Abstract
Strictures in Crohn's disease (CD) are a hallmark of long‐standing intestinal damage, brought about by inflammatory and non‐inflammatory pathways. Understanding the complex pathophysiology related to inflammatory infiltrates, extracellular matrix deposition, as well as muscular hyperplasia is crucial to produce high‐quality scoring indices for assessing CD strictures. In addition, cross‐sectional imaging modalities are the primary tool for diagnosis and follow‐up of strictures, especially with the initiation of anti‐fibrotic therapy clinical trials. This in turn requires such modalities to both diagnose strictures with high accuracy, as well as be able to delineate the impact of each histomorphologic component on the individual stricture. We discuss the current knowledge on cross‐sectional imaging modalities used for stricturing CD, with an emphasis on histomorphologic correlates, novel imaging parameters which may improve segregation between inflammatory, muscular, and fibrotic stricture components, as well as a future outlook on the role of artificial intelligence in this field of gastroenterology.
Keywords: computer tomography enterography, Crohn's disease, cross‐sectional imaging, fibrosis, intestinal ultrasound, magnetic resonance enterography, radiomics, strictures
INTRODUCTION
Crohn's Disease (CD) is a variant of inflammatory bowel diseases (IBD) leading to fistulizing and stricturing phenotypes. Medical therapy approaches for fibrostenosis are short‐lived and inevitably lead to endoscopic intervention or surgical resection. Stricturing is most common in the small bowel and particularly in the terminal ileum. 1 The diagnosis of strictures in CD may or may not be triggered by obstructive symptoms and utilizes diagnostic imaging or endoscopy. Recent data suggests that early intensive medical therapies may decrease the rate of stricturing complications. 2 , 3 However, up to 21% of patients present with stricturing CD at diagnosis, and 10% of patients without baseline findings of strictures may develop symptomatic strictures over a 5‐year follow‐up. 4 The etiology behind this persistent disease progression may be due to either incomplete control of mucosal inflammation or inflammation‐independent mechanisms of intestinal damage. 1
Given the transmural nature of CD, endoscopic biopsies are insufficient to detect the composition of the intestinal wall within strictures. This is relevant because strictures involve all bowel wall layers, and comprise a mix of inflammation, muscular hyperplasia and fibrosis. 5 In response to these limitations, cross‐sectional imaging modalities are being developed. The segregation of fibrostenotic‐predominant from inflammatory‐predominant strictures may help better determine which patients would most benefit from aggressive anti‐inflammatory therapy and which would likely need surgical or endoscopic intervention. Nowadays, such segregation may aid in enrolling patients in contemporary clinical trials of anti‐fibrotic agents for stricturing CD.
Herein, we review the imaging modalities used in the diagnosis and management of stricturing CD, mainly intestinal ultrasound (IUS), computed tomography (CT) and magnetic resonance imaging (MR), while highlighting their clinical applicability, their shortcomings as well as a future outlook into what is yet to come in this field of CD, such as machine learning (ML) and radiomics.
HISTOMORPHOLOGIC CORRELATES OF STRICTURE TYPES INVOLVED IN CROHN’S DISEASE
Traditionally, intestinal fibrosis is described as a progressive pathway driven by chronic inflammation leading to mesenchymal cell activation, accumulation of extracellular matrix, scarring, and eventual stricturing. 1 This pathogenetic view, however, has recently been challenged with evidence supporting inflammation‐independent mechanisms of fibrosis. 6 In addition, smooth muscle hyperplasia, especially in the muscularis mucosa and the muscularis propria, is increasingly believed to grossly contribute to luminal narrowing in CD strictures. A semiquantitative analysis of 48 CD stricture surgical specimens revealed that smooth muscle hyperplasia/hypertrophy correlated with chronic inflammation independent of fibrosis, and that some strictures may arise from nonfibrotic smooth muscle‐mediated narrowing. 7 Interestingly, purely inflammatory or fibrostenotic strictures are exceedingly rare, 8 , 9 and these elements almost always co‐occur and even correlate in severity. 5 Hence, inflammation, muscular hyperplasia, and fibrosis must all be considered for histomorphologic evaluation. In approximately 85% of cases, internal penetrating disease is accompanied by stricturing disease. 10 Of importance, fibrosis and fibromuscular changes in strictures are similar irrespective of the presence of penetrating disease. Inflammation, however, is more severe in penetrating CD. 11
Several stricture histopathologic scoring systems have been created but they are hampered by significant heterogeneity, lack of formal validity or reliability testing, and missing assessment of components such as muscle hypertrophy/hyperplasia. 12 As histopathology scoring systems represent the gold standard for evaluation of imaging techniques that will ultimately support stricture clinical trials, they should reasonably assesses all key aspects of CD strictures. The Stenosis Therapy and Anti‐Fibrotic Research (STAR) systematic review and expert consensus has set in motion the standardization and validation of a histopathological scoring system for small bowel strictures in CD. 12
RADIOLOGIC DEFINITIONS FOR CD STRICTURES
The advent of anti‐fibrotic trials in CD made it important to standardize definitions of image‐based stricture diagnosis. Criteria for CD associated strictures were highly heterogenous, if not lacking at all. 13 The CrOhN's disease anti‐fibrotic STRICTure therapies (CONSTRICT) group of international IBD experts recently suggested standardized radiological definitions for a small bowel stricture diagnosis. 14 For clinical purposes, at least 2 of 3 criteria are required: localized luminal narrowing (>50% luminal narrowing compared to an adjacent healthy bowel loop), bowel wall thickening (25% increase in wall thickness relative to the adjacent non‐affected bowel) and pre‐stenotic dilation (≥3 cm). Clinical trials necessitate all 3 features be present. Similarly, the Society of Abdominal Radiology and the American Gastroenterological Association defined strictures as persistent luminal narrowing with ≥3 cm upstream bowel dilation, irrespective of imaging findings of active inflammation. 15
DIAGNOSIS OF CD STRICTURES
All cross‐sectional imaging modalities, IUS, MR and CT, are able to detect strictures with high accuracy, irrespective of the stricture definitions used (Table 1). 13 The choice of imaging test depends on local availability, cost‐effectiveness, radiation exposure concerns, patient comorbidities, phenotype, and preferences, among other factors.
TABLE 1.
Description of characteristics, diagnostic accuracy and limitations of different cross‐sectional imaging techniques as modalities for the detection of Crohn's disease strictures, with emphasis on markers suggestive of inflammation or fibrosis
| Test | Characteristics | Sensitivity/Specificity for stricture detection | Unique/Strength | Limitations | Markers of inflammation | Markers of fibrosis |
|---|---|---|---|---|---|---|
| IUS (SE, SWE, CEUS, CDI) | Quick, widely available | 80%–100%, 63%–75%, Detection improved with contrast enhancement 88%–100% and 88%–100% 20 , 21 , 22 , 58 | Likely best tolerated by patients; can be performed by trained GI or GI technician | Limited widespread North American experience; interobserver variability | Color Doppler signal, Peri‐enteric inflammatory fat, IV contrast enhancement. | Thickened bowel with lack of vascularity or contrast enhancement, pre‐stenotic dilation, stratification present, possibly increased elastographic parameters |
| SWE: has the characteristics of real‐time, good repeatability, and relatively objective results | SE: high operator dependence and variability | |||||
| IV‐CEUS: real‐time assessment of vascular distribution in the intestinal wall with highest temporal resolution | Potential limited duodenal and rectal visualization, and limited in obese patients | |||||
| Can miss subtle areas of fibrosis | ||||||
| CTE | Rapid exam, widely available, requires oral contrast | 85%–100%, 100% 9 , 16 | Widely available | Radiation exposure | Mural hyperenhancement, mesenteric stranding and hypervascularity, ulceration | Minimal mural hyperenhancement, sub‐mucosal fat, no mesenteric inflammation, no ulceration |
| Mesenteric hypervascularization can predict fibrosis, but also correlates with intestinal inflammation | ||||||
| MRE (DWI, MT‐MR, DCE‐MRI) | No radiation exposure, restricted availability, longer examination times, requires oral contrast | 75%–100%, 91%–96% 19 , 20 , 59 | DWI, MT‐MR and other parameters help characterize fibrosis best so far | Limited availability in various centers | Early layered or homogeneous mural hyperenhancement, mesenteric stranding and engorged vasa recta, T2 hyperintensity of bowel wall compared to muscle, restricted diffusion and ulceration | Delayed mural hyperenhancement relative to normal bowel wall enhancement, no mesenteric inflammation, T1 and T2 isointensity or hypointensity, no ulceration, elevated magnetization transfer ratio |
| Patient comfort and issues with motion artifact (claustrophobia and length of test, especially with Gadolinium use in some parameters) |
Abbreviations: CDI: color doppler imaging; CEUS: contrast‐enhanced ultrasound; CTE: computed tomography enterography; DCE‐MRI: dynamic contrast enhanced‐magnetic resonance imaging; DWI: diffusion‐weighted imaging; GI: gastrointestinal; IUS: Intestinal Ultrasound; IV: intravenous; MRE: magnetic resonance enterography; MT‐MR: magnetization transfer magnetic resonance; SE: strain elastography; SWE: shear wave elastography.
CT enterography (CTE)/enteroclysis
CT enterography (CTE) has high sensitivity and specificity (up to 100%) for detecting small bowel strictures 13 , 16 and the use of oral and intravenous (IV) contrast improves its detection rate (Figure 1). The use of neutral or low‐density contrast, either orally (enterography) or by nasoenteric tube (enteroclysis), allows for superior small bowel wall identification and characterization compared with standard high attenuation enteric CT contrast. Computed tomography is almost universally available, has fast examination time, and relatively low cost. In healthcare systems that apply a Beveridge model (e.g., the United Kingdom, Spain, New Zealand, Veterans Health Administration in the United States [U.S.], etc.) or a Bismarck model (e.g., Germany, Belgium, Japan, some employer‐based healthcare plans in the U.S. etc.), CT is readily available. In contrast, long waiting lists and delays in imaging might be expected in national health insurance models (e.g., Canada, Taiwan, South Korea, Medicare in the U.S.). Further, CT is available to those who can afford it in systems with out‐of‐pocket models such as those predominant in less‐developed (e.g., India, China, Africa, South America), as well as uninsured or underinsured populations in the U.S. Despite the U.S. having elements of all of the above models, CT overall is highly accessible and easily approved where pre‐certification from insurance companies is required. 17 Finally, the use of CT technology has to be weighed against recurrent radiation exposure risks. 13 Based on theoretical epidemiological data, cumulative effective doses of as low as 50 millisieverts (the equivalent of five CT abdomen and pelvis scans) are presumed to cause potentially harmful radiation exposure consequences such as solid tumor development. According to several studies, about 5%–34.7% of CD patients receive such high doses of radiation exposure, largely driven by CT imaging. The creation of IBD radiation diaries has been proposed to improve physicians' recognition of patients' total radiation exposures. 18 Nonetheless, when the clinical presentation of the patient demands a rapid assessment, then CT remains the most readily available and consistent means of evaluation.
FIGURE 1.
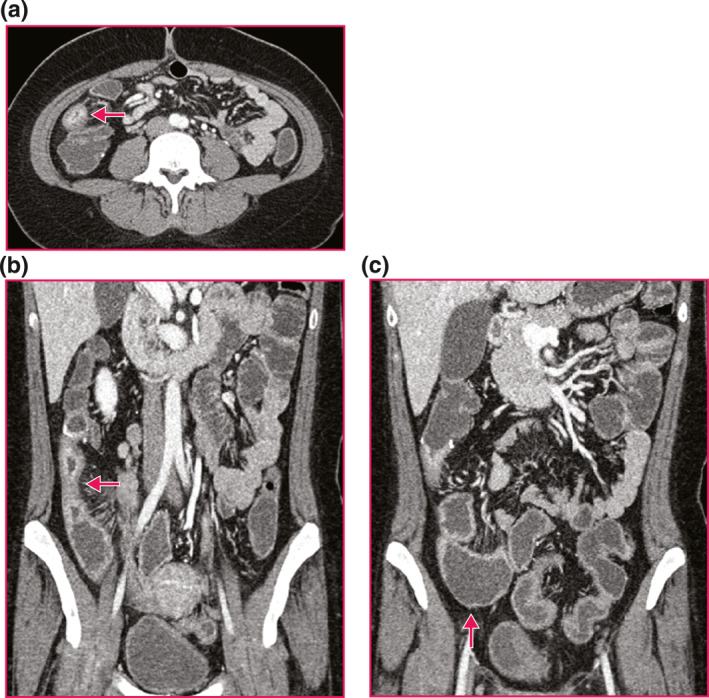
Representative computed tomography (CT) enterography imaging of a Crohn's disease stricture. (a) Neo‐terminal ileum (terminal ileum (TI)) Stricture. Axial, post contrast enhanced CT enterography of a neo‐TI stricture (arrow) demonstrates stratified mural hyperenhancement, wall thickening and luminal narrowing. (b) Neo‐TI Stricture. Coronal, post contrast enhanced CT enterography of a neo‐TI stricture (arrow) demonstrates stratified mural hyperenhancement, wall thickening and luminal narrowing. (c) Upstream Dilation Proximal to Neo‐TI Stricture. Coronal, post contrast enhanced CT enterography proximal to neo‐TI stricture demonstrates upstream dilation to 4.1 cm (arrowhead).
MR enterography (MRE)
The sensitivity and specificity ranges of MR enterography (MRE) for stricture detection are essentially equivalent to those of CTE, with 75%–100% and 91%–96%, respectively (Figure 2). 13 , 19 , 20 In addition, MR Exams include multiphasic acquisitions over 20–25 min of time; this longer duration allows for assessing if the lumen stays consistently narrow with consistent upstream dilation or stasis, thus adding another layer of certainty that a stricture is present. Given its accuracy and lack of ionizing radiation exposure, the CONSTRICT group recommended MRE as the ideal test for assessing and following‐up on CD strictures. 13 However, MRE is time‐consuming, expensive, not universally available and may cause significant discomfort to claustrophobic patients. Although a significant MR scanner density is observed in economically developed countries such as in Europe, a critical access limitation still exists in countries of Asia and Sub‐Saharan Africa. Additionally, in the U.S., pre‐certification from insurance companies remains more challenging.
FIGURE 2.
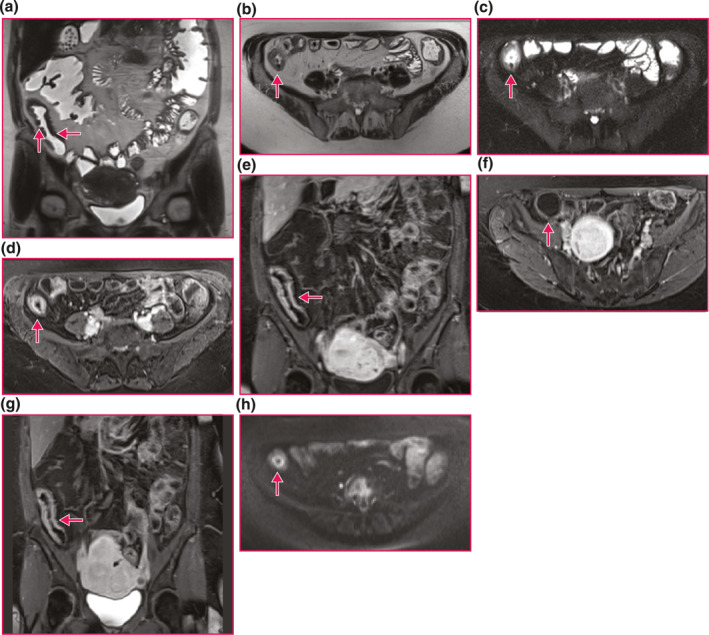
Representative magnetic resonance imaging (MR) enterography imaging of a Crohn's disease stricture. (a) Naïve terminal ileum (TI) Stricture. Coronal half‐Fourier single‐shot turbo spin‐echo sequence of a naïve TI stricture (horizontal arrow) demonstrates wall thickening and lumen narrowing. Ulcers are also present (vertical arrow). (b) Naïve TI Stricture. Axial half‐Fourier single‐shot turbo spin‐echo sequence of a naïve TI stricture (arrow) demonstrates wall thickening and luminal narrowing. (c) Naïve TI Stricture. Axial, fat saturation, half‐Fourier single‐shot turbo spin‐echo sequence of a naïve TI stricture (arrow) demonstrates wall thickening, luminal narrowing and increased signal in the wall indicating edema from active inflammation. (d) Naïve TI Stricture. Axial volumetric interpolated breath‐hold examination sequence of a naïve TI stricture (arrow) in the enteric phase post contrast enhancement demonstrates stratified mural hyperenhancement, wall thickening and luminal narrowing. (e) Naïve TI Stricture. Coronal volumetric interpolated breath‐hold examination sequence of a naïve TI stricture (arrow) in the enteric phase post contrast enhancement demonstrates a more uniform but still stratified mural hyperenhancement, wall thickening and luminal narrowing. (f) Upstream Dilation Proximal to Naïve TI Stricture. Axial volumetric interpolated breath‐hold examination sequence proximal to a naïve TI stricture in the enteric phase post contrast demonstrates upstream dilation to 3.4 cm (arrowhead). (g) Naïve TI Stricture. Coronal volumetric interpolated breath‐hold examination sequence of a naïve TI stricture (arrow) in the delayed phase (7 min) post contrast enhancement demonstrates a more uniform but still stratified mural hyperenhancement, wall thickening and luminal narrowing. One investigation suggests that delayed phase enhancement indicates fibrosis predominance. (h) Naïve TI Stricture. Axial high B value (B = 800 s/mm2) diffusion weighted sequence of a naïve TI stricture (arrow) demonstrates a high signal stratified pattern indicating restricted diffusion from active inflammation.
Intestinal US (IUS)
IUS is the least expensive modality, and likely the most tolerated procedure, and can safely be used within the same IBD clinic visit by a trained gastroenterologist. For such reasons, IUS is becoming more widespread for long‐term follow‐up, yet remains mostly used in specialized academic centers as it requires significant expertise. The sensitivity and specificity ranges of transabdominal IUS are 80%–100% and 63%–75%, respectively. 21 , 22 IV contrast enhancement (CEUS) improves those to 88%–100% and 88%–100%, respectively (Figure 3). 13
FIGURE 3.
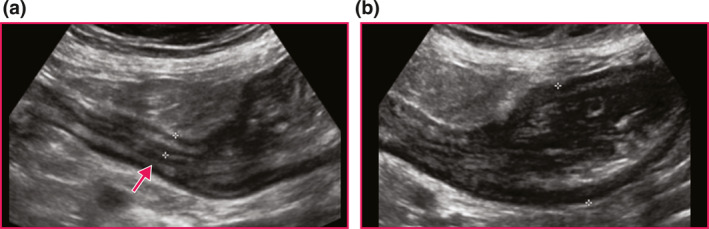
Representative fibrostenotic Disease on Intestinal Ultrasound using a naïve Crohn's disease stricture at terminal ileum (TI) prior to resection. (a) Longitudinal view of stricture with thickened bowel wall measuring 7.3 mm, narrowed lumen along the entire length of the stricture (arrow), and surrounding inflammatory fat. (b) Pre‐stenotic dilation measured at 3.9 cm with dysfunctional peristalsis.
CHARACTERIZATION OF CD STRICTURES ON ROUTINE CROSS‐SECTIONAL IMAGING
Beyond the ability of diagnosing a stricture, several studies have attempted to dissect inflammation, fibrosis and even muscularization within a given stricture. 23 Single or combined parameters within each imaging modality are being explored, with more promise for MRE‐multiparametric scores at demonstrating sufficient accuracy in segregating histopathological stricture components. These studies still need to be validated. 23 , 24
CT enterography/enteroclysis
Two small studies used contrast‐enhanced CT to assess imaging findings with comparison to surgical specimens. A series of 22 patients found that both histological inflammation and fibrosis were greater in strictures radiologically identified as inflammatory compared to those without active inflammation. 8 , 13 In another series of 44 patients, the sensitivity for both inflammation and fibrostenosis identification was high, but the fibrostenosis score was based upon a thick, nonenhancing wall, luminal stricturing and pre‐stenotic dilation. 9 Overall, CTE may not be accurate enough at distinguishing inflammation from fibrosis. 13
The diagnostic accuracy of 18F‐Fluorodeoxyglucose (18F‐FDG) positron emission tomography (PET)/CT (and well as PET/MRE) in CD strictures has been evaluated in small cohorts. 25 , 26 Areas under the curve (AUC) were reportedly higher for PET/MRE (0.77) than PET/CT (0.51) for differentiating fibrotic from non‐fibrotic strictures, yet the discrimination between inflammation and fibrosis remains suboptimal, and the high radiation exposure of PET would limit the routine clinical application of this technique. 13 , 26 Studies that assess multiparametric MRE with PET features should be reconsidered in light of emerging novel indices for strictures on cross sectional imaging and histopathology.
MR enterography and novel MR‐based modalities
MRE‐defined stricture components have been studied in small cohorts, where presence of inflammation was found to correlate with mural thickness and T2‐weighted fat‐saturated intramural signal intensity, while fibrosis was more commonly associated with layered enhancement. 27 However, these same parameters correlated with both inflammation and fibrosis in similar studies. 13 A devised acute inflammation score (AIS) 28 showed promising sensitivity and specificity for detection of inflammation and fibrosis. The cohort, however, mostly comprised active inflammation samples (AIS > 4.1 in 64/67) and only had 3 patients with exclusively inactive fibrotic strictures. The role of muscular hypertrophy was not assessed. Magnetic resonance imaging (MRI) scans performed 7 min after contrast administration (delayed enhancement) can distinguish mild‐to‐moderate from moderate‐to‐severe fibrosis, a finding that still requires validation. 29 Different MR techniques, diffusion‐weighted imaging (DWI), magnetization transfer (MT) imaging, and MR with dynamic contrast enhancement (DCE) were explored. 30 , 31 DWI values appear to inversely correlate with degrees of fibrosis, whereas magnetization transfer magnetic resonance (MT‐MR) signals parallels the extent of collagen deposition. 32 , 33 , 34 These novel parameters also showed fair inflammation/fibrostenosis segregation in patients with prior ileocolic resection. 35 Efforts are underway to validate these findings. One such recent example involved using multiparametric MRE‐based scores for histologic fibrosis and inflammation assessment, and described a composite score of magnetic resonance index of activity (MaRIA), apparent diffusion coefficient, and measurement of delayed gain of enhancement to predict histologic fibrosis (ROC = 0.91) while MaRIA alone was best at predicting active histologic inflammation (ROC = 0.97). Contrary to prior studies, a good correlation was not found between MT‐MR and either inflammation or fibrosis. 24
Dynamic contrast enhanced‐magnetic resonance imaging and DWI were investigated for smooth muscle hypertrophy in relation to inflammation and fibrosis, using histopathological specimens as the gold standard. 36 Factors such as decreased volume transfer coefficient (Ktrans), maximum enhancement (ME), initial slope of increase, as well as increased mural thickening corelated well with moderate‐to‐severe active inflammation. Initial slope of increase and mural thickness were able to describe muscle hypertrophy in sections with mild/absent inflammation, and a parameter that combined ME and thickness was able to accurately describe muscular alterations with active inflammation. 36 The study also validated that wall thickness on T2‐WI parallels muscular hypertrophy more so than fibrosis. 37
A recent pilot study assessed the use of magnetic resonance elastography for predicting fibrosis as well as the clinical course of patients with CD. 38 A high correlation between the stiffness value measured on MR elastography and radiologist morphological analysis using a visual analog scale was established. A bowel stiffness of ≥3.57 kPa predicted the occurrence of a clinical event (abdominal surgery, hospitalization or emergency department consultation for abdominal pain or bowel obstruction) with AUC 0.82.
However, most of these studies are either small, 13 have only been evaluated in animal models, 33 or lack external validation for specific parameters (such as MT ratio in MT‐MR for detecting fibrosis). 34
IUS techniques
Elastography, namely strain (SE) and shear wave elastography (SWE), are currently studied IUS techniques for stricture characterization. 13 Whereas SE is the measure of bowel stiffness in response to the application of an external compression, SWE interprets the speed of acoustic emissions where stiffer tissues allow for faster propagation. 39 Fibrosis of the bowel correlated with higher point SWE velocity and strain ratio. 40 , 41 More specifically, increasing SWE measurements in stiffer bowels correlated with smooth muscle hyperplasia. 42 The addition of microbubble IV‐CEUS was explored to differentiate stricture types. However, standardization and reproducibility of data ranges and thresholds are required to use this technique confidently. 43
A recent meta‐analysis assessed the diagnostic role of different modes of IUS in distinguishing CD stricture types. 44 Fibrostenotic stricturing correlated with higher mean bowel wall thickness, harder strain value and lower peak enhancement than inflammatory stricturing. However, included studies suffered from significant design heterogeneity, limitations in gold standards, poorly pre‐specified thresholds, as well as unclear blinding of histopathological stricture assessment. Nonetheless, the systematic review showed potential promise with combining multiple IUS parameters to assess fibrosis or predict stricture‐related surgeries. 45
Computational imaging approaches involving artificial intelligence and radiomics
Radiomics involves computational extraction of appearance, intensity, or shape descriptors from radiographic images, and can predict outcomes of interest when integrated in ML algorithms. Radiomics has gained notable traction in the field of oncology, for example, investigators trained and validated a radiomics‐driven ML model using baseline T2‐Weighted MRI images of primary rectal cancers to predict histopathologic response to neoadjuvant chemoradiation. 46 Clinical applications of radiomics have recently begun to be explored in CD. 47 (Figure 4) These include semi‐automation of measuring bowel wall thickness on MRE and CTE, evaluating intraluminal stricture areas along the course of diseased segments, as well as assessing small bowel structural damage; all of which have shown good consistency with radiologist assessments. 48 , 49 Currently, ML and radiomics' accuracy in stratifying the severity of inflammation/fibrosis/smooth muscle hyperplasia on cross‐sectional imaging is being studied. One study used 212 resected bowel lesions from 167 CD patients to train and test a CTE‐based radiomic model for characterizing intestinal fibrosis (none‐to‐mild vs. moderate‐to‐severe) with good performance (AUC 0.724–0.816 across 3 centers). This remained consistent regardless of center‐specific CT‐scanners, stricture location, presence of fistula or abscesses. 50 While the model outperformed radiologist assessment (AUCs<0.6), radiological definitions were not based on CONSTRICT criteria, and anastomotic strictures were excluded. Furthermore, inflammation severity was not assessed extensively, so the impact of inflammation on the accuracy of detecting fibrosis was not studied. From a radiomics perspective, there were concerns with wide 95% confidence intervals for AUCs which may reflect unstable diagnostic performance with small sample sizes. 51 A later study suggested that deep learning models (i.e. multi‐layer neural networks) may be more efficient than radiomics while maintaining similar performance characteristics, and still outperforming radiologist assessments. 52 Although an expert IBD pathologist scored the histopathological samples in both studies, neither employed a validated scoring system, nor comprehensively evaluated all histomorphological components of a stricture.
FIGURE 4.
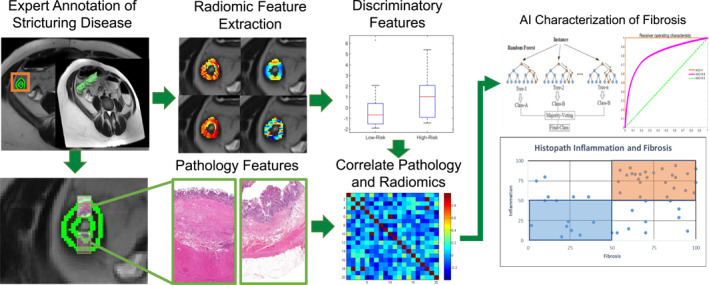
Overall experimental workflow for developing Crohn's disease stricturing characterization model. First, an expert radiologist identifies a region of stricturing disease. Quantitative radiomic features are then extracted on a per‐pixel basis withing the region of interest (green). A subset of features that strongly discriminate between low and high fibrosis, inflammation, etc. are selected. Meanwhile, an expert pathologist evaluates pathology sections taken from the same stricturing regions and provides scoring to characterize the disease based on pre‐determined standardized criterion. Finally, radiomic and pathology features are correlated and used to train an artificial intelligence (AI) model to predict extent of each pathology within the stricturing region.
FUTURE OUTLOOK
A deeper understanding is needed for the pathophysiological interplay between inflammation, fibrosis, and muscular hyperplasia on the overall narrowing of the stricture. A validated histopathological index score is critical to reliably validate cross‐sectional imaging modalities which guide CD‐stricture medical and surgical care. Histomorphological heterogeneity within strictures, and the fact that most internal penetrating disease coexists with strictures, 11 also translates into challenges within cross‐sectional imaging to detect these separate elements. Addressing these challenges would critically help reshaping the scope of CD care, especially with anti‐fibrotic therapies on the horizon.
IUS, CTE and MRE have all shown excellent performance in detecting strictures, though MR‐based modalities may offer promising new parameters to segregate fibrostenosis from inflammation. Upcoming techniques such as the Type I Collagen‐Targeted MR Imaging Probe, may offer even better abilities for staging fibrosis in CD. 53
Meanwhile, AI‐based technologies (such as deep learning and radiomics) suggest significant promise for complementing radiologist evaluations, and could help enable deeper interrogation of phenotypes in stricturing CD while leveraging routine imaging sequences.
Ideally, future cross‐sectional imaging and AI‐based techniques should target subtle radiological characteristics that precede clinically‐significant stricturing and are difficult to visualize by an experienced radiologist. For instance, CD imaging assessments largely ignore the perienteric fat, which has a uniform appearance on imaging yet is believed to exert pro‐stricture function in CD 54 ; this may be explored by AI‐based techniques. Combining these findings with clinical metadata, such as identified risk factors of progression to stricturing disease from the TREAT registry, the pediatric RISK inception cohort, and the ACCENT I trial, 55 , 56 will be critical to set guidelines for screening as well as targeting of early anti‐fibrotic therapy in CD. While waiting for reliable biomarkers to predict and assess strictures in CD, 57 radiomics could also be an asset in identifying patients who would benefit from closer follow‐up. For example, radiomics could be used in to help predict patients at high‐risk of stricture recurrence and need for earlier medical intervention.
The clinical dilemma of stricturing CD requires a multifaceted approach to better understand its pathophysiology, to standardize histomorphologic indices, and to explore cross‐sectional imaging modalities with unique parameters that correlate with the different components of a CD stricture. As we are enrolling patients in the first anti‐fibrosis trials in stricturing CD, the need to standardize histopathology and radiology definitions of strictures is pivotal. Together with technical advances in artificial intelligence (AI), the landscape of care for stricturing CD, from targeted medical therapy, to timing of endoscopic and surgical intervention, and even early detection of stricture, is bound to be impacted. A current view of the management and follow up for stricturing CD is summarized in Figure 5.
FIGURE 5.
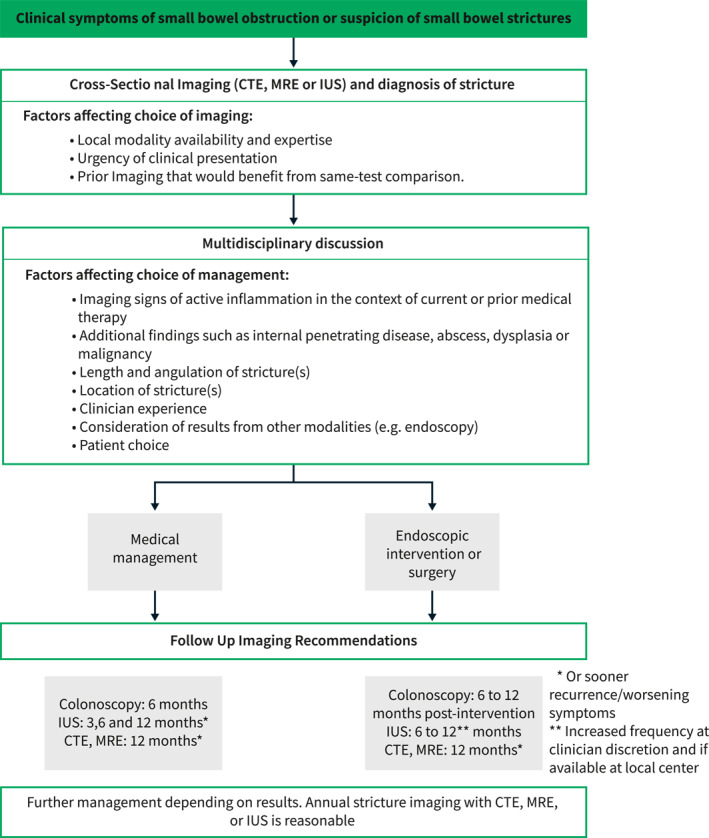
A experts' decision tree regarding the use of cross‐sectional imaging for diagnosing and following up fibrostenotic disease in CD. The above algorithm reflects the opinion of the author team.
CONFLICT OF INTEREST
Florian Rieder is consultant to Agomab. Allergan, AbbVie, Boehringer Ingelheim, Celgene, Cowen, Falk Pharma, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Origo, Pfizer, Pliant, Prometheus, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Theravance, Thetis, UCB and received funding from the National Institute of Health, Helmsley Charitable Trust, Crohn's and Colitis Foundation, Rainin Foundation, UCB, Boehringer‐Ingelheim, Pliant, Morphic, BMS, 89Bio. Joseph Sleiman receives funding from Pfizer. Prathyush Chirra does not receive funding in conflict with this project. Satish E Viswanath receives funding from Pfizer. Ilyssa O Gordon does not receive and direct funding, but the Cleveland Clinic receives funding on her behalf from Celgene, UCB, GB004, Pliant, Morphic Therapeutics, and Helmsley Charitable Trust. Namita S Gandhi receives funding from Pfizer. Cathy Lu has received consultant/speaker fees from Abbvie, Janssen, Ferring, Takeda, and Fresenius Kabi. Mark E Baker Receives salary support to the Institution from Pfizer and Helmsley Charitable Trust.
ACKNOWLEDGMENTS
This work was supported via NIDDK R01DK123233 to F.R.,1F31DK130587‐01A1 to P.C., NCI 1U01CA248226‐01 to S.E.V, CDMRP W81XWH‐21‐1‐0345 to S.E.V.
Sleiman J, Chirra P, Gandhi NS, Baker ME, Lu C, Gordon IO, et al. Crohn's disease related strictures in cross‐sectional imaging: More than meets the eye? United European Gastroenterol J. 2022;10(10):1167–78. 10.1002/ueg2.12326
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current review.
REFERENCES
- 1. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):340–50. 10.1053/j.gastro.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma C, Moran GW, Benchimol EI, Targownik LE, Heitman SJ, Hubbard JN, et al. Surgical rates for Crohn's disease are decreasing: a population‐based time trend analysis and validation study. Am J Gastroenterol. 2017;112(12):1840–8. 10.1038/ajg.2017.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding NS, Yip WM, Choi CH, Saunders B, Thomas‐Gibson S, Arebi N, et al. Endoscopic dilatation of Crohn's anastomotic strictures is effective in the long term, and escalation of medical therapy improves outcomes in the biologic era. J Crohns Colitis. 2016;10(10):1172–8. 10.1093/ecco-jcc/jjw072 [DOI] [PubMed] [Google Scholar]
- 4. Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, et al. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population‐based inception cohort: an Epi‐IBD study. Gut. 2019;68(3):423–33. 10.1136/gutjnl-2017-315568 [DOI] [PubMed] [Google Scholar]
- 5. Rimola J, Capozzi N. Differentiation of fibrotic and inflammatory component of Crohn's disease‐associated strictures. Int Res. 2020;18(2):144–50. 10.5217/ir.2020.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zidar N, Langner C, Jerala M, Bostjancic E, Drobne D, Tomazic A. Pathology of fibrosis in crohn's disease‐contribution to understanding its pathogenesis. Front Med (Lausanne). 2020;7:167. 10.3389/fmed.2020.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in crohn's fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis. 2017;11(1):92–104. 10.1093/ecco-jcc/jjw126 [DOI] [PubMed] [Google Scholar]
- 8. Adler J, Punglia DR, Dillman JR, Polydorides AD, Dave M, Al‐Hawary MM, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis. 2012;18(5):849–56. 10.1002/ibd.21801 [DOI] [PubMed] [Google Scholar]
- 9. Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol. 2007;102(11):2541–50. 10.1111/j.1572-0241.2007.01537.x [DOI] [PubMed] [Google Scholar]
- 10. Jurgens M, Brand S, Laubender RP, Seiderer J, Glas J, Wetzke M, et al. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn's disease independent of the IL23R genotype. J Gastroenterol. 2010;45(7):721–31. 10.1007/s00535-010-0231-7 [DOI] [PubMed] [Google Scholar]
- 11. de Sousa H Tavares, Gullo I, Castelli C, Dias CC, Rieder F, Carneiro F, et al. Ileal Crohn's disease exhibits similar transmural fibrosis irrespective of phenotype. Clin Transl Gastroenterol. 2021;12(4):e00330. 10.14309/ctg.0000000000000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon IO, Bettenworth D, Bokemeyer A, Srivastava A, Rosty C, de Hertogh G, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn's disease: a systematic review. Gastroenterology. 2020;158(1):137–50. 10.1053/j.gastro.2019.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettenworth D, Bokemeyer A, Baker M, Mao R, Parker CE, Nguyen T, et al. Assessment of Crohn's disease‐associated small bowel strictures and fibrosis on cross‐sectional imaging: a systematic review. Gut. 2019;68(6):1115–26. 10.1136/gutjnl-2018-318081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rieder F, Bettenworth D, Ma C, Parker CE, Williamson LA, Nelson SA, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti‐fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther. 2018;48(3):347–57. 10.1111/apt.14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruining DH, Zimmermann EM, Loftus EV, Jr. , Sandborn WJ, Sauer CG, Strong SA, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn's disease. Radiology. 2018;286(3):776–99. 10.1148/radiol.2018171737 [DOI] [PubMed] [Google Scholar]
- 16. Vogel J, da Luz Moreira A, Baker M, Hammel J, Einstein D, Stocchi L, et al. CT enterography for Crohn's disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007;50(11):1761–9. 10.1007/s10350-007-9005-6 [DOI] [PubMed] [Google Scholar]
- 17. Wallace LS. A view of health care around the world. Ann Fam Med. 2013;11(1):84. 10.1370/afm.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2165–78. 10.3748/wjg.v22.i7.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinha R, Murphy P, Sanders S, Ramachandran I, Hawker P, Rawat S, et al. Diagnostic accuracy of high‐resolution MR enterography in Crohn's disease: comparison with surgical and pathological specimen. Clin Radiol. 2013;68(9):917–27. 10.1016/j.crad.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 20. Kumar S, Hakim A, Alexakis C, Chhaya V, Tzias D, Pilcher J, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn's disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015;30(1):86–91. 10.1111/jgh.12724 [DOI] [PubMed] [Google Scholar]
- 21. Pallotta N, Vincoli G, Montesani C, Chirletti P, Pronio A, Caronna R, et al. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn's disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012;18(1):74–84. 10.1002/ibd.21678 [DOI] [PubMed] [Google Scholar]
- 22. Maconi G, Carsana L, Fociani P, Sampietro GM, Ardizzone S, Cristaldi M, et al. Small bowel stenosis in Crohn's disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther. 2003;18(7):749–56. 10.1046/j.1365-2036.2003.01673.x [DOI] [PubMed] [Google Scholar]
- 23. Rieder F, Latella G, Magro F, Yuksel ES, Higgins PD, Di Sabatino A, et al. European Crohn's and colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn's disease. J Crohns Colitis. 2016;10(8):873–85. 10.1093/ecco-jcc/jjw055 [DOI] [PubMed] [Google Scholar]
- 24. Coimbra A, Rimola J, Cuatrecasas M, De Hertogh G, Van Assche G, Vanslembrouck R, et al. Magnetic resonance enterography and histology in patients with fibrostenotic Crohn's disease: a multicenter study. Clin Transl Gastroenterol. 2022;13(7):e00505. 10.14309/ctg.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacene HA, Ginsburg P, Kwon J, Nguyen GC, Montgomery EA, Bayless TM, et al. Prediction of the need for surgical intervention in obstructive Crohn's disease by 18F‐FDG PET/CT. J Nucl Med. 2009;50(11):1751–9. 10.2967/jnumed.109.065466 [DOI] [PubMed] [Google Scholar]
- 26. Pellino G, Nicolai E, Catalano OA, Campione S, D'Armiento FP, Salvatore M, et al. PET/MR versus PET/CT imaging: impact on the clinical management of small‐bowel Crohn's disease. J Crohns Colitis. 2016;10(3):277–85. 10.1093/ecco-jcc/jjv207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Punwani S, Rodriguez‐Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, et al. Mural inflammation in Crohn disease: location‐matched histologic validation of MR imaging features. Radiology. 2009;252(3):712–20. 10.1148/radiol.2523082167 [DOI] [PubMed] [Google Scholar]
- 28. Loch FN, Kamphues C, Beyer K, Klauschen F, Schineis C, Weixler B, et al. Diagnostic accuracy of magnetic resonance enterography for the evaluation of active and fibrotic inflammation in Crohn’s disease. Front Surg. 2022;9:872596. 10.3389/fsurg.2022.872596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rimola J, Planell N, Rodriguez S, Delgado S, Ordas I, Ramirez‐Morros A, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110(3):432–40. 10.1038/ajg.2014.424 [DOI] [PubMed] [Google Scholar]
- 30. Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion‐weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn's disease: a systematic review and meta‐analysis. Inflamm Bowel Dis. 2016;22(3):669–79. 10.1097/mib.0000000000000607 [DOI] [PubMed] [Google Scholar]
- 31. Allocca M, Fiorino G, Bonifacio C, Peyrin‐Biroulet L, Danese S. Noninvasive multimodal methods to differentiate inflamed vs fibrotic strictures in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2019;17(12):2397–415. 10.1016/j.cgh.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 32. Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn's disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24(3):619–29. 10.1007/s00330-013-3015-7 [DOI] [PubMed] [Google Scholar]
- 33. Lu B, Lin J, Du J, He S, Cao Q, Huang L, et al. Native T1 mapping and magnetization transfer imaging in grading bowel fibrosis in Crohn's disease: a comparative animal study. Biosensors. 2021;11(9):302. 10.3390/bios11090302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meng J, Huang S, Sun C, Zhang ZW, Mao R, Yang YH, et al. Comparison of three magnetization transfer ratio parameters for assessment of intestinal fibrosis in patients with Crohn's disease. Korean J Radiol. 2020;21(3):290–7. 10.3348/kjr.2019.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pozzessere C, Boudiaf M, Cirigliano A, Dohan A, Mazzei MA, Barat M, et al. MR‐enterography: role in the assessment of suspected anastomotic recurrence of Crohn disease after ileocolic resection. Radiol Med. 2022;127(3):238–50. 10.1007/s11547-022-01452-1 [DOI] [PubMed] [Google Scholar]
- 36. Yu L, Hu S, Huang FC, Wu YC, Zheng XY. Evaluation of dynamic contrast‐enhanced magnetic resonance imaging and diffusion‐weighted imaging for predicting muscular hyperplasia/hypertrophy in Crohn's disease. Abdom Radiol. 2022;47(5):1714–24. 10.1007/s00261-022-03422-7 [DOI] [PubMed] [Google Scholar]
- 37. Wagner M, Ko HM, Chatterji M, Besa C, Torres J, Zhang X, et al. Magnetic resonance imaging predicts histopathological composition of ileal Crohn's disease. J Crohns Colitis. 2018;12(6):718–29. 10.1093/ecco-jcc/jjx186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avila F, Caron B, Hossu G, Ambarki K, Kannengiesser S, Odille F, et al. Magnetic resonance elastography for assessing fibrosis in patients with Crohn's disease: a pilot study. Dig Dis Sci. 2022;67(9):4518–24. 10.1007/s10620-021-07311-9 [DOI] [PubMed] [Google Scholar]
- 39. Slosarz D, Poniewierka E, Neubauer K, Kempinski R. Ultrasound elastography in the assessment of the intestinal changes in inflammatory bowel disease‐systematic review. J Clin Med. 2021;10(18):4044. 10.3390/jcm10184044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orlando S, Fraquelli M, Coletta M, Branchi F, Magarotto A, Conti CB, et al. Ultrasound elasticity imaging predicts therapeutic outcomes of patients with Crohn's disease treated with anti‐tumour necrosis factor Antibodies. J Crohns Colitis. 2018;12(1):63–70. 10.1093/ecco-jcc/jjx116 [DOI] [PubMed] [Google Scholar]
- 41. Ding SS, Fang Y, Wan J, Zhao CK, Xiang LH, Liu H, et al. Usefulness of strain elastography, ARFI imaging, and point shear wave elastography for the assessment of Crohn disease strictures. J Ultrasound Med. 2019;38(11):2861–70. 10.1002/jum.14989 [DOI] [PubMed] [Google Scholar]
- 42. Lu C, Gui X, Chen W, Fung T, Novak K, Wilson SR. Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in Crohn's disease strictures. Inflamm Bowel Dis. 2017;23(3):421–30. 10.1097/mib.0000000000001020 [DOI] [PubMed] [Google Scholar]
- 43. Ripolles T, Rausell N, Paredes JM, Grau E, Martinez MJ, Vizuete J. Effectiveness of contrast‐enhanced ultrasound for characterisation of intestinal inflammation in Crohn's disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7(2):120–8. 10.1016/j.crohns.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 44. Xu C, Jiang W, Wang L, Mao X, Ye Z, Zhang H. Intestinal ultrasound for differentiating fibrotic or inflammatory stenosis in Crohn's disease: a systematic review and meta‐analysis. J Crohns Colitis. 2022. [DOI] [PubMed] [Google Scholar]
- 45. Quaia E, Gennari AG, Cova MA, van Beek EJR. Differentiation of inflammatory from fibrotic ileal strictures among patients with Crohn's disease based on visual analysis: feasibility study combining conventional B‐mode ultrasound, contrast‐enhanced ultrasound and strain elastography. Ultrasound Med Biol. 2018;44(4):762–70. 10.1016/j.ultrasmedbio.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 46. Antunes JT, Ofshteyn A, Bera K, Wang EY, Brady JT, Willis JE, et al. Radiomic features of primary rectal cancers on baseline T2 ‐weighted MRI are associated with pathologic complete response to neoadjuvant chemoradiation: a multisite study. J Magn Reson Imaging: JMRI. 2020;52(5):1531–41. 10.1002/jmri.27140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbur I, Kurowski J, Bera K, Thawani R, Achkar J.‐P, Fiocchi C, et al. Automated segmentation and radiomic characterization of visceral fat on bowel MRIs for Crohn's disease. SPIE; 2018. [Google Scholar]
- 48. Stidham RW, Enchakalody B, Waljee AK, Higgins PDR, Wang SC, Su GL, et al. Assessing small bowel stricturing and morphology in Crohn's disease using semi‐automated image analysis. Inflamm Bowel Dis. 2020;26(5):734–42. 10.1093/ibd/izz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naziroglu RE, Puylaert CAJ, Tielbeek JAW, Makanyanga J, Menys A, Ponsioen CY, et al. Semi‐automatic bowel wall thickness measurements on MR enterography in patients with Crohn's disease. Br J Radiol. 2017;90(1074):20160654. 10.1259/bjr.20160654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, Liang D, Meng J, Zhou J, Chen Z, Huang S, et al. Development and validation of a novel computed‐tomography enterography radiomic approach for characterization of intestinal fibrosis in Crohn's disease. Gastroenterology. 2021;160(7):2303–16. 10.1053/j.gastro.2021.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang B, Zhang S. The potential of radiomics in the assessment of intestinal fibrosis in Crohn's disease. Gastroenterology. 2021;161(6):2065–6. 10.1053/j.gastro.2021.06.052 [DOI] [PubMed] [Google Scholar]
- 52. Meng J, Luo Z, Chen Z, Zhou J, Chen Z, Lu B, et al. Intestinal fibrosis classification in patients with Crohn's disease using CT enterography‐based deep learning: comparisons with radiomics and radiologists. Eur Radiol. 2022. 10.1007/s00330-022-08842-z [DOI] [PubMed] [Google Scholar]
- 53. Li Z, Lu B, Lin J, He S, Huang L, Wang Y, et al. A type I collagen‐targeted MR imaging Probe for staging fibrosis in Crohn's disease. Front Mol Biosci. 2021;8:762355. 10.3389/fmolb.2021.762355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, et al. The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn's disease. Inflamm Bowel Dis. 2019;25(3):421–6. 10.1093/ibd/izy331 [DOI] [PubMed] [Google Scholar]
- 55. Lichtenstein GR, Olson A, Travers S, Diamond RH, Chen DM, Pritchard ML, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn's disease. Am J Gastroenterol. 2006;101(5):1030–8. 10.1111/j.1572-0241.2006.00463.x [DOI] [PubMed] [Google Scholar]
- 56. Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389(10080):1710–8. 10.1016/s0140-6736(17)30317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steiner CA, Berinstein JA, Louissaint J, Higgins PDR, Spence JR, Shannon C, et al. Biomarkers for the prediction and diagnosis of fibrostenosing Crohn's disease: a systematic review. Clin Gastroenterol Hepatol. 2022;20(4):817–46. 10.1016/j.cgh.2021.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Onali S, Calabrese E, Petruzziello C, Zorzi F, Sica G, Fiori R, et al. Small intestine contrast ultrasonography vs computed tomography enteroclysis for assessing ileal Crohn's disease. World J Gastroenterol. 2012;18(42):6088–95. 10.3748/wjg.v18.i42.6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pous‐Serrano S, Frasson M, Palasi Gimenez R, Sanchez‐Jorda G, Pamies‐Guilabert J, Llavador Ros M, et al. Accuracy of magnetic resonance enterography in the preoperative assessment of patients with Crohn's disease of the small bowel. Colorectal Dis. 2017;19(5):O126–O33. 10.1111/codi.13613 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current review.


