Abstract
The vaccine potential of a combination of three pneumococcal virulence proteins was evaluated in an active-immunization–intraperitoneal-challenge model in BALB/c mice, using very high challenge doses of Streptococcus pneumoniae. The proteins evaluated were a genetic toxoid derivative of pneumolysin (PdB), pneumococcal surface protein A (PspA), and a 37-kDa metal-binding lipoprotein referred to as PsaA. Mice immunized with individual proteins or combinations thereof were challenged with high doses of virulent type 2 or type 4 pneumococci. The median survival times for mice immunized with combinations of proteins, particularly PdB and PspA, were significantly longer than those for mice immunized with any of the antigens alone. A similar effect was seen in a passive protection model. Thus, combinations of pneumococcal proteins may provide the best non-serotype-dependent protection against S. pneumoniae.
Streptococcus pneumoniae continues to be a major cause of life-threatening invasive diseases such as pneumonia, meningitis, and bacteremia, as well as other highly prevalent albeit less serious infections, such as otitis media and sinusitis (3, 16, 41). Pneumococcal infections are prevalent throughout the world, and children under the age of 5 years, the elderly, and immunocompromised individuals are particularly susceptible (3, 16, 24). Mortality from pneumococcal disease is particularly high in developing countries, where pneumococcal pneumonia has been estimated to account for 20 to 25% of all deaths in children under the age of 5 years (46). Global management of pneumococcal disease is also being complicated by the alarming rate at which this organism is acquiring resistance to multiple antimicrobials (19).
The limitations of the currently available polyvalent vaccine formulations comprising purified pneumococcal capsular polysaccharide (PS) are well documented. These include the facts that the PS vaccines confer strictly serotype-specific protection and that the present formulation contain only 23 of the 90 known serotypes. PS are also T-cell-independent antigens and are poorly immunogenic in children under 2 years of age (15, 20). Protein-PS conjugate vaccines that are currently undergoing clinical trials, although highly immunogenic (21, 23, 36) have more limited serotype coverage. Moreover, they are likely to be expensive, and this may limit their deployment in developing countries where they are needed most. Pneumococcal conjugate vaccines have been shown to be capable of eliminating nasopharyngeal carriage of vaccine serotypes, but there is evidence from some studies that there is a concomitant increase in carriage of non-vaccine serotypes (30). These included types known to be capable of causing invasive disease, and so the actual reduction in the overall incidence of pneumococcal disease achieved by introduction of conjugate vaccines with limited serotype coverage may be less than expected.
The known and potential shortcomings of existing vaccination strategies have necessitated research into development of new cheap and effective vaccines against pneumococcal disease. Studies in our laboratories have been directed towards understanding the mechanism of pathogenesis of S. pneumoniae with a view to developing vaccines based on protein antigens common to all serotypes. Such proteins, being T-cell-dependent antigens, are likely to be highly immunogenic in human infants and, moreover, to elicit immunological memory. In addition, they may provide a degree of protection against all serotypes. So far, the three proteins which have shown the greatest promise as vaccine antigens are the thiol-activated toxin pneumolysin (9), pneumococcal surface protein A (PspA) (13, 48), and a 37-kDa metal-binding lipoprotein referred to as PsaA (7, 14). Each of these proteins has been shown to elicit a significant level of protection in animal models against one or more S. pneumoniae serotypes (1, 10, 11, 25, 35, 42, 43, 47; E. W. Ades, J. S. Sampson, D. E. Briles, J. D. King, B. De, R. C. Huebner, and G. M. Carlone, Program Abstr. Pneumococcal Vaccines World 1998 Conf., p. 29, session 4, 1998). Comparative sequence analyses indicate that the genes encoding pneumolysin and PsaA are highly conserved among diverse capsular serotypes of S. pneumoniae (7, 27, 40), but there is marked heterogeneity in the region encoding the amino-terminal portion of PspA (13, 48, 49). Nevertheless, PspA contains conserved epitopes which result in protection against diverse capsular and PspA types after immunization with a single PspA antigen (10, 11, 25, 43).
All available evidence suggests that pneumolysin, PspA, and PsaA contribute to the virulence of S. pneumoniae (4–8, 14, 26, 29, 32, 34) but act at different stages of the pathogenic process (31, 33, 39). Thus, immunization with a combination of these proteins may provide a higher degree of protection than immunization with any of the antigens alone. This possibility was examined in the present study. For this study we used high challenge doses in an effort to enhance our ability to detect additive protective effects of immunizations with combinations of pneumolysin, PspA, and PsaA over that achieved with any of these antigens alone.
Preparation of antigens.
Pneumococcal antigens were purified from recombinant Escherichia coli expressing the respective cloned gene. The original source of the gene in each case was a capsular type 2 strain, D39 (2). For pneumolysin, a mutated gene encoding a derivative with a Trp-433→Phe substitution was used. This genetic toxoid, designated PdB, has only 0.1% residual cytotoxic activity relative to the native toxin but retains full immunogenicity, and it was purified as previously described (1, 36). PsaA was expressed as a His6-tagged fusion protein and purified by Ni-nitrilotriacetic acid affinity chromatography (37). A 43-kDa N-terminal portion of PspA was also expressed as a His6-tagged fusion protein and purified by Ni-nitrilotriacetic acid affinity chromatography. This truncated PspA has been shown to elicit cross-protective immunity against pneumococcal challenge in mice (43, 49). All antigens were >95% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with Coomassie brilliant blue R250.
Immunization of mice and antibody responses.
For each experiment, eight groups of 5- to 6-week-old male BALB/c mice (14 or 15 per group) were immunized intraperitoneally with either PdB alone, PsaA alone, PspA alone, PdB plus PsaA, PdB plus PspA, PsaA plus PspA, PdB plus PsaA plus PspA, or a placebo. Each mouse received three doses of 5 μg of each antigen alone or in combination in 50 μg of alum adjuvant (Imject alum no. 77161; Pierce, Rockford, Ill.) at 10- to 12-day intervals. The mice given the placebo received an identical course of saline plus alum.
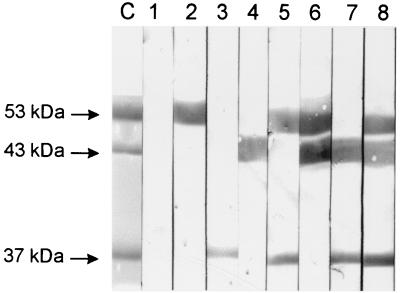
Sera were collected from mice by retro-orbital bleeding 1 week after the third immunization. The sera were pooled on a group-by-group basis and analyzed by enzyme-linked immunosorbent assay and Western blotting for specific antibodies to each of the purified protein antigens. As shown in Table 1, strong, antigen-specific antibody responses were generated in mice immunized with the pneumolysin toxoid (PdB), PsaA, and PspA when administered alone. Furthermore, there was no obvious dimunition in antigen-specific antibody titer when the antigens were administered in combination with others, indicating that there was no detectable antagonistic effect of combining the antigens. Western blot analysis of the purified proteins also demonstrated specific antibody responses to each of the antigens (Fig. 1). Similar results were obtained with whole-cell lysates of D39, with the exception that anti-PspA reacted with the full-length PspA of approximately 86 kDa (data not shown).
TABLE 1.
Antibody titers obtained from mice immunized with PdB, PspA, and PsaA
| Immunization group | Antibody titer (ELISA)a against:
|
||
|---|---|---|---|
| PdB | PsaA | PspA | |
| Placebo | —b | — | — |
| PdB | 28,000 | — | — |
| PsaA | — | 18,500 | — |
| PspA | — | — | 150,000 |
| PdB-PsaA | 40,000 | 18,500 | — |
| PdB-PspA | 50,000 | — | 62,000 |
| PsaA-PspA | — | 30,500 | 140,000 |
| PdB-PsaA-PspA | 45,000 | 38,000 | 50,000 |
ELISA titers were determined as the reciprocal of the dilution of serum giving 50% of the highest absorbance reading above the background at 405 nm.
—, <200.
FIG. 1.
Western blot of purified PdB (53 kDa), truncated PspA (43 kDa), and PsaA (37 kDa), showing specificity of antibody responses to the various protein antigens. The proteins were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose. They were then reacted with specific antisera generated from mice immunized with the various protein combinations. Lane C, Coomassie blue-stained gel showing the relative positions of the proteins. Lanes 1 to 8, nitrocellulose membrane reacted with normal mouse serum (lane 1), anti-PdB (lane 2), anti-PsaA (lane 3), anti-PspA (lane 4), anti-PdB-PsaA (lane 5), anti-PdB-PspA (lane 6), anti-PsaA-PspA (lane 7), and anti-PdB-PsaA-PspA (lane 8).
Challenge.
Intraperitoneal-challenge experiments were carried out 2 weeks after the third immunization of mice, and two separate experiments were performed in parallel using two S. pneumoniae strains. These were D39, a virulent type 2 strain (2), and WCH43, a virulent type 4 clinical isolate from the Women's and Children's Hospital, North Adelaide, South Australia, Australia.
Before challenge, the bacteria were grown at 37°C overnight on blood agar and then inoculated into serum broth consisting of 10% (vol/vol) horse serum in meat extract broth. They were then grown statically for 3 h at 37°C to give approximately 108 CFU/ml. Serotype-specific capsule production was confirmed by quellung reaction using antisera obtained from Statens Seruminstitut, Copenhagen, Denmark. Each immunized BALB/c mouse was then infected with approximately 107 CFU of either the capsular type 2 strain (D39) or the type 4 strain (WCH43). This dose was equivalent to approximately 105 times the 50% lethal dose (LD50) of both strains for BALB/c mice.
The survival of the intraperitoneally challenged mice was closely monitored for 21 days. Differences in median survival time between groups were analyzed by the Mann-Whitney U test (one tailed). Differences in the overall survival rate between groups were analyzed by the Fisher exact test.
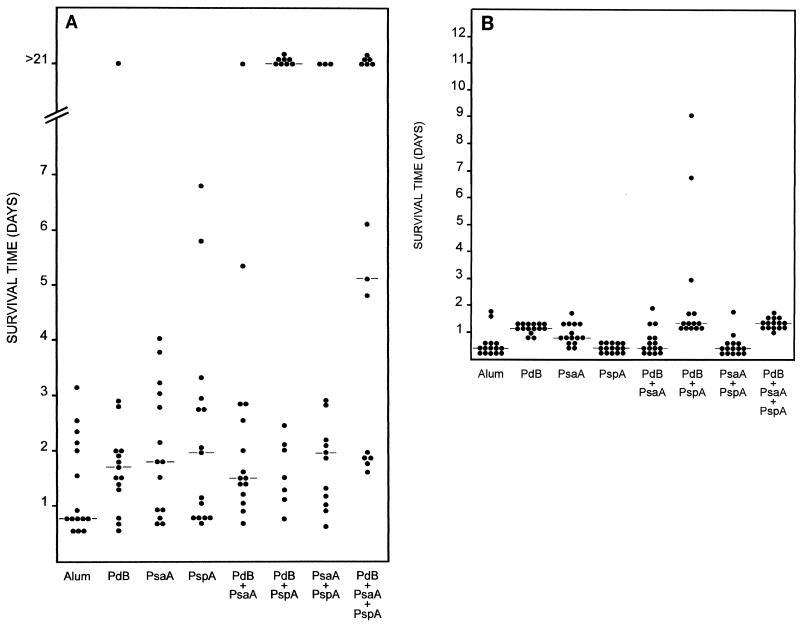
Figure 2A shows the results obtained when the mice were challenged with the highly virulent capsular type 2 strain D39. Given the high challenge dose, it is not surprising that the median survival time for mice that received the alum placebo was less than 1 day and that all of the mice in this group died in just over 3 days. Although mice that received either PdB, PsaA, or PspA alone survived longer than those in the placebo group, the median survival times of 1.7, 1.8, and 2 days, respectively, were not significantly different from that of the placebo group (Table 2). However, all groups of mice that received combinations of the antigens had significantly longer median survival times than the placebo group. Interestingly, mice that received a combination of PdB and PsaA or PsaA and PspA did not survive significantly longer than those that received the single antigens alone. In contrast, mice that were immunized with PdB plus PspA survived significantly longer than those that were immunized with PdB alone (P = 0.01), PsaA alone (P < 0.025), or PspA alone (P < 0.01). The level of protection obtained with the PdB-PspA combination was very similar to that obtained with the PdB-PsaA-PspA combination. However, mice that received the PdB-PsaA-PspA combination survived significantly longer than those that received the alum placebo (P < 0.001), PdB alone (P < 0.01), PsaA alone (P < 0.01), PspA alone (P < 0.01), PdB-PsaA (P < 0.01), or PsaA-PspA (P < 0.05). In addition, mice that received PspA in combination with PsaA and/or PdB survived longer than mice that were immunized with a combination of PdB and PsaA, suggesting that PspA may provide slightly superior protection than PdB or PsaA within the context of the challenge strain and dosage used.
FIG. 2.
Survival times for mice after intraperitoneal challenge. Groups of 14 or 15 BALB/c mice were immunized with the indicated antigens and challenged 2 weeks after the third immunization with approximately 107 CFU of D39 (type 2) (A) or WCH43 (type 4) (B). The broken lines indicate the median survival time for each group.
TABLE 2.
Statistical comparison of median survival timesa
| Type 4 challenge immunization |
P value for comparison with the following type 2 challenge immunization
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Alum | PdB | PsaA | PspA | PdB-PsaA | PdB-PspA | PsaA-PspA | PdB-PsaA-PspA | |
| Alum | NSb | NS | NS | <0.05 | =0.001 | <0.025 | <0.001 | |
| PdB | <<0.001 | NS | NS | NS | =0.01 | NS | <0.01 | |
| PsaA | <<0.01 | <0.05 | NS | NS | <0.025 | NS | <0.01 | |
| PspA | NS | <<0.001 | <<0.001 | NS | <0.01 | NS | <0.01 | |
| PdB-PsaA | NS | <<0.001 | <0.025 | <0.025 | <0.01 | NS | <0.01 | |
| PdB-PspA | <<0.001 | <0.01 | <0.001 | <<0.001 | <<0.001 | NS | NS | |
| PsaA-PspA | NS | <<0.001 | <0.001 | NS | NS | <<0.001 | <0.05 | |
| PdB-PsaA-PspA | <<0.001 | NS | <<0.01 | <<0.001 | <<0.001 | NS | <<0.001 | |
Groups of immunized mice were challenged either by a capsular type 2 strain (D39) or by a capsular type 4 strain (WCH43). Differences were analyzed using the Mann-Whitney U test (one tailed).
NS, not significant (P ≥ 0.05).
The overall survival rates for mice immunized with PdB-PspA and PdB-PsaA-PspA combinations were significantly greater than the survival rates for mice in the placebo group (P << 0.005 and P < 0.01, respectively). Similarly, there were significant differences in the survival rates for mice that received PdB-PspA and PdB-PsaA-PspA antigens versus those that were immunized with either PdB alone or PdB-PsaA (P < 0.01 and P < 0.05, respectively). However, there was no significant difference in the survival rates between mice immunized with the placebo and those that were immunized with PdB alone, PsaA alone, PspA alone, PdB plus PsaA, or PsaA plus PspA.
In the second challenge experiment, mice immunized with the various antigen combinations were challenged intraperitoneally with the type 4 strain WCH43. This strain is as virulent for BALB/c mice as D39, with the challenge inoculum of 107 CFU corresponding to approximately 105 times the LD50. In this experiment, the median survival time for mice in the placebo group was also less than 1 day (Fig. 2B). Mice that received PspA alone were not significantly protected compared with those that received the alum placebo (Table 2). However, mice immunized with either PdB alone or PsaA alone had significantly longer median survival times than the group that received either the placebo (P << 0.001 and P << 0.01, respectively) or PspA (P << 0.001 in both cases). It was somewhat surprising that the median survival time for mice immunized with a combination of either PdB and PsaA or PsaA and PspA was not significantly different from that for mice in the placebo group. As a corollary, mice immunized with PsaA alone were significantly better protected against the type 4 challenge than mice that received a combination of either PdB and PsaA (P < 0.025) or PsaA and PspA (P < 0.001). However, the median survival time for mice that received a combination of PdB and PspA was significantly longer than that for those that received PdB alone (P < 0.01), PsaA alone (P < 0.001), or PspA alone (P << 0.001). With the exception of mice immunized with PdB alone and PdB-PspA, the median survival time for mice immunized with PdB-PsaA-PspA was significantly longer than the median survival time for mice that received the placebo (P << 0.001), PsaA alone (P << 0.01), PspA alone (P << 0.001), PdB-PsaA (P << 0.001), or PsaA-PspA (P << 0.001). Collectively, the results from the two challenge experiments support the hypothesis that immunization with a combination of pneumococcal proteins may give superior protection over immunization with a single antigen alone.
Passive-immunization studies.
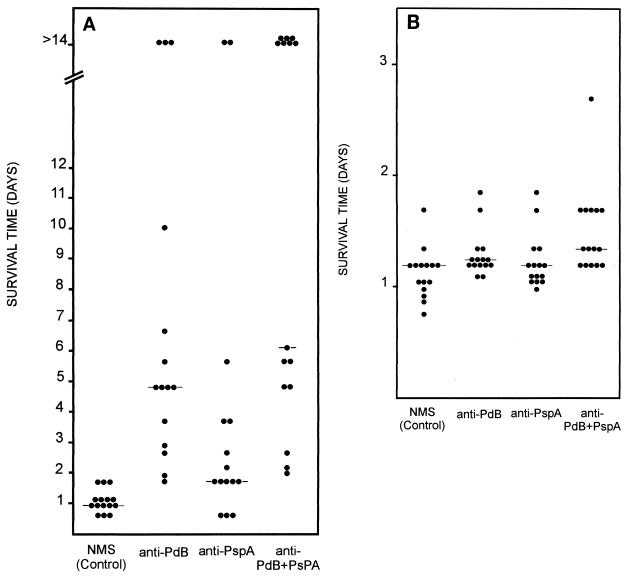
Passive-immunization–intraperitoneal-challenge experiments were conducted to determine whether the protection afforded by immunization of mice with the various protein antigen combinations was antibody mediated. One hundred microliters of pooled sera from mice immunized with either PdB alone, PspA alone, a combination of PdB and PspA, or an alum placebo was administered by intraperitoneal injection into groups of 15 naive mice. Before injection, anti-PdB and anti-PspA sera were concentrated and/or adjusted to a titer of 50,000. This was followed 1 h later by intraperitoneal challenge with 106 CFU of D39 or 105 CFU of WCH43. The inoculum was approximately 104 and 103 LD50s of strain D39 and strain WCH43, respectively, for BALB/c mice. In the D39 challenge, the median survival times for mice that received either anti-PdB, anti-PspA, or anti-PdB-PspA sera were significantly longer than that for mice that received sera from the placebo group (P << 0.001, P < 0.025, and P << 0.001, respectively) (Fig. 3A). Moreover, the median survival time for mice that received anti-PdB-PspA serum was significantly longer than that for mice that received only anti-PdB serum (P << 0.05) or that for mice that received only anti-PspA serum (P << 0.001). In the WCH43 challenge, the median survival time for mice that received either anti-PdB or anti-PdB-PspA serum was significantly longer than that for mice which received placebo serum (P << 0.05 and P << 0.001, respectively). Interestingly, a significant difference between the anti-PspA group and the placebo group was not observed (Fig. 3B). However, the median survival time for mice that received anti-PdB-PspA serum was significantly longer than that for mice that received anti-PdB serum alone (P < 0.025) or that for mice that received anti-PspA alone (P << 0.01), confirming that both PdB and PspA antibodies contribute to protection. Thus, the results with passive immunization are consistent with those obtained in the active-immunization–challenge experiments, confirming that the protection of mice with the various protein antigen combinations is, at least in part, antibody mediated.
FIG. 3.
Passive immunization and intraperitoneal challenge. Groups of 15 BALB/c mice were injected intraperitoneally with sera (titer = 50,000) obtained from mice immunized with the indicated antigens and then were challenged 1 h later with approximately 106 CFU of D39 (type 2) (A) or with approximately 105 CFU of WCH43 (type 4) (B). The broken lines indicate the median survival time for each group. NMS, normal mouse serum.
Discussion.
The widespread impact of pneumococcal disease throughout the world has prompted considerable efforts to develop cheap, effective pneumococcal vaccines. Appreciable levels of success have been achieved with the polyvalent PS vaccines and, most recently, with protein-PS conjugate vaccine formulations. However, the problems of serotype specificity of protection, geographical and temporal variations in serotype distribution, and the cost of conjugate vaccine formulations remain (15, 20, 21, 23, 36). These problems are further exacerbated by the possibility that nasopharyngeal replacement carriage of non-vaccine serotypes in vaccinated individuals will be reflected in increased rates of disease caused by these types (30).
In the present study, we proposed that immunization with a combination of virulence protein antigens of S. pneumoniae may give superior protection against a wider variety of strains over immunization with any of the protein antigens alone. Because these proteins appear to function at different stages of the pathogenic process (31, 33, 39), it was anticipated that a combined vaccine would elicit a higher degree of protection than any single antigen alone. Intraperitoneal challenge of actively immunized mice with particularly massive doses of two different challenge strains has provided unequivocal evidence that immunization with a combination of the proteins gives superior protection over immunization with any single antigen. The protection data obtained from the passive-immunization–intraperitoneal-challenge experiments also indicate that the protection is, at least in part, antibody mediated.
The three protein antigens evaluated in this study have been well characterized and shown to contribute to the pathogenesis of S. pneumoniae (33). These proteins have also been shown to be protective in different animal models (1, 10, 11, 25, 35, 42, 43, 47; Ades et al., Program Abstr. Pneumococcal Vaccines World 1998 Conf., 1998). Strong, antigen-specific antibody responses were mounted against these antigens, either alone or when administered in combination, indicating that these antigens are immunogenic and that there are no obvious deleterious or antagonistic consequences of combining these antigens for immunization of mice.
The protection observed due to immunization with PdB is presumed to be a consequence of direct neutralization of the pneumolysin toxin, thereby arresting bacteremia and hindering the exponential growth of the organisms in vivo. On the other hand, protection imparted by immunization with PspA is probably a consequence of the blocking of PspA's ability to inhibit complement fixation (28, 44), thereby facilitating clearance of the virulent pneumococci. Thus, immunization with both antigens might be expected to provide additive protection, as was observed in the present study.
In our high-dose intraperitoneal model, little demonstrable benefit could be attributed to anti-PsaA antibodies, since we could not show a significant level of protection in PsaA-immunized mice. However, there have been reports where PsaA has been found to confer significant levels of protection against nasopharyngeal carriage (Ades et al., Program Abstr. Pneumococcal Vaccines World 1998 Conf., 1998). Moreover, Talkington et al. have reported protection against systemic challenge with a type 3 strain (42). Being a lipoprotein, PsaA is presumably located on the outer face of the cell membrane, beneath both the cell wall and the capsule. Moreover, X-ray crystallographic analysis has shown that the dimensions of PsaA are such that it cannot be exposed on the outer surface of the organism (22). Thus, antibodies against PsaA are unlikely to be opsonic and presumably must diffuse through the capsule and cell wall layers in order to interact with the lipoprotein and block its biological function (metal ion transport). Pneumococci are known to undergo a reversible phase variation involving alteration in the levels of PS production; translucent phase variants produce less PS and exhibit enhanced nasopharyngeal colonization, whereas opaque phase variants produce more PS and exhibit much greater systemic virulence (18, 45). Thus, during nasopharyngeal colonization, PsaA may be more accessible to exogenous antibody, whereas the presence of a thicker caspule after systemic invasion may preclude interaction between antigen and antibody.
The protection data from the present study are encouraging and substantiate the claim for serious consideration of the combination protein vaccine approach for combating infections caused by S. pneumoniae. A logical extension of this study will include an evaluation of protection afforded by protein combinations, especially pneumolysin and PspA, in other model systems involving different challenge routes. Further studies would necessarily include an assessment of the protective efficacies of combinations including other recently characterized virulence-associated proteins of S. pneumoniae, such as CbpA (also known as SpsA and PspC) (12, 17, 38). These studies will be critical for the design of new vaccination strategies against pneumococcal disease.
Acknowledgments
We thank Robert Fulgham for assistance with purification of PspA.
This work was supported by grants from the National Health and Medical Research Council of Australia and the World Health Organization.
REFERENCES
- 1.Alexander J E, Lock R A, Peeters C C A M, Poolman J T, Andrew P W, Mitchell T J, Hansman D, Paton J C. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltimore R S, Shapiro E D. Pneumococcal infections. In: Evans A S, Brachman P S, editors. Bacterial infections of humans: epidemiology and control. New York, N.Y: Plenum Medical Book Co.; 1991. pp. 525–546. [Google Scholar]
- 4.Benton K A, Paton J C, Briles D E. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 5.Berry A M, Alexander J E, Mitchell T J, Andrew P W, Hansman D, Paton J C. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect Immun. 1995;63:1969–1974. doi: 10.1128/iai.63.5.1969-1974.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry A M, Ogunniyi A D, Miller D C, Paton J C. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect Immun. 1999;67:981–985. doi: 10.1128/iai.67.2.981-985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry A M, Paton J C. Sequence heterogeneity of PsaA, a 37-kDa putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry A M, Yother J, Briles D E, Hansman D, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulnois G J, Paton J C, Mitchell T J, Andrew P W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 10.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles D E, Tart R C, Wu H Y, Ralph B A, Russell M W, McDaniel L S. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann NY Acad Sci. 1998;797:118–126. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Walter A, Briles D E, Hollingshead S K. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun. 1999;67:6533–6542. doi: 10.1128/iai.67.12.6533-6542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crain M J, Waltman W D, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dintilhac A, Alloing G, Granadel C, Claverys J-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 15.Douglas R M, Paton J C, Duncan S J, Hansman D. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 16.Garenne M, Ronsmans C, Campbell H. The magnitude of mortality from acute respiratory infections in children under 5 years in developing countries. World Health Stat Q. 1992;46:180–191. [PubMed] [Google Scholar]
- 17.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim J O, Weiser J N. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 19.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koskela M, Leinonen M, Häivä V M, Timonen M, Mäkelä P H. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr Infect Dis. 1986;5:45–50. doi: 10.1097/00006454-198601000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kuo J, Douglas M, Ree H K, Lindberg A A. Characterization of a recombinant pneumolysin and its use as a protein carrier for pneumococcal type 18C conjugate vaccines. Infect Immun. 1995;63:2706–2713. doi: 10.1128/iai.63.7.2706-2713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence M C, Pilling P A, Epa V C, Berry A M, Ogunniyi A D, Paton J C. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure. 1998;6:1553–1561. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee C-J, Lock R A, Mitchell T J, Andrew P W, Boulnois G J, Paton J C. Protection of infant mice from challenge with Streptococcus pneumoniae type 19F by immunization with a type 19F polysaccharide-pneumolysoid conjugate. Vaccine. 1994;12:875–878. doi: 10.1016/0264-410x(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 24.Leowski J. Mortality from acute respiratory infections in children under 5 years of age: global estimates. World Health Stat Q. 1986;39:138–144. [PubMed] [Google Scholar]
- 25.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular serotype. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel L S, Yother J, Vijayakamur M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell T J, Mendez F, Paton J C, Andrew P W, Boulnois G J. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 1990;18:4010. doi: 10.1093/nar/18.13.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeleman C, Geelen S P M, Aerts P C, Daha M R, Mollnes T E, Roord J J, Posthuma G, van Dijk H, Fleer A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak R, Braun J S, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex PsaA. Mol Microbiol. 1998;29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 30.Obaro S K, Adegbola R A, Banya W A S, Greenwood B M. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–272. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 31.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 32.Paton J C. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 1998;6:85–87. doi: 10.1016/s0966-842x(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 33.Paton J C, Andrew P W, Boulnois G J, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 34.Paton J C, Berry A M, Lock R A. Molecular analysis of putative pneumococcal virulence proteins. Microb Drug Resist. 1997;3:1–10. doi: 10.1089/mdr.1997.3.1. [DOI] [PubMed] [Google Scholar]
- 35.Paton J C, Lock R A, Hansman D. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983;40:548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton J C, Lock R A, Lee C-J, Li J P, Berry A M, Mitchell T J, Andrew P W, Hansman D, Boulnois G J. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991;59:2297–2304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilling P A, Lawrence M C, Berry A M, Ogunniyi A D, Lock R A, Paton J C. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr D. 1998;54:1464–1466. doi: 10.1107/s0907444998005812. [DOI] [PubMed] [Google Scholar]
- 38.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 39.Rubins J B, Charboneau D, Paton J C, Mitchell T J, Andrew P W, Janoff E N. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest. 1995;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampson J S, Furlow Z, Whitney A M, Williams D, Facklam R, Carlone G M. Limited diversity of Streptococcus pneumoniae psaA among pneumococcal vaccine serotypes. Infect Immun. 1997;65:1967–1971. doi: 10.1128/iai.65.5.1967-1971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shann F. Etiology of severe pneumonia in children in developing countries. Pediatr Infect Dis J. 1986;5:247–252. doi: 10.1097/00006454-198603000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Talkington D F, Brown B G, Tharpe J A, Koenig A, Russell H. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 43.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 44.Tu A-H T, Fulgham R L, McCrory M A, Briles D E, Szalai A J. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. Global programme for vaccines and immunization (vaccine research and development). Report of The Technical Review Group meeting, July 1997–June 1998; achievements and plan of activities: meningococcal and pneumococcal disease vaccines. Geneva, Switzerland: World Health Organization; 1997. pp. 26–30. [Google Scholar]
- 47.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 48.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]