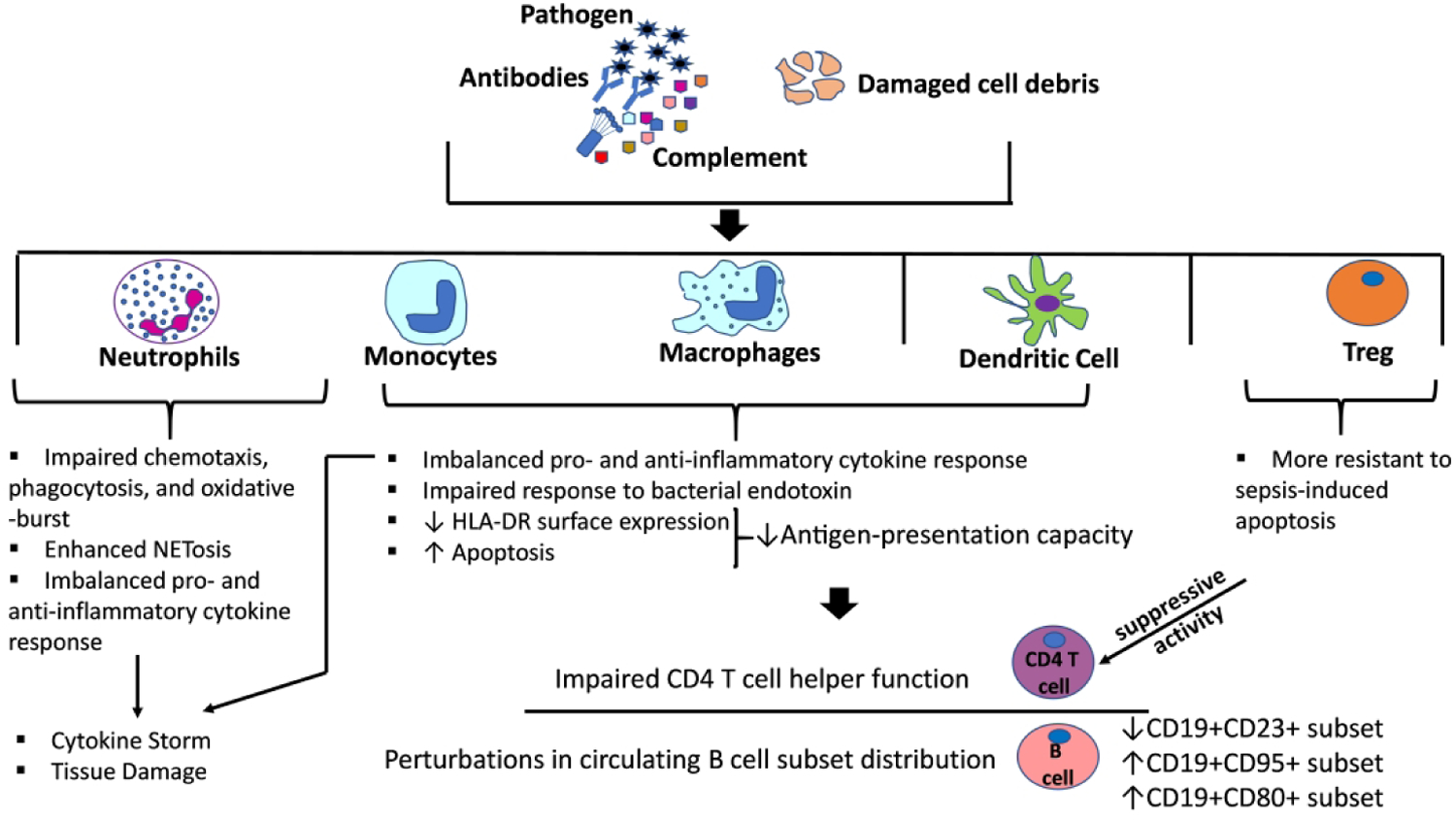
The illustration depicts the dysregulation at the levels of the innate immune response (complement, neutrophils, monocytes-macrophages) as well as the adaptive immune response (T cells and B cells) that characterizes the immunopathology observed in the setting of sepsis.
Multiple cells of both the innate and adaptive immune system have been found to be dysfunctional during sepsis. Neutrophils, which play a key role in host defense against bacterial pathogens, have been shown to have altered chemotaxis and recruitment to the site of infection, impaired phagocytic activity, defects in the production of reactive oxygen species (ROS), and altered secretion of pro-inflammatory cytokines12. Each of these alterations in neutrophil function may contribute to failure to control the initial infection. Concurrently, exaggerated production of neutrophil extracellular traps (NETs), a chromatin mesh that serves to contain pathogens, may stimulate autoinflammation and exacerbate end-organ damage13. Reduced expression of monocyte human leukocyte antigen (HLA)-DR, an important antigen-presenting molecule on innate immune cells, often correlates with decreased responsiveness to ex vivo stimulation with bacterial endotoxin and may increase the risk of secondary infection in patients with sepsis. In addition, increased apoptosis of antigen presenting cells (APCs) has been demonstrated in septic patients with secondary infections and may play a role in T-cell anergy7,14.
CD4+ T-cells, key components of the adaptive immune system, are significantly reduced in non-survivors of sepsis due to apoptosis and splenic removal15. Among remaining CD4+ cells, cytokine production in both T helper 1 (Th1) and T helper 2 (Th2) subpopulations is diminished. T-regulatory cells (Treg), a potentially immunosuppressive subset of CD4+ T cells, may play a role in suppressing excessive inflammation in sepsis. However, evidence suggests that Tregs may be more resistant to sepsis-related apoptosis than other T-cell populations and may therefore contribute to immunoparalysis16. Still, the role of Treg cells in perpetuating sepsis-induced immune suppression has not been as clearly established in children as it has in adults. Similar to CD4+ T-cells, depletion of B-cells, especially the CD5+ B1a-type cells, is also associated with worse outcomes in septic patients17. Deletion of innate response activator (IRA) B cells is associated with hypercytokinemia and delayed clearance of pathogenic bacteria18. The timeline of these changes, based on adult data, has been recently reviewed by Martin et al.19 In brief, there is leukocytosis (increase in neutrophil and monocyte populations) in the first days after sepsis onset followed by a state of lymphopenia with decreased numbers of both adaptive and innate immune cells. Even when cell counts recover, there can be lasting immunoparalysis characterized by reduced functionality of immune cells that increases the risk of secondary infections and death15,19,20.
There are developmental differences in host innate and adaptive immune responses from the neonatal period to adulthood that may impact how sepsis affects children6. For example, an imbalance in the inflammatory and compensatory anti-inflammatory responses may play a greater role in septic shock and MODS in children compared to adults21. Inflammatory response is attenuated in neonatal mice with sepsis22, and while macrophages from young children produce greater amounts of both pro-inflammatory TNF-α and anti-inflammatory IL-10 compared to adults, the resulting IL-10/TNF ratio is higher in children23. Multiple studies have demonstrated that adaptive and innate immune suppression in children within the first 2 days of septic shock is associated with adverse outcomes24,25. And similar to adults, clinical studies have demonstrated that higher initial pro-inflammatory responses in children are associated with impairment of innate immunity, represented by decreased monocyte HLA-DR expression, and adverse infection-related outcomes26.
In summary, dysregulation in both the innate and adaptive immune responses during sepsis leads to a range of phenotypes consisting of both hyperinflammation and immunosuppression. This heterogeneity in sepsis phenotypes is likely responsible for the failure of multiple immunomodulatory therapies trialed in patients with sepsis. Precise and rapid characterization of the unique disturbances in the immune response in children with sepsis may promote personalized therapy and improved outcomes.
Biomarkers and functional immunologic assays
In order to characterize sepsis phenotypes in children and develop a personalized treatment plan, biomarkers and immunologic assays to assess the degree of immune dysregulation are needed. The goal of testing would be to diagnose and quantify impaired immunologic function (i.e., hyperinflammation and immunoparalysis) and identify which patients would benefit from immunomodulatory treatments. To date, however, no one specific marker of immune activation or suppression has been consistently associated with risk of poor outcome.
Single cytokine levels can be measured or tracked in the setting of sepsis, although a lag in availability of results hinders clinical applicability. Following trends in IL-6 or TNF levels may help determine the likelihood of recovery, with decreasing IL-6 levels associated with improved prognosis in septic adults27 28. An overproduction of anti-inflammatory cytokines, such as IL-10 or a high IL-10/TNF ratio, may be a predictor of severity and fatal outcome29.
Lymphopenia, a low circulating absolute number of lymphocytes, has been observed in patients following sepsis and may be secondary to apoptosis and/or decreased bone marrow production30,31. The degree and duration of lymphopenia is correlated with delayed hospital-acquired infections and death32. Lymphopenia is relatively easy to follow with peripheral blood count testing and may help to identify patients for whom immunostimulatory therapies could be useful33. In children with septic shock, infectious complications were associated with lower lymphocyte counts and reduced ability of lymphocytes to respond to stimulation24.
Functional immune testing, however, remains the gold standard for lymphocyte assessment because it directly measures the capacity of a cell population to respond to an immune challenge34. T-cell anergy or lack of proliferation can be assessed by mitogen stimulation. However, assays require time for incubation and access to flow cytometry with fluorescent probes, limiting their clinical use in rapidly evolving diseases such as sepsis. Quantification of co-inhibitory receptor expression, such as programmed cell death 1 (PD-1), may be an alternate and more accessible way to assess lymphocyte anergy in sepsis35. Overexpression of PD-1 and its ligand PDL-1 has been associated with decreased lymphocyte proliferation capacity, late infectious complications, and mortality36.
The antigen presenting molecule HLA-DR should be expressed on the surface of the vast majority of circulating monocytes. This can be quantified by flow cytometry as a percentage of circulating cells or molecules of HLA-DR per cell. Low HLA-DR is considered a hallmark of sepsis-induced immunosuppression37,38. In adults, HLA-DR <30% or <8,000 molecules/cell is associated with increased risk for nosocomial infection and mortality. The threshold of HLA-DR expression associated with adverse outcomes in children with sepsis is likely similar, though less clear due to limited data26. The trajectory of HLA-DR expression may be more important than an absolute threshold, with lack of improvement in a low initial HLA-DR expression over the first week after sepsis onset being predictive of mortality, including in a study of children39. Unfortunately, monocyte HLA-DR lacks standardization across individual laboratories and is subject to inconsistencies if processing is delayed. In addition, although a marker of immunologic state, it is not directly a functional assay.
HLA-DR expression correlates with TNF-α secretion after ex vivo endotoxin challenge, a potentially more functional assessment of monocyte responsiveness. Whole blood is incubated with lipopolysaccharide (LPS) and TNF-α production is measured. Marked reduction in TNF-α production indicates a decreased innate immune response capacity and has been associated with nosocomial infection, prolonged organ dysfunction, and mortality in children25,40,41. However, care must be taken when performing this assay, as monocytes are prone to rapid adaptation to changes in their microenvironment, such as ex vivo testing conditions42.
The discussed measures of immune status and function are somewhat accessible, albeit to different degrees, and have been associated individually with outcomes, most with some study in children. Many of these assays link to immunomodulatory therapeutic agents addressed next. Further innovative approaches including gene expression profiling and complex endotyping are discussed under precision approaches to come.
Immunomodulatory Therapies in Sepsis
Since the 1960s, sepsis-induced inflammation has been a major therapeutic target. Initial anti-inflammatory therapies included high dose corticosteroids, antagonists to pro-inflammatory cytokines and endotoxins, TLR blockers, and platelet activating factor inhibitors43–45. However, in the past two decades, numerous studies have shown that specifically targeting the sepsis induced pro-inflammatory response has not led to clear improvement in outcomes. This may be because the hyperinflammatory phase in sepsis confers some benefit, including initiating broad-based and timely immune activation46, or because these therapies were given non-selectively to heterogeneous populations with different sepsis phenotypes. Additionally, our knowledge of the immunoparalysis phase of sepsis as well as the molecular signature of sepsis-induced immune dysregulation has greatly expanded. Utilizing an evidence-based, directed therapy approach to specifically target the major mechanism of immune dysregulation may improve outcomes. We focus herein on the current evidence and available immunomodulatory therapies (Figure 3), while acknowledging the advancements to come in precision endotyping and need for utilization of these subtypes in therapeutic trials and analyses.
Figure 3: Targeted immunomodulatory therapies in sepsis.
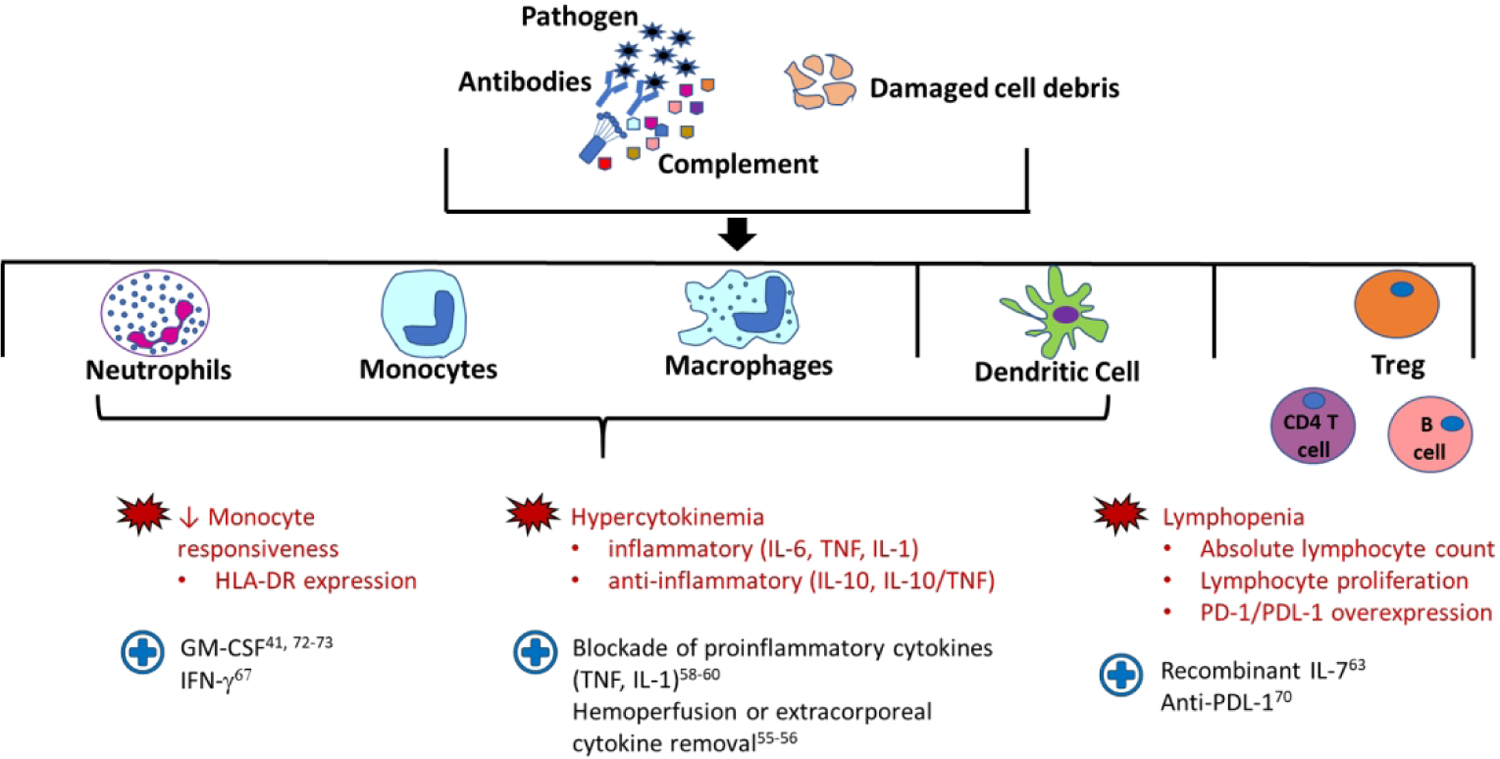
Schematic depicting therapies trialed to target specific areas of immune dysregulation in sepsis. Treg: regulatory T cell, HLA-DR: human leukocyte antigen-DR, IFN: interferon, TNF: tumor necrosis factor, IL: interleukin, PD: programmed cell death, PDL: programmed cell death ligand.
Immunosuppressive therapies
Given that sepsis is essentially a dysregulated immune response mediated by both pro- and anti-inflammatory cytokines, a large body of literature has developed surrounding the use of generalized and individual cytokine-directed therapies. CytoSorb® (CytoSorbents Corporation, New Jersey, USA) is a potential anti-cytokine therapy that has demonstrated utility in a pilot study47. The concept of using hemoperfusion to remove damaging immune mediators is not novel. Polymyxin B hemoperfusion (PMX-HP) directed at removing LPS has been studied extensively. Since endotoxin mediated tissue injury and resultant hyperinflammation is a major source of sepsis related mortality, LPS is a seemingly important target. However, a recent metanalysis confirmed findings from smaller studies that found there was no significant difference in mortality among those who received PMX-HP and those who received standard therapy48. The trial of PMX-HP set a precedent for consideration of other immune-filtration modalities as possible treatment options in sepsis. CytoSorb® is an extracorporeal device with a high-flow, low-resistance cytokine adsorbent, containing specially developed polymer beads with a huge adsorption surface and adsorption spectrum between 5 and 60 kDa49. In a proof of concept pilot study, extracorporeal cytokine removal was applied for 24 hours in the early stage of septic shock. The trial met safety endpoints and the authors reported that even with a single treatment there were statistically significant reductions in vasopressor requirements, serum procalcitonin, and big-endothelin 1 (BigET-1), compared to controls47. However, additional studies are needed to determine how these short-term clinical and biochemical improvements may impact outcomes and whether extracorporal cytokine removal has any benefit in pediatric sepsis.
There is more literature available surrounding the use of cytokine-directed therapy including TNF and IL-1 blockade, as well as IL-7, IL-15 and IFN-γ agonists. As mentioned previously, blockade of pro-inflammatory cytokines such as TNF and IL-1 in heterogeneous populations of patients with sepsis has not led to clear improvement in outcomes50,51. However, in a post-hoc analysis of a subset of adult patients with sepsis and features of macrophage activation syndrome, treatment with IL-1 receptor antagonist significantly decreased mortality52. This suggests that improved phenotyping prior to immunomodulation is necessary to identify patients most likely to benefit from cytokine-directed therapies.
Immunostimulatory therapies
Immunostimulatory therapies to counter the immunoparalysis stage of sepsis, which is associated with significant late-stage mortality53, are receiving increasing attention. As outlined above, immunoparalysis is characterized by dysfunction and apoptosis of numerous immune cells including lymphocytes, monocytes, and macrophages54. To protect against fatal opportunistic infections associated with T cell lymphopenia, treatment with IL-7 has been proposed. Interleukin-7 is antiapoptotic and is necessary for lymphocyte survival and expansion. In addition, it induces proliferation of both CD4+ and CD8+ T cells. The IRIS-7 trial was a prospective, randomized, double-blind, placebo-controlled trial of recombinant human IL-7 (CYT107) in patients with septic shock and severe lymphopenia. The study suggested that CYT107 was safe and well tolerated. Treatment with CYT107 resulted in a 3- to 4-fold increase in the absolute lymphocyte count and in circulating CD4+ and CD8+ T cells, but there was no improvement in 28-day mortality55. Interleukin-15 is important for natural killer (NK) cell, NKT cell, and memory CD8+ T-cell development and function, and has therefore been proposed for use as an immunostimulatory therapy in sepsis-induced immunoparalysis56. The use of IL-15 in sepsis has only been studied in the mouse model57, though clinical trials have demonstrated safety and efficacy in cancer patients58. Guo et al reported that mice treated with high-dose IL-15 immediately following the onset of sepsis had worse outcomes, but the study did not test its impact later in disease when immunoparalysis may be expected57. Given these mixed results, further clinical trials need to be performed with close attention to safety outcomes surrounding the use of IL-15. A recent case series, which included two pediatric patients, demonstrated that IFN-γ therapy was well-tolerated and improved immune host defense in sepsis-induced immunosuppression, as measured by HLA-DR expression in monocytes59. A larger, multicenter placebo-controlled trial assessing the effect of IFN-γ therapy on immune function in patients with sepsis has been completed, though results have not yet been published (NCT01649921). Results from such studies will shed further light on the efficacy of IFN-γ therapy in sepsis.
Due to the potential role of T-cell exhaustion in sepsis-induced immunosuppression, antagonizing inhibitory immune checkpoints, which limit T-cell function, has been proposed. Immune checkpoint inhibitors are currently approved and in use for treatment of multiple cancers. As previously mentioned, increased PD-1 and PDL-1 have been documented in septic patients compared to healthy controls and in sepsis non-survivors compared to sepsis survivors15,60. Preclinical data supports the use of immune checkpoint inhibitors to improve survival from secondary fungal infections following recovery from polymicrobial sepsis, but inconsistent protection is reported when they are used in primary polymicrobial sepsis61. A phase 1b study of anti-PDL-1 in 24 patients with sepsis suggested it was safe, did not lead to hypercytokinemia, and resulted in a dose-dependent increase in monocyte HLA-DR expression62, but further clinical trials have yet to be performed.
Impaired function of APCs has also been implicated in sepsis-induced immunosuppression. Tolerance to endotoxin and reduced antigen presentation capacity in peripheral blood mononuclear cells (PBMCs), defined by ex vivo stimulation or HLA-DR expression, has been documented in children and adults with sepsis63. Reduced HLA-DR has been associated with impaired monocyte function, increased secondary infections, and increased mortality in adults with sepsis64. Consequently, granulocyte-macrophage colony-stimulating factor (GM-CSF), a myelopoietic growth factor that impacts the survival, proliferation, and activation of neutrophils and monocytes as well as numerous other immune cells, has been promoted as a potential therapy to restore antigen presentation and reduce immunoparalysis in sepsis. In a small, randomized trial of septic neonates with neutropenia (n=60), GM-CSF therapy increased neutrophil, monocyte, lymphocyte, and platelet counts and was associated with decreased mortality65. A trial in septic children with MODS with severe reduction in TNF response who were treated with GM-CSF had fewer nosocomial infections40. However, trials in children and adults that did not attempt to select for participants with immunoparalysis did not show similar benefits64,66. A multicenter trial in children with sepsis and endotoxin tolerance is currently enrolling (NCT03769844).
Precision approaches to pediatric sepsis care
Sepsis is complex and heterogenous at the individual level, and to move the needle in development of effective therapeutics, we need to stratify patients into clearer endotypes based on biological features (host factors, comorbidities, genetics)67. Beyond single biomarkers, cell counts, and specific functional assays, broad screening and omics approaches have been used to identify and endotype patients with sepsis. Several investigators have published leukocyte derived mRNA transcriptomic gene expression and discovery-based biomarker studies utilizing modeling to identify subgroups of children with sepsis who have immunologic abnormalities in similar pathways and/or are at risk for particular outcomes68,69,70. Sweeney et al pooled pediatric/neonatal transcriptomic sepsis datasets and noted three clusters: inflammopathic, adaptive, and coagulopathic71. However, the therapeutic implications of these endotypes remain unclear and warrant further study. Additional considerations for endotyping critically-ill children with sepsis include the potential utility of sampling local inflammatory environments (e.g., the upper airway and lung in acute respiratory distress syndrome)72; linking immunologic and other system (e.g., coagulation, hepatobiliary) abnormalities; and prediction of disease trajectories73. Advanced consensus endotype definitions and multi-center cohorts are needed to solidify models for 1) development of rapid platforms/multiplex assays in a clinically actionable timeline and 2) interventional trials to tailor therapeutics in a precision medicine approach to clinical care for children with sepsis. To this point, accessibility and turnaround time of immune profiling and functional assays remains a limitation to applying results to clinical care. Newer technologies are being developed that utilize microfluidic platforms, single cell proteomics (e.g., Olink®, IsoPlexis©), or rapid transcriptomic patterns that may provide more rapid results that can be used in clinical decision-making, monitoring patients over time, and/or assessing response to therapy.
Conclusion
Sepsis is a disorder of immune dysregulation triggered by infection1. Although accumulation of environmental and infectious exposures modifies the immune system throughout childhood, little is known about how differences in innate and adaptive immunity based on age, comorbidities, prior exposures, and genetics impact the pathophysiology of pediatric sepsis and this remains an area for advancement. Accurate phenotyping of immune function during sepsis and endotyping of disease subtypes may be particularly important in children. Caution must be taken when extrapolating results from adult trials and animal models. To advance effective therapeutics for pediatric sepsis, we must move from undifferentiated immunomodulatory therapies (i.e., a one size fits all) to individual immune phenotyping and directed therapy. Advancements in the ability to perform multidimensional endotyping at the bedside will be central to developing a personalized approach to children with sepsis diagnosis and management.
Figure 2: Representative monocyte HLA-DR expression before and after GM-CSF treatment in sepsis.
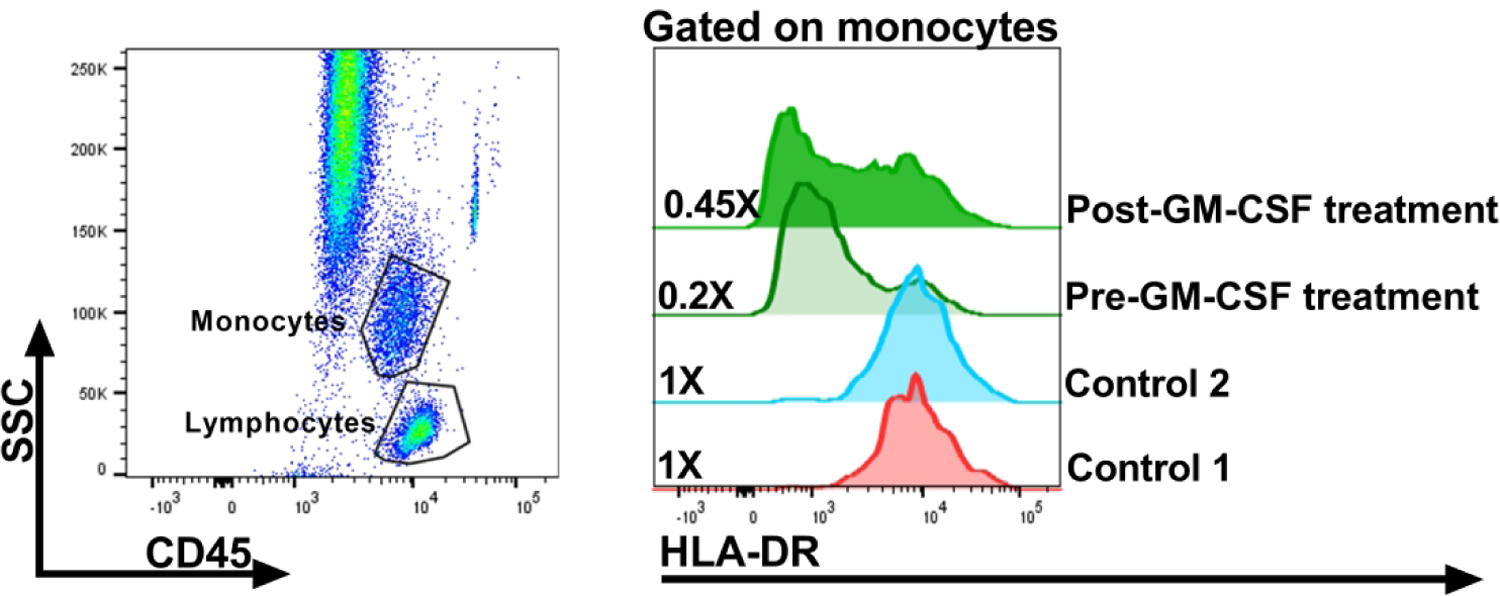
This is an example of polychromatic flow-cytometry utilized to assess monocyte-associated HLA-DR expression pre and post GM-CSF treatment in a sepsis patient. Briefly, whole blood samples from a patient and commercially available flow-cytometry control samples (Streck, Omaha, NE) were stained with fluorochrome-conjugated monoclonal antibodies targeting the CD45, CD3 and HLA-DR surface markers, and hierarchical gating was utilized to evaluate HLA-DR expression on monocytes. Monocytes were identified based on CD45 expression and light scatter characteristics (side-scatter [SSC]) (left panel). Surface expression of HLA-DR on monocytes is demonstrated by utilizing the stacked histogram plot (right panel). The numerical values in the plot depict the frequency of monocytes expressing HLA-DR and response post GM-CSF therapy. Laboratory assays done under IRB-approved protocol at Lurie Children’s.
Impact bullet points:
Immunologic responses during sepsis vary significantly among patients and evolve over the course of illness. The resulting spectrum of immunoparalysis that can occur due to sepsis can increase morbidity and mortality in children and adults.
This narrative review summarizes the current literature surrounding biomarkers and functional immunologic assays for immune dysregulation in sepsis, with a focus on immunomodulatory therapies that have been evaluated in sepsis.
A precision approach toward diagnostic endotyping and therapeutics including gene expression will allow for optimal clinical trials to evaluate efficacy of individualized and targeted treatments for pediatric sepsis.
Acknowledgements:
We would like to thank the Program in Inflammation, Immunity and the Microbiome (PrIIMe) team and acknowledge the Lurie Children’s Immunology Lab. The following authors are supported by National Institutes of Health awards: NHLBI K08HL143127 (Coates), NIAID K08AI123524 (Arshad), and NIAID K23AI139337 (Mithal).
Footnotes
Competing interest declaration: The authors have no competing interests to disclose.
References:
- 1.Singer M et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315, 801–810, doi: 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann-Struzek C et al. The global burden of paediatric and neonatal sepsis: a systematic review. The Lancet. Respiratory medicine 6, 223–230, doi: 10.1016/s2213-2600(18)30063-8 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 191, 1147–1157, doi: 10.1164/rccm.201412-2323OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon AK, Hollander GA & McMichael A Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282, 20143085, doi: 10.1098/rspb.2014.3085 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgountzou A & Papadopoulos NG Postnatal Innate Immune Development: From Birth to Adulthood. Front Immunol 8, 957, doi: 10.3389/fimmu.2017.00957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn J, Cornell TT, Wong HR, Shanley TP & Wheeler DS The host response to sepsis and developmental impact. Pediatrics 125, 1031–1041, doi: 10.1542/peds.2009-3301 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delano MJ & Ward PA The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev 274, 330–353, doi: 10.1111/imr.12499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X-H, Yin Y-J & Zhang J-X Sepsis and immune response. World J Emerg Med 2, 88–92 (2011). [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R & Janeway C Jr. Innate immunity. The New England journal of medicine 343, 338–344, doi: 10.1056/nejm200008033430506 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Chousterman BG, Swirski FK & Weber GF Cytokine storm and sepsis disease pathogenesis. Seminars in immunopathology 39, 517–528, doi: 10.1007/s00281-017-0639-8 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Delano MJ & Ward PA Sepsis-induced immune dysfunction: can immune therapies reduce mortality? The Journal of clinical investigation 126, 23–31, doi: 10.1172/jci82224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolaczkowska E & Kubes P Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology 13, 159–175, doi: 10.1038/nri3399 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Kaplan MJ & Radic M Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189, 2689–2695, doi: 10.4049/jimmunol.1201719 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler MS et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PloS one 12, e0182427–e0182427, doi: 10.1371/journal.pone.0182427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boomer JS et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605, doi: 10.1001/jama.2011.1829 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao C, Ma T, Chai Y-F & Shou S-T The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med 6, 5–9, doi: 10.5847/wjem.j.1920-8642.2015.01.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monserrat J et al. Early alterations of B cells in patients with septic shock. Critical care (London, England) 17, R105, doi: 10.1186/cc12750 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chousterman BG & Swirski FK Innate response activator B cells: origins and functions. International immunology 27, 537–541, doi: 10.1093/intimm/dxv028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin MD, Badovinac VP & Griffith TS CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Front Immunol 11, 1364, doi: 10.3389/fimmu.2020.01364 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen IJ, Sjaastad FV, Griffith TS & Badovinac VP Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J Immunol 200, 1543–1553, doi: 10.4049/jimmunol.1701618 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doughty L, Carcillo JA, Kaplan S & Janosky J The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest 113, 1625–1631, doi: 10.1378/chest.113.6.1625 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Wynn JL et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28, 675–683, doi: 10.1097/SHK.0b013e3180556d09 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Barsness KA et al. Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg 39, 912–915; discussion 912–915, doi: 10.1016/j.jpedsurg.2004.02.009 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Muszynski JA et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care 18, R145, doi: 10.1186/cc13980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muszynski JA et al. Early Immune Function and Duration of Organ Dysfunction in Critically III Children with Sepsis. Am J Respir Crit Care Med 198, 361–369, doi: 10.1164/rccm.201710-2006OC (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remy S et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study. Ann Intensive Care 8, 36, doi: 10.1186/s13613-018-0382-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skirecki T, Borkowska-Zielinska U, Zlotorowicz M & Hoser G Sepsis immunopathology: perspectives of monitoring and modulation of the immune disturbances. Arch Immunol Ther Exp (Warsz) 60, 123–135, doi: 10.1007/s00005-012-0166-1 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Chaudhry H et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 27, 669–684 (2013). [PMC free article] [PubMed] [Google Scholar]
- 29.Gogos CA, Drosou E, Bassaris HP & Skoutelis A Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181, 176–180, doi: 10.1086/315214 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss RS et al. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol 174, 5110–5118, doi: 10.4049/jimmunol.174.8.5110 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Girardot T, Rimmele T, Venet F & Monneret G Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 22, 295–305, doi: 10.1007/s10495-016-1325-3 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Drewry AM et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 42, 383–391, doi: 10.1097/SHK.0000000000000234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felmet KA, Hall MW, Clark RS, Jaffe R & Carcillo JA Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 174, 3765–3772, doi: 10.4049/jimmunol.174.6.3765 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Monneret G & Venet F Sepsis-induced immune alterations monitoring by flow cytometry as a promising tool for individualized therapy. Cytometry B Clin Cytom 90, 376–386, doi: 10.1002/cyto.b.21270 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS & Green JM A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care 16, R112, doi: 10.1186/cc11404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guignant C et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 15, R99, doi: 10.1186/cc10112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monneret G et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32, 1175–1183, doi: 10.1007/s00134-006-0204-8 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Benlyamani I, Venet F, Coudereau R, Gossez M & Monneret G Monocyte HLA-DR Measurement by Flow Cytometry in COVID-19 Patients: An Interim Review. Cytometry A 97, 1217–1221, doi: 10.1002/cyto.a.24249 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Manzoli TF, Troster EJ, Ferranti JF & Sales MM Prolonged suppression of monocytic human leukocyte antigen-DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary Intensive Care Unit. J Crit Care 33, 84–89, doi: 10.1016/j.jcrc.2016.01.027 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Hall MW et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 37, 525–532, doi: 10.1007/s00134-010-2088-x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall MW et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 41, 224–236, doi: 10.1097/CCM.0b013e318267633c (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liddiard K & Taylor PR Understanding local macrophage phenotypes in disease: shape-shifting macrophages. Nat Med 21, 119–120, doi: 10.1038/nm.3798 (2015). [DOI] [PubMed] [Google Scholar]
- 43.The Effectiveness of Hydrocortisone in the Management of Severe Infections: A Double-Blind Study. JAMA 183, 462–465, doi: 10.1001/jama.1963.63700060029012 (1963). [DOI] [Google Scholar]
- 44.Ono S, Tsujimoto H, Hiraki S & Aosasa S Mechanisms of sepsis-induced immunosuppression and immunological modification therapies for sepsis. Ann Gastroenterol Surg 2, 351–358, doi: 10.1002/ags3.12194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuttle KM, McDonald MD & Anderson EJ Re-Evaluating Biologic Pharmacotherapies That Target the Host Response during Sepsis. International Journal of Molecular Sciences 20, 6049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedeva C, Menassa J & Puthalakath H Sepsis: Inflammation Is a Necessary Evil. Front Cell Dev Biol 7, 108, doi: 10.3389/fcell.2019.00108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawchar F et al. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care 49, 172–178, doi: 10.1016/j.jcrc.2018.11.003 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Lin WT, Lai CC, Wang JJ & Chao CM Effect of polymyxin B hemoperfusion on the outcome of patients with sepsis and septic shock. J Infect 80, 350–371, doi: 10.1016/j.jinf.2019.11.013 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Cytosorbents.
- 50.Fisher CJ Jr. et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med 334, 1697–1702, doi: 10.1056/NEJM199606273342603 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Fisher CJ Jr. et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 (1994). [PubMed] [Google Scholar]
- 52.Shakoory B et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 44, 275–281, doi: 10.1097/CCM.0000000000001402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao C, Yu M & Chai Y Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis 10, 782, doi: 10.1038/s41419-019-2015-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotchkiss RS & Crouser E Imaging Apoptosis in Sepsis--A Technology We Would Die for! Crit Care Med 43, 2506–2508, doi: 10.1097/CCM.0000000000001289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francois B et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3, doi: 10.1172/jci.insight.98960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinhagen F et al. Immunotherapy in sepsis - brake or accelerate? Pharmacol Ther 208, 107476, doi: 10.1016/j.pharmthera.2020.107476 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Guo Y et al. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. J Immunol 198, 1320–1333, doi: 10.4049/jimmunol.1601486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romee R et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527, doi: 10.1182/blood-2017-12-823757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payen D et al. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect Dis 19, 931, doi: 10.1186/s12879-019-4526-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shao R et al. Monocyte programmed death ligand-1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care 20, 124, doi: 10.1186/s13054-016-1301-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Busch LM, Sun J, Cui X, Eichacker PQ & Torabi-Parizi P Checkpoint inhibitor therapy in preclinical sepsis models: a systematic review and meta-analysis. Intensive Care Medicine Experimental 8, 7, doi: 10.1186/s40635-019-0290-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotchkiss RS et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit Care Med 47, 632–642, doi: 10.1097/CCM.0000000000003685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathias B, Szpila BE, Moore FA, Efron PA & Moldawer LL A Review of GM-CSF Therapy in Sepsis. Medicine (Baltimore) 94, e2044, doi: 10.1097/MD.0000000000002044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bo L, Wang F, Zhu J, Li J & Deng X Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care 15, R58, doi: 10.1186/cc10031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilgin K et al. A randomized trial of granulocyte-macrophage colony-stimulating factor in neonates with sepsis and neutropenia. Pediatrics 107, 36–41, doi: 10.1542/peds.107.1.36 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Carr R, Modi N & Doré C G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev 2003, Cd003066, doi: 10.1002/14651858.Cd003066 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanski NL & Wong HR Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 16, 20–31, doi: 10.1038/s41581-019-0199-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong HR et al. The pediatric sepsis biomarker risk model. Crit Care 16, R174, doi: 10.1186/cc11652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong HR et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 191, 309–315, doi: 10.1164/rccm.201410-1864OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbas M & El-Manzalawy Y Machine learning based refined differential gene expression analysis of pediatric sepsis. BMC Med Genomics 13, 122, doi: 10.1186/s12920-020-00771-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sweeney TE et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis Across Multiple Datasets Reveals Three Robust Clusters. Crit Care Med 46, 915–925, doi: 10.1097/CCM.0000000000003084 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grunwell JR et al. Machine Learning-Based Discovery of a Gene Expression Signature in Pediatric Acute Respiratory Distress Syndrome. Crit Care Explor 3, e0431, doi: 10.1097/CCE.0000000000000431 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banerjee S, Mohammed A, Wong HR, Palaniyar N & Kamaleswaran R Machine Learning Identifies Complicated Sepsis Course and Subsequent Mortality Based on 20 Genes in Peripheral Blood Immune Cells at 24 H Post-ICU Admission. Front Immunol 12, 592303, doi: 10.3389/fimmu.2021.592303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


