Abstract
Crohn's disease (CD) is a chronic immune‐mediated inflammatory condition which can negatively impact a patient's quality of life. The traditional management strategy for CD has focused on symptomatic control, however, this approach fails to prevent organ damage and to change the progressive course of this disease. Thus, the field has moved towards a treat‐to‐target strategy that includes identifying individualized objective targets, choosing a therapy based on individual factors that include disease severity and risk, closely monitoring disease activity at predefined time points, and optimizing therapies as needed. Due to the increasing number of therapies approved for CD, this review explores the various factors which should be considered in the sequencing of treatment options together with using the treat‐to‐target framework to control disease activity early in its course and provide holistic patient care.
Keywords: biologic treatments, Crohn's disease, inflammatory bowel disease, sequencing, treat‐to‐target
INTRODUCTION
Crohn's disease (CD), a type of inflammatory bowel disease (IBD), is a chronic, immune‐mediated inflammatory condition which primarily affects the gastrointestinal tract. 1 Uncontrolled, this inflammation may result in cumulative damage to the bowel and the development of disease‐related complications, including stricture formation with possible obstruction, fistulae and abscesses, and in those with CD involving the large intestine, an increased risk of colorectal cancer. 1 These and other disease ramifications negatively impact a patient's quality of life and ability to perform activities of daily living. 2
Traditional IBD management strategies have focused on symptomatic control, but this does not prevent bowel damage or alter the progressive course of this disease. 3 Studies demonstrate that up to 50% of patients in clinical remission continue to have evidence of objective inflammation. 4 As such, newer strategies of management have shifted from controlling symptoms alone to managing both patient symptoms and inflammation defined by endoscopic and transmural healing. 5 The goals of management include clarifying the disease type and severity, inducing remission rapidly, 6 and maintaining steroid‐free remission. Ultimately, the aim is to change the natural course of the disease, avoid hospitalization and surgery, avoid drug‐related and disease‐related complications, reduce the costs of care, and provide patients with stable functional remission.
The need to identify effective therapies and to control disease activity at early stages in the disease course has led to the adoption of the treat‐to‐target strategy for managing IBD. 7 This strategy involves identifying an objective target agreed upon by the patient and physician, choosing the initial therapies based on disease severity and risk profile, assessing the target after a predefined amount of time, and optimizing therapy to achieve the target. 7 Furthermore, the inclusion of less conventional treatment targets including traditional and novel extra‐intestinal manifestations allows for a more holistic approach to patient care. 6 This leads to the notion of choosing the right drug, for the right patient, at the right time. Additionally, with the increasing therapeutic armamentarium at the physician's disposal, this allows for a greater level of personalization in patient care.
In this article, we discuss the treat‐to‐target model for CD management and the multiple factors influencing the sequencing of therapies for patients with CD.
TREAT‐TO‐TARGET IN CROHN'S DISEASE
Rationale behind treat‐to‐target
Early and appropriate therapy, particularly in patients with CD, is associated with improved short and long‐term outcomes including a reduction in hospitalization and surgery. 8 It is thought that using a proactive treatment and monitoring strategy will increase the likelihood of achieving disease control, endoscopic healing, and disease‐related complications. Taken together, this should also reduce health‐related costs and burden on the health system. 9
Multiple studies have investigated whether a treat‐to‐target strategy can achieve improved outcomes when compared with routine clinical management. One of the first studies introducing this concept in CD was a retrospective study by Bouguen et al. In this study, the authors investigated whether adjusting therapy based on the presence of endoscopic lesions resulted in higher rates of endoscopic healing, defined as no ulcerations. They found that patients undergoing more frequent endoscopic evaluation (HR 2.35 95% CI 1.15–4.97, p = 0.019) and therapeutic optimization (HR 4.28 95% CI 1.9–11.5, p = 0.0003) were significantly more likely to achieve endoscopic healing. 10
The prospective, ‘Randomized Evaluation of an Algorithm for Crohn's Treatment (REACT)’ study showed that patients randomized to the early combined immunosuppression arm with an anti‐tumor necrosis factor agent (anti‐TNF) and an antimetabolite based on clinical symptoms (Harvey‐Bradshaw Index ≤4) had lower rates of major adverse outcomes (occurrence of surgery, hospital admission or serious disease related complications) when compared with patients receiving conventional management (HR, 0.73; 95% CI, 0.62–0.86; p = 0.0003). 11 The limitations of this study include using clinical symptoms alone to guide management as opposed to using objective measures of remission as a treatment target. The multi‐center randomized CALM study, in comparison, used objective biochemical markers, C‐reactive protein (CRP) and fecal calprotectin, to guide therapeutic decisions in the treat‐to‐target arm, with a primary endpoint of ‘mucosal healing’, defined as a CD Endoscopic Index of Severity score of <4. Therapeutic decisions based on these objective markers achieved superior clinical and endoscopic outcomes in patients with CD. 12 Interestingly the recent randomized, prospective trial, STARDUST, which compared symptom‐based to treat‐to‐target management based on endoscopic findings in patients receiving Ustekinumab found no difference in the endoscopic response. 13 However, when stratified by baseline disease severity (either endoscopic or history of bowel damage), patients with a more severe disease phenotype at baseline achieved higher rates of endoscopic healing. Overall, these data support the use of treat‐to‐targets in CD particularly in patients with a history of severe/complicated disease.
The possible limitations of the treat‐to‐target strategy include that it can be labor intensive and costly in terms of the repeated testing and the time patients need to invest in the treatment and monitoring plans. However, there are some data showing its cost‐effectiveness. 9 Additionally, as shown in the STARDUST trial, the treat‐to‐target strategy may not be appropriate for all patients.
Selecting the right target for the right patient
The treat‐to‐target strategy involves obtaining a baseline assessment of disease activity and severity, communicating with the patient to identify individualized targets, choosing an initial therapy based on individual risk factors, reassessing the target early with monitoring during both the active and quiescent phase, and optimizing treatment strategies. Biochemical markers of inflammation include C‐reactive protein (CRP), the Endoscopic Healing Index (EHI), and fecal calprotectin. Endoscopic options include colonoscopy, esophagogastroduodenoscopy, and capsule endoscopy, and radiologic imaging options include computed tomography enterography (CTE), magnetic resonance enterography (MRE), and intestinal ultrasound. These provide objective markers for inflammation and provide multiple options for disease monitoring, 14 but all of these must be benchmarked at a baseline time of known active disease in an individual patient. Benchmarking usually occurs at the time of ileocolonoscopy and labs and before treatment is initiated or changed. If the patient has not achieved the target at the agreed upon timepoint, assess adherence, adjust dosing, add a therapy, or change the therapy and then reassess the target.
The Selecting Therapeutic Targets in Inflammatory Bowel Disease Endpoints (STRIDE) consortium has provided consensus on the various goals or targets when developing a treatment and monitoring strategy with the patient. These are separated into short, intermediate, and long‐term targets, which if achieved, will lead to sustained functional remission. The short‐term goals include symptomatic response and remission. Intermediate goals include normalization of inflammatory markers such as CRP and fecal calprotectin and, importantly, achieving normal growth and development in children. 6 Long‐term goals include endoscopic healing, normalized quality of life, and the absence of disability 6 (defined using the IBD‐Disability Index 15 ). The group has defined clinical remission in CD as the resolution of abdominal pain and altered bowel habits and endoscopic remission as the resolution of ulceration at ileocolonoscopy or the resolution of inflammation by cross sectional imaging if ileocolonoscopy cannot adequately assess inflammation. 16 While not formally included in STRIDE, another objective goal includes transmural healing in patients with CD, which has been associated with improved long‐term outcomes including lower rates of hospitalization, therapy escalation, steroid use, and surgery. 17 , 18 From a practical point of view, timing of disease assessment and target assessment is an evolving priority. Although prior assessments were recommended in a timeframe of months, more recent updates and our own practice suggests that most effective therapies can be reassessed using benchmarked targets including fecal calprotectin and bowel wall thickness as assessed by intestinal ultrasound in 6 weeks or even sooner. 19 , 20 , 21
In addition to these clear goals, other less considered elements should be examined. The induction of remission should be rapid—from a patient perspective this would allow time to return to daily activities and reduce disability‐related loss of functionality. Another important aspect of rapid induction of remission is reducing the exposure to steroid therapy and ensuring that any therapy or goal is achieved off steroids. Other goals should include addressing the common extra‐intestinal manifestations (EIMs) of joint pain or skin involvement as well as the less commonly considered EIMs such as pain, fatigue, mental health disorders, and sexual dysfunction. 6 Fatigue has been associated with poor health‐related quality of life, 22 and patients have reported that CD negatively impacts their sexual function. 23 Additionally, high rates of anxiety and depression in patients with active and quiescent CD suggests the need for standardization of screening for mental health disorders in these patients. 24 By clearly defining and achieving these goals, we can hopefully modify the natural history of IBD. Furthermore, as these goals may vary from patient to patient, they may provide a rationale for therapeutic sequencing patterns in CD management. In Figure 1, we provide a practical approach to incorporating treat‐to‐target.
FIGURE 1.
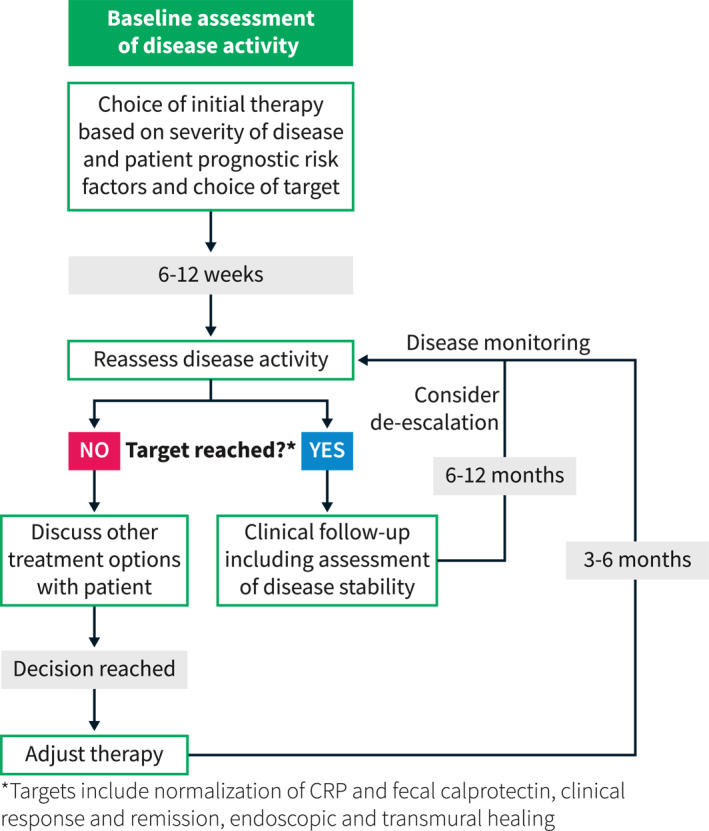
A practical approach to incorporating treat‐to‐target decision making into clinical practice. Modified from Christensen and Rubin. 25
SEQUENCING THERAPIES IN CD
The ‘step‐up’ strategy
Traditionally, the management of IBD has followed the so‐called ‘step‐up’ strategy which focused primarily on gastrointestinal symptomatic relief and endoscopic remission. In this strategy, aminosalicylates, steroids, and immunomodulators such as thiopurines and methotrexate were used as first line therapies. If these therapies failed or were not adequate, treatments were changed to advanced therapies such as biologics or small molecules. This strategy for disease management has numerous shortfalls. The primary deficit is that it lacks any degree of risk stratification and personalization of therapy based on the individual patients and their specific disease characteristics, and patients must get sicker or suffer disease progression or a complication before they can be moved up to other options. Furthermore, newer therapies are positioned agnostically to existing ones. As such, disease and patient related factors (family history of IBD, young age, smoking history, history of appendectomy, extent of inflammation, perianal or penetrating disease, endoscopic findings of deep or large ulcerations, histologic findings such as granulomas and poor or inadequate response to initial induction therapy), which may influence a specific therapeutic decision, are not taken into account. Lastly, the ‘step‐up’ strategy by definition does not provide guidance for therapy de‐escalation and assumes that the therapy used for induction will dictate what is later used for maintenance.
Considerations beyond the ‘step up approach’
When choosing a CD therapy, it is important to consider a variety of factors including the patient's disease activity, severity and duration, co‐morbid illnesses, the accessibility and affordability of the treatment, the patient's lifestyle and preferences, and the involvement of extra‐intestinal manifestations. It is necessary to discuss these factors and choose objective targets in a shared decision‐making process with the patient. Another important aspect to consider is that the benefit to risk ratio is not a constant throughout a patient's illness. During the acute phase in patients who are symptomatic or have risk factors for severe disease, a more aggressive approach may be warranted. This is in contrast to other situations where safety considerations (elderly, or immune suppressed patients), patient preference (mode of administration, frequency of dosing) and access to care (insurance coverage and cost and time to patient) may weigh in more in the deliberation.
Biologic therapies as a first line treatment
Clinical trials have repeatedly demonstrated that early initiation of a biologic therapy either alone or in combination with an immune modulator is superior to immune modulator alone regardless of the biologic chosen. 26 , 27 However, a question regarding the sequencing of biologics in patients with moderate to severe CD remains. The SEAVUE study, a randomized, double blind, parallel group, active‐controlled phase 3b trial, found that ustekinumab and adalimumab are bot highly effective in treating moderately to‐severely active Crohn's disease in biologically naïve patients. 28 A network meta‐analysis of 18 clinical trials by Singh and colleagues found that in biologic‐naïve patients, infliximab and adalimumab ranked the highest for inducing clinical remission and endoscopic improvement in patients with moderate to severe CD. 29 In patients with prior anti‐TNF exposure, ustekinumab was superior for the induction of clinical remission and may be the preferred second‐line agent. 30 Similarly, a comparison of ustekinumab with vedolizumab as a second line therapy after anti‐TNF found that ustekinumab is more effective in maintenance, but both are just as effective in induction. 31 These data highlight that factors other than clinical disease severity should help guide therapy choice, and perhaps the use of certain therapies for induction and other for maintenance is feasible.
Clinical severity guiding initial therapy choice
The treat‐to‐target paradigm assesses multiple factors affecting the patient and provides a more personalized and rational approach to drug selection. A symptomatic patient who is experiencing a significantly reduced quality of life will have very different treatment goals compared to a patient who is in clinical remission but still has objective evidence of disease. This highlights that no single strategy is a perfect fit for all patients, and there needs to be a certain adaptability in the physician's approach. In the above example, a symptomatic patient will require a therapy with rapid induction qualities with the target being symptomatic remission and return to day‐to‐day functioning. As such, steroids may be the primary induction agent used and the response to steroids could inform downstream decisions regarding subsequent therapy.
Considering disease phenotype and location
Therapy considerations also differ depending on disease phenotype and location. Perianal involvement, for example, provides unique therapeutic considerations. In these patients, in whom improvement or resolution of perianal disease is the target, an aggressive therapy approach with the use of anti‐TNF as a first line agent in combination with an immune modulator and antibiotics is most approprite. 32 , 33 In this scenario, once the target is reached—decrease drainage, closure of fistula openings—therapy can be de‐escalated with the cessation of antibiotics and then continued monitoring performed. While vedolizumab and ustekinumab have also shown to be effective in treating fistulizing CD, the most robust data are for anti‐TNFs and as such these should ideally be used as first line therapies in such circumstances. 34 , 35 Additionally, it has been shown that patients with colonic CD have greater response rates to vedolizumab when compared with patients with ileal involvement. These data indicate that disease location should be taken into account when deciding upon medications particularly when considering use of vedolizumab. 36
EIMS and co‐existing immune conditions affect therapy choice
Another factor to consider when choosing a therapy is the presence of co‐existing immune conditions or EIMs where therapeutic targets are different and include resolution of skin lesions, joint pain and inflammation, eye inflammation or neurological outcomes. For example, in patients with concomitant plaque psoriasis or psoriatic arthritis, the primary drug considerations should be anti‐TNF, 37 , 38 ustekinumab, 39 , 40 methotrexate, 41 or risankizumab, 42 , 43 all shown to be effective for both conditions. Additionally, JAK inhibitors can be considered in patients with psoriatic arthritis, 37 but by label, after anti‐TNF therapy has been used. Rizankizumab has been recently approved for use in patients with moderate to severe CD. However, its sequencing in relation to ustekinumab is unclear at this time. Despite this, in patients with moderate‐to‐severe plaque psoriasis, risankizumab was more effective in achieving a clinical response that ustekinumab indicating that in patients with both conditions, rizankizumab may be preferable. 43 Patients with rheumatoid arthritis should receive either an anti‐TNF, 44 JAK inhibitor, 45 or methotrexate. 46 It is notable that the cytokine IL‐23 is not expressed on joints and the anti‐integrin therapy vedolizumab may not treat an independent or parallel joint process either. Currently, JAK inhibitors are not approved for the treatment of CD. However, the phase 2 and 3 trials of upadacitinib in CD are promising and indicate its use in co‐existing immune conditions is a viable treatment option. 47 Other EIMS and co‐existing immune conditions are detailed in Figure 2. Additional considerations for sequencing medications in Crohn's disease are outlined in Figure 3.
FIGURE 2.
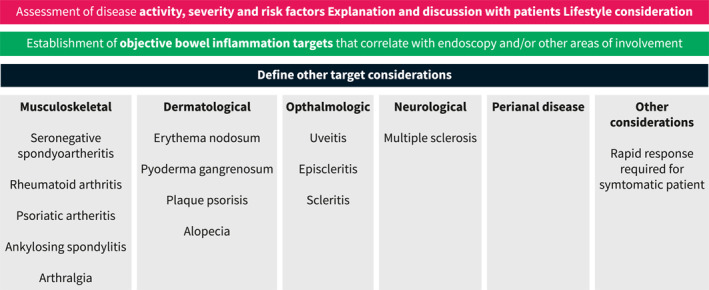
Considering therapeutic options based on co‐existing immune conditions or extraintestinal manifestations
FIGURE 3.
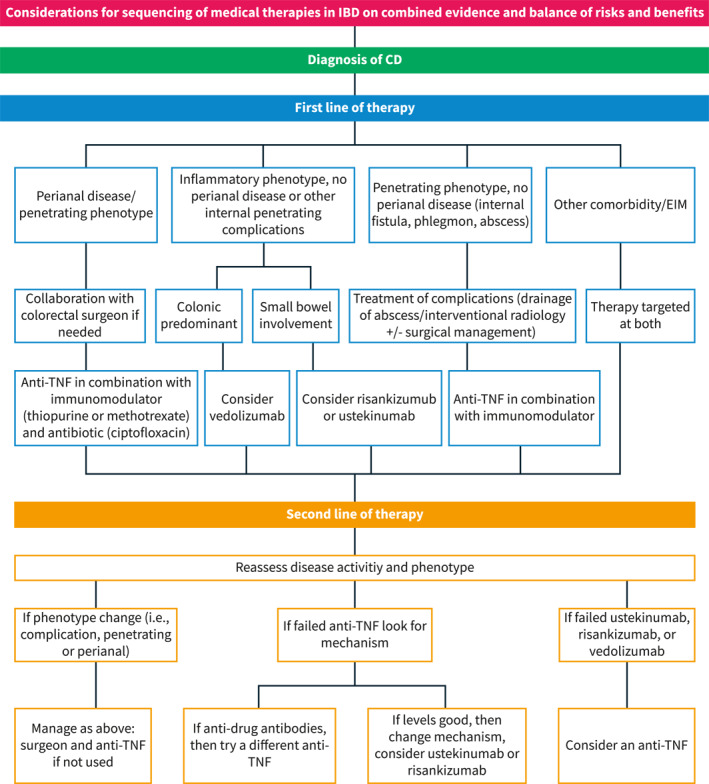
Considerations for sequencing of medical therapies in Crohn's disease
Future directions for therapeutic sequencing algorithms
Prediction of response to therapy is the subject of ongoing investigation. This interesting and much desired goal is being looked at from multiple directions. The gut microbiome is of interest and studies have shown that certain gut microbial signatures may be predictive of response to vedolizumab, infliximab and ustekinumab. 48 , 49 , 50 Of interest is whether gut microbial manipulation prior to initiating a biologic can increase response rates. Other studies have shown certain genomic and proteomic markers can predict treatment response in CD patients to anti‐TNFs. 51 , 52 , 53 , 54 , 55 Future strategies include biomarker driven selections and considering combination therapies including vedolizumab or anti‐IL‐23 plus a JAK inhibitor, or anti‐IL‐23 plus anti‐TNF. Initial data from real‐world studies have shown early promise and safety of combination biologic therapy. 56 , 57
CONCLUSION
The treat‐to‐target management strategy in CD promotes the open communication between the patient and provider to identify personalized targets and choose an initial therapy with continuous monitoring of the targets and optimization of therapies. It incorporates both patients' reported symptoms and inflammation assessed through benchmarked biomarkers and endoscopy to guide treatment options with the goal of controlling the inflammation, preventing organ damage and improving quality of life. With the increasing number of available treatment options for CD, it is important to consider a variety of factors before choosing a therapy. Choosing therapies based on activity and severity of the disease, co‐morbid illnesses, the phase of the disease, and accessibility and affordability provide a rational approach to sequencing therapies and may result in improved disease‐related outcomes.
CONFLICT OF INTEREST
NMG has no relevant disclosures. NAC has served as a consultant for Seres Pharmaceuticals and Iterative Scopes. DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Arena Pharmaceuticals, Bristol‐Myers Squibb, Genentech/Roche, Gilead Sciences, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Pfizer, Prometheus Biosciences, Takeda, and Techlab Inc.
Garcia NM, Cohen NA, Rubin DT. Treat‐to‐target and sequencing therapies in Crohn's disease. United European Gastroenterol J. 2022;10(10):1121–8. 10.1002/ueg2.12336
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated, or the article describes entirely theoretical research.
REFERENCES
- 1. Torres J, Mehandru S, Colombel JF, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741–55. 10.1016/s0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 2. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10–20. 10.1016/j.crohns.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 3. Peyrin‐Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, et al. Clinical disease activity, C‐reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014;63(1):88–95. 10.1136/gutjnl-2013-304984 [DOI] [PubMed] [Google Scholar]
- 4. Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18(9):1634–40. 10.1002/ibd.21925 [DOI] [PubMed] [Google Scholar]
- 5. Colombel JF, Narula N, Peyrin‐Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–61. e355. 10.1053/j.gastro.2016.09.046 [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, D'Haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and strategies to support a treat‐to‐target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–66. 10.1093/ecco-jcc/jjz131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D’Haens GR, et al. Deep remission at 1 Year prevents progression of early crohn's disease. Gastroenterology. 2020;159(1):139–47. 10.1053/j.gastro.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lakatos PL, Kaplan GG, Bressler B, Khanna R, Targownik L, Jones J, et al. Cost‐effectiveness of tight control for crohn's disease with adalimumab‐based treatment: economic evaluation of the CALM trial from a Canadian perspective. J Can Assoc Gastroenterol. 2022;5(4):169–76. 10.1093/jcag/gwac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(6):978–85. 10.1016/j.cgh.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 11. Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386(10006):1825–34. 10.1016/s0140-6736(15)00068-9 [DOI] [PubMed] [Google Scholar]
- 12. Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vanasek T, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–89. 10.1016/s0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Vermeire S, D'Haens G, Panés J, Dignass A, Magro F, et al. Treat to target versus standard of care for patients with Crohn's disease treated with ustekinumab (STARDUST): an open‐label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol. 2022;7(4):294–306. [DOI] [PubMed] [Google Scholar]
- 14. Peyrin‐Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14(3):348–54. e317. 10.1016/j.cgh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 15. Chan W, Shim HH, Lim MS, Sawadjaan FLB, Isaac SP, Chuah SW, et al. Symptoms of anxiety and depression are independently associated with inflammatory bowel disease‐related disability. Dig Liver Dis. 2017;49(12):1314–19. 10.1016/j.dld.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 16. Peyrin‐Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat‐to‐target. Am J Gastroenterol. 2015;110(9):1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 17. Fernandes SR, Rodrigues RV, Bernardo S, Cortez‐Pinto J, Rosa I, da Silva JP, et al. Transmural healing is associated with improved long‐term outcomes of patients with crohn's disease. Inflamm Bowel Dis. 2017;23(8):1403–9. 10.1097/mib.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 18. Castiglione F, Imperatore N, Testa A, De Palma GD, Nardone OM, Pellegrini L, et al. One‐year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026–39. 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 19. Narula N, Wong ECL, Dulai PS, Marshall JK, Colombel JF, Reinisch W. Week 6 calprotectin best predicts likelihood of long‐term endoscopic healing in crohn's disease: a post‐hoc analysis of the UNITI/IM‐UNITI trials. J Crohns Colitis. 2021;15(3):462–70. 10.1093/ecco-jcc/jjaa189 [DOI] [PubMed] [Google Scholar]
- 20. Pauwels RWM, van der Woude CJ, Erler NS, de Vries AC. Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease. Therap Adv Gastroenterol. 2020;13:1756284820979765. 10.1177/1756284820979765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Voogd F, Bots S, Gecse K, Gilja OH, D'Haens G, Nylund K. Intestinal ultrasound early on in treatment follow‐up predicts endoscopic response to anti‐TNFα treatment in Crohn's Disease. J Crohns Colitis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen BL, Zoëga H, Shah SA, LeLeiko N, Lidofsky S, Bright R, et al. Fatigue is highly associated with poor health‐related quality of life, disability and depression in newly‐diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39(8):811–22. 10.1111/apt.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marín L, Mañosa M, Garcia‐Planella E, Gordillo J, Zabana Y, Cabre E, et al. Sexual function and patients' perceptions in inflammatory bowel disease: a case‐control survey. J Gastroenterol. 2013;48(6):713–20. 10.1007/s00535-012-0700-2 [DOI] [PubMed] [Google Scholar]
- 24. Mikocka‐Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22(3):752–62. 10.1097/mib.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 25. Christensen B, Rubin DT. In: Baumgart D, editor. Crohn’s disease and ulcerative colitis. 2nd ed. Springer Nature; 2017. [Google Scholar]
- 26. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362(15):1383–95. 10.1056/nejmoa0904492 [DOI] [PubMed] [Google Scholar]
- 27. Ben‐Horin S, Novack L, Mao R, Guo J, Zhao Y, Sergienko R, et al. Efficacy of biologic drugs in short‐duration versus long‐duration inflammatory bowel disease: a systematic review and an individual‐patient data meta‐analysis of randomized controlled trials. Gastroenterology. 2022;162(2):482–94. 10.1053/j.gastro.2021.10.037 [DOI] [PubMed] [Google Scholar]
- 28. Sands BE, Irving PM, Hoops T, et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic‐naive patients with moderately to severely active Crohn's disease: a multicentre, randomised, double‐blind, parallel‐group, phase 3b trial. Lancet. 2022;399(10342):2200–11. [DOI] [PubMed] [Google Scholar]
- 29. Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta‐analysis: first‐ and second‐line biologic therapies for moderate‐severe Crohn's disease. Aliment Pharmacol Ther. 2018;48(4):394–409. 10.1111/apt.14852 [DOI] [PubMed] [Google Scholar]
- 30. Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107(2):262–72. 10.1038/ajg.2011.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parrot L, Dong C, Carbonnel F, Meyer A. Systematic review with meta‐analysis: the effectiveness of either ustekinumab or vedolizumab in patients with Crohn's disease refractory to anti‐tumour necrosis factor. Aliment Pharmacol Ther. 2022;55(4):380–8. 10.1111/apt.16714 [DOI] [PubMed] [Google Scholar]
- 32. Lichtenstein GR, Loftus EV, Jr. , Isaacs KL, Regueiro MD, Gerson LB, Sands BE. Correction: ACG clinical guideline: management of crohn's disease in adults. Am J Gastroenterol. 2018;113(7):1101. 10.1038/s41395-018-0120-x [DOI] [PubMed] [Google Scholar]
- 33. West RL, van der Woude CJ, Hansen BE, Felt‐Bersma RJF, van Tilburg AJP, Drapers JAG, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab: a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2004;20(11‐12):1329–36. 10.1111/j.1365-2036.2004.02247.x [DOI] [PubMed] [Google Scholar]
- 34. Feagan BG, Schwartz D, Danese S, Rubin DT, Lissoos TW, Xu J, et al. Efficacy of vedolizumab in fistulising crohn's disease: exploratory analyses of data from GEMINI 2. J Crohns Colitis. 2018;12(5):621–6. 10.1093/ecco-jcc/jjy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Godoy Brewer GM, Salem G, Afzal MA, Limketkai BN, Haq Z, Tajamal M, et al. Ustekinumab is effective for perianal fistulising Crohn's disease: a real‐world experience and systematic review with meta‐analysis. BMJ Open Gastroenterol. 2021;8(1):e000702. 10.1136/bmjgast-2021-000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vermeire S, D'Haens G, Baert F, Danese S, Kobayashi T, Loftus EV, et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active crohn's disease: results from the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16(1):27–38. 10.1093/ecco-jcc/jjab133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila‐Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–50. 10.1056/nejmoa1615975 [DOI] [PubMed] [Google Scholar]
- 38. Lebwohl M, Blauvelt A, Paul C, Sofen H, Weglowska J, Piguet V, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double‐blind, etanercept‐ and placebo‐controlled study (CIMPACT). J Am Acad Dermatol. 2018;79(2):266–76. e265. 10.1016/j.jaad.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 39. McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double‐blind, placebo‐controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9. 10.1016/s0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- 40. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–74. 10.1016/s0140-6736(08)60725-4 [DOI] [PubMed] [Google Scholar]
- 41. Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann‐Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque‐type psoriasis (METOP): a 52 week, multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;389(10068):528–37. 10.1016/s0140-6736(16)32127-4 [DOI] [PubMed] [Google Scholar]
- 42. Kristensen LE, Keiserman M, Papp K, McCasland L, White D, Lu W, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24‐week results from the randomised, double‐blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–31. 10.1136/annrheumdis-2021-221019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60. 10.1056/nejmoa1607017 [DOI] [PubMed] [Google Scholar]
- 44. Elliott MJ, Maini RN, Feldmann M, Kalden J, Antoni C, Smolen J, et al. Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–10. 10.1016/s0140-6736(94)90628-9 [DOI] [PubMed] [Google Scholar]
- 45. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507. 10.1056/nejmoa1109071 [DOI] [PubMed] [Google Scholar]
- 46. Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gomor B, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatol. 2000;39(6):655–65. 10.1093/rheumatology/39.6.655 [DOI] [PubMed] [Google Scholar]
- 47. Sandborn WJ, Feagan BG, Loftus EV, Jr. , Peyrin‐Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with crohn's disease. Gastroenterology. 2020;158(8):2123–38. e2128. 10.1053/j.gastro.2020.01.047 [DOI] [PubMed] [Google Scholar]
- 48. Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, et al. Gut microbiome function predicts response to anti‐integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21(5):603–10. e603. 10.1016/j.chom.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018;3(1). 10.1128/msystems.00188-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doherty MK, Ding T, Koumpouras C, Telesco SE, Monast C, Das A, et al. Fecal microbiota signatures are associated with response to ustekinumab therapy among crohn's disease patients. mBio. 2018;9(2). 10.1128/mbio.02120-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang MH, Friton JJ, Raffals LE, Leighton JA, Pasha SF, Picco MF, et al. Novel genetic risk variants can predict anti‐TNF agent response in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(8):1036–43. 10.1093/ecco-jcc/jjz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Medina‐Medina R, Iglesias‐Flores E, Benítez JM, Marin‐Pedrosa S, Salgueiro I, Ferrin G, et al. P008 Proteomic markers of response to anti‐TNF drugs in patients with Crohn's disease. J Crohn's Colitis. 2019;13(Supplment_1):S090. 10.1093/ecco-jcc/jjy222.132 [DOI] [Google Scholar]
- 53. Bank S, Julsgaard M, Abed OK, Burisch J, Broder Brodersen J, Pedersen NK, et al. Polymorphisms in the NFkB, TNF‐alpha, IL‐1beta, and IL‐18 pathways are associated with response to anti‐TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49(7):890–903. 10.1111/apt.15187 [DOI] [PubMed] [Google Scholar]
- 54. Belarif L, Danger R, Kermarrec L, Nerriere‐Daguin V, Pengam S, Durand T, et al. IL‐7 receptor influences anti‐TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest. 2019;129(5):1910–25. 10.1172/jci121668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pierre N, Baiwir D, Huynh‐Thu VA, Mazzucchelli G, Smargiasso N, De Pauw E, et al. Discovery of biomarker candidates associated with the risk of short‐term and mid/long‐term relapse after infliximab withdrawal in Crohn's patients: a proteomics‐based study. Gut. 2020;70(8):1450–7. 10.1136/gutjnl-2020-322100 [DOI] [PubMed] [Google Scholar]
- 56. Kwapisz L, Raffals LE, Bruining DH, Pardi DS, Tremaine WJ, Kane SV, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19(3):616–17. 10.1016/j.cgh.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 57. Eronen H, Kolehmainen S, Koffert J, Koskinen I, Oksanen P, Jussila A, et al. Combining biological therapies in patients with inflammatory bowel disease: a Finnish multi‐centre study. Scand J Gastroenterol. 2022;57(8):936–41. 10.1080/00365521.2022.2045350 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable—no new data generated, or the article describes entirely theoretical research.


