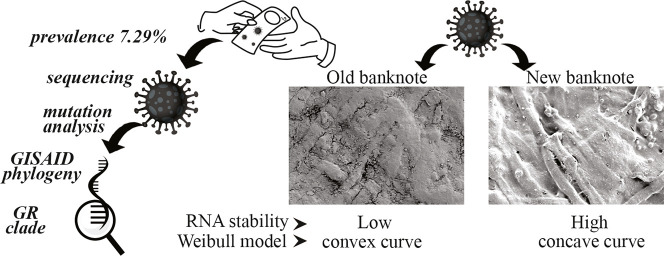
Abstract
Originating in December 2019 in China, SARS-CoV-2 has emerged as the deadliest pandemic in humankind's history. Along with direct contact and droplet contaminations, the possibility of infections through contaminated surfaces and fomites is investigating. This study aims to assess SARS-CoV-2 viral RNA's prevalence by real-time one-step reverse transcriptase PCR on banknotes circulating in Bangladesh. We also evaluated the persistence of the virus on banknotes spiked with SARS-CoV-2 positive diluted human nasopharyngeal samples. Among the 425 banknote samples collected from different entities, 7.29% (n = 31) were tested positive for targeted genes. Twenty-four positive representative samples were assessed for n gene fragments by conventional PCR and sequenced. All the samples that carry viral RNA belonged to the GR clade, the predominantly circulating clade in Bangladesh. In the stability test, the n gene was detected for up to 72 h on banknotes spiked with nasopharyngeal samples, and CT values increase significantly with time (p < 0.05). orf1b gene was observed to be less stable, especially on old banknotes, and usually went beyond detectable limit within 8 to 10 h. The stability of virus RNA well fitted by the Weibull model and concave curve for new banknotes and convex curve for old banknotes revealed. Handling banknotes is unavoidable; hence, these findings imply that proper hygiene practice is needed to limit SARS-CoV-2 transmission through banknotes.
Keywords: Coronavirus, COVID-19, Currency, Fomite, Pandemic, Transmission
Graphical abstract
1. Literature review
Severe acute respiratory virus (SARS), a member of Betacoronavirus, was responsible for two previous epidemics in just two decades; in November 2002 as SARS in China and in June 2012 as MERS (Middle East respiratory syndrome coronavirus) in Saudi Arabia (Zaki et al., 2012; Zhong et al., 2003). At the end of December 2019, another SARS-like outbreak was confirmed in China that turned pandemic quickly. WHO named the disease COVID-19 (WHO, 2020), and the virus was announced as SARS-CoV-2 as suggested by the International Committee on Taxonomy of Viruses (Gorbalenya et al., 2020). As of January 09, 2021, the pandemic claimed over 1.9 million deaths, with 87.59 million confirmed cases; new areas or populations are infected daily (WHO, 2021). Several researchers reported that the virus quickly accumulated mutations and became predominant or co-exited together (Islam et al., 2020a; Islam et al., 2020b; Nextstrain, 2020; GISAID, 2020; Rambaut et al., 2020). GISAID proposed nomenclature represents the worldwide phylogenetic circulating diversity under seven major clades: GV, GH, GR, G, V, L, and S.
Community transmission is playing a significant role in the SARS-CoV-2 pandemic. The direct transmission routes include coughing or sneezing mediated infectious droplets and mechanical ventilation, including talking or singing (Stadnytskyi et al., 2020; van Doremalen et al., 2020). Similarly, direct physical contact is also responsible for SARS-CoV-2 transmission. Another unreported and poorly understood indirect route of virus transmission is contaminated surfaces and fomites. Transmission through contaminated surfaces is rare but cannot be denied. Various respiratory viruses, e.g., SARS-CoV, Influenza, Rhinovirus, etc., can persist in multiple fomites without losing infectivity for a certain period (Thomas et al., 2008). After the SARS-CoV-2 pandemic, researchers are reporting the presence of SARS-CoV-2 RNA on various surfaces such as patients' masks (Li et al., 2020); door handles and sanitizer dispenser (Razzini et al., 2020); different surface areas of the hospital (Santarpia et al., 2020); sewage pools (Wang et al., 2020). Unlike previous pandemic-causing coronaviruses (e.g., SARS and MERS), SARS-CoV-2 has a higher basic reproduction number (Ro), thus is highly contagious with a low incubation period but a higher infection rate (Xie and Chen, 2020). It is pertinent to identify the potential infection source in the community, characterize the route, and break the transmission chain to control the outbreak. Like other fomites, the banknote is an ideal source of pathogenic microbes (Gabriel et al., 2013; Hiko et al., 2016; Jalali et al., 2015; Maritz et al., 2017; Pal and Bhadada, 2020; Thomas et al., 2008). Microbes remain infective in papers, and stability depends on the surrounding environment's initial loads, temperature, and moisture content (Pastorino et al., 2020). The banknote is the most ubiquitous and transferable object in present world. Developing countries, including Bangladesh, have limited users of virtual banking and are dependent on paper banknotes. However, few countries decontaminate their paper notes at regular intervals and assess the microbial contamination, which is seemingly not possible for many nations. The reasons behind microbial contamination in paper notes are material composition, specifically moisture absorbing and dust retaining capacity, and high frequency of exchange (Vriesekoop et al., 2010). Paper-based banknotes' rough surface also provides the necessary support for microbes to settle down and be accumulated. A recent report showed that SARS-CoV-2 survives on banknotes for 28 days at 20 °C (Riddell et al., 2020). However, to the best of our knowledge, there is no data regarding the prevalence of SARS CoV-2 on banknotes as it may survive longer (Riddell et al., 2020). Although microbial can survive on different surfaces as linear regression (Riddell et al., 2020), many researchers documented the microbial survival as nonlinear, concave, or convex with sigmoid shapes (Buzrul and Alpas, 2007; Coroller et al., 2006; Jahid et al., 2013; Mafart et al., 2002). In this research, we aimed to determine the prevalence of SARS-CoV-2 RNA on circulatory Bangladeshi banknotes and assess the stability of SARS-CoV-2 on spiked banknotes.
2. Material and methods
2.1. Ethics approval
The work has been conducted in the Genome Centre of Jashore University of Science and Technology, providing SARS-CoV-2 real-time reverse-transcriptase polymerase chain reaction (RT-PCR) diagnostic service of the national COVID-19 response and surveillance program (https://dghs.gov.bd/images/docs/Notice/rt_pcr_lab.pdf). Jashore University of Science and Technology's institutional ethical review board has reviewed the research project with an exemption of consent from the patients (ref: ERC/FBS/JUST/2020-42). The nonissuable banknotes used in this study were collected from a public bank, withdrawn from circulation for destruction.
2.2. Sample collection and processing
We have collected circulating banknotes of varying denominations as an exchange of payment from retail shops, ticket vendors and auto-rickshaw drivers, etc., at places in two Southern Districts, namely Khulna, Jashore Bangladesh. During the collection of banknotes, we avoid selection bias. Hence, the banknotes are not equally distributed but representing the natural circulation frequencies among denominations. Sample collectors were asked to put the paper banknotes directly into a biohazard sample collection bag and zipped that, and transported them to the laboratory, preferably within 2 h. Each banknote was removed from the bag in a biosafety Class IIa cabinet and kept on a sterile polystyrene petry dish. We swabbed both sides of the banknotes with nylon flocked round (oral) swab (biovencer Healthcare Pvt. Ltd., India) soaked with 0.9% saline containing Rnasin (Sunsure Biotech, China). The swab head is cut and put into 80.0 μL 0.9% saline containing Rnasin in a DNase/RNase-free microcentrifuge tube and mixed vigorously. We aliquoted 20.0 μL suspension into 20.0 μL of ice-cold RNA extraction buffer (QuickExtract™ RNA Extraction kit, Lucigen) and vortexed for 1 min. The extract was chilled immediately on ice and kept at −20 °C till real-time RT-PCR amplification of SARS-CoV-2 specific genes. To assess the SARS-CoV-2, we used the methodology as described later in section 2.4.
2.3. Preparation of banknote notes spiked with SARS-CoV-2 positive sample
2.3.1. Experimental setup
To assess the stability of SARS-CoV-2 RNA on the paper banknote, we have selected three SARS-CoV-2 positive human samples from the Genome Centre of Jashore University of Science and Technology containing both nasal and oro-pharyngeal swab collected in 2.0 mL 0.9% saline water. The Center is routinely diagnosing the COVID-19 patients' samples by real-time RT-PCR method. Among SARS-CoV-2 positive (CT = 22) routine diagnosis samples, we selected three. We used six different banknotes, three were visibly old and dirty circulated for years, and the other three were new and clean but tore apart. We cut the banknotes (nonissuable) into circular pieces of 28 sq. mm (3 mm of radius) size, and three random parts from each banknote were tested for SARS-CoV-2 RNA by real-time RT-PCR method and found negative. All the pieces of banknotes were placed in a polystyrene Petri dish and spiked with ten microlitres of two-times diluted SARS-CoV-2 positive samples (we spiked 39 pieces of each banknote type with each of the three types of positive samples), allowed to dry in a biosafety IIa cabinet. When the banknotes were visibly dry, the Petri dishes were placed inside a clear biohazard bag and sealed. We set all experient inside the biosafety Class IIa cabinet at room temperature with negative pressure. After setting the whole experiment, three replica spiked banknote pieces were picked from each set and kept in micro-centrifuge tubes at different time intervals (i.e., zero hours, 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h). The temperature was monitored and recorded throughout the experiment by a thermometer (Model: SHX-RPT-6, Shanghai, China). Researchers took suitable biosafety precautions (e.g., overhead gown with N95 respirator) throughout the work and handled suspected samples and spiked banknotes only in BSC IIa facilities.
2.3.2. RNA extraction from the spiked banknote
After each time interval, we collected pieces of spiked banknotes and put each of the pieces in twenty microlitres of 0.9% saline in microcentrifuge tubes. RNA extracted, as mentioned in section 2.2, and detection of SARS-CoV-2 as in section 2.4.
2.4. Detection of SARS-CoV-2 RNA
We used the primer and probe sequences and protocol designed and utilized by Chu et al. (2020). The sequences of primers and probes used for this study are enlisted in Table A2. Due to the lack of suitable positive control, we used RNA extracted from a previously characterized in our laboratory (the whole genome sequenced, accession no. EPI_ISL_561377) positive sample as positive control and 0.9% saline prepared with RNase free water as a negative control in this assay. We used The GoTaq® Probe 1-Step RT-qPCR Reaction Mix (Promega, USA) for the duplex reaction. We prepared the reaction mixture following the kit's protocol, except for using 9 μL RNA extract as a template and preparing a final reaction volume of 25 μL (instead of 20 μL). We optimized the thermal Cycling parameters as followed: Reverse transcription at 45 °C for 30 min; Reverse transcriptase inactivation and GoTaq® DNA Polymerase activation at 95 °C for 2 min, and 45 cycles of the regime of denaturation at 95 °C for 15 s and annealing with extension at 60 °C for 1 min. We performed The PCR reaction in a QuantStudio™ 3 Real-Time PCR System (The Applied Biosystems, USA) in a 96-well plate (0.2 mL) and analyzed it in Quantstudio design and analysis software (v1.3.3). We interpreted the sigmoid curve for either orf1b or n gene or both with a CT value of ≤36 as positive. We repeated samples with CT values between 36 and 39 were and above those were considered negative in the prevalence study. For the assay of stability of SARS-CoV-2 RNA on banknotes, we created standard curves for n- and orf genes by a serial of 2-fold diluted inoculum following the identical RNA extraction and real-time RT-PCR protocol.
2.5. Preparation of cDNA for targeted PCR
According to the manufacturer, we prepared cDNA for the representative 21 SARS-CoV-2 positive samples from the leftover RNA using the ProtoScript® II First Strand cDNA Synthesis Kit (NEB, UK) instruction with some modifications. In short, we omitted the ‘denaturation of RNA secondary structure’ step described in the manual, wherein we mixed 6 μL of extracted RNA with 2 μL of random primers and used other components as mentioned in the protocol. The mixture was then annealed and incubated at 42 °C and 48 °C for 5 and 20 min, respectively, followed by deactivating enzyme at 80 °C for 5 min and immediate chilling on ice. The final reaction mix was 20 μL for each cDNA synthesis reaction.
2.6. Determining the intactness of viral RNA genome on banknotes
For checking the virus's intactness and possibly the infectivity, we targeted an 850 bp large segment of the viral genome, which spans the receptor-binding region (RBD) of the spike protein-coding sequence. The forward and reverse primers are S_F2 (GCTGTAGACTGTGCACTTGACCC) and S_R2 (GTAGTGTCAGCAATGTCTCTGCC), respectively. We carried out the PCR in 10 μL reaction volume comprising of 4 μL cDNA, 5 μL hot-start color master mixture (GoTaq® G2 Green Master; Promega, USA), 0.5 μM of each forward and reverse primer. The thermocycling conditions were as followed: the initial denaturation at 95 °C for 1 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 50 s and a final extension at 72 °C for 5 min. Finally, we electrophoresed PCR products on a 1% (w/v) agarose gel stained with ethidium bromide (UltraPure™ Ethidium Bromide, 10 mg/mL; Thermo Fisher, USA) and visualized using a gel documentation system (Bio-Rad, USA).
2.7. Targeted Sanger sequencing of SARS-CoV-2 genes
We used the randomly amplified cDNA as a template for the PCR targeting N protein-coding sequence for detecting the viral phylogenetic clade (M. T. Islam et al., 2020). After purifying the PCR products with the ExoSAP-IT™ PCR product cleanup reagent as per manufacturers instruction (Thermo Fisher Scientific, USA). The BigDye Terminator v3.1cycle sequencing ready reaction kit (Thermo Fisher Scientific) was used in a way to optimize the cost than as mentioned in the Islam et al. (2020). Instead of 0.5 μL, 0.25 μL (per 10 μL reaction) undiluted BigDye Terminator v3.1 Ready Reaction mix was used together with 1 μL 5× sequencing buffer, 0.3 μL primer, 3.0 μL template DNA, and 5.7 μL nuclease-free water. We set up the cycle sequencing PCR condition according to the kit protocol. Accession IDs to the submitted sequences as an archetype are available in the GISAID EpiFlu™ database (Table A5). We performed further bioinformatics analyses considering Wuhan-Hu-1 (NC_045512.2) as the reference sequence using Molecular Evolutionary Genetics Analysis (MEGA X) software (Kumar et al., 2018).
2.8. Scanning electron micrograph
We air-dried representative disc of new and old banknotes, mounted on a scanning electron microscope (SEM) sample loading disc with carbon tape. We sputter-coated the loaded samples with gold using the timed gold sputter preset recipe in Q150RS plus machine (Quorum technologies ltd., UK) at the following conditions: sputter current of 20 mA, sputter time of 120 s with tooling factor 2.30. We examined and took the micrograph in a field-emission scanning electron microscope, FESEM (Gemini Sigma 300, Zeiss, Germany), and collected digitized images by Smart SEM software (Zeiss, Germany).
2.9. Nonlinear regression analysis
Generally, the Weibull model is used to estimate different parameters, which is a nonlinear model. The equation is the following:
| (1) |
where α is scale parameters (unit is min or sec) and n is shape parameters (unitless) (van Boekel, 2002). Nt is the number of microorganisms (CFU/mL or cm2) after survival time t. N0 is the initial number of microorganisms, and t is the survival time of microorganisms (h). n values equal to 1 correspond to linear survival curves, n values >1 correspond to downward concave survival curves, n values <1 conforms to convex survival curves.
However, Eq. (1) can be reparametrized by Coroller et al. (2006) as the following equation:
Here, α = log10(N 01/N 02) (the difference between two subpopulations). ẟ1 and δ2 are the scale parameters of the first and second subpopulation, respectively. ρ is the shape parameter. For the old banknotes, the Eq. (1) can be reparametrized by Mafart et al., (2002) as follows:
| (2) |
Here, ρ is the shape parameter, and δ is the scale parameter (Mafart et al., 2002).
If the model fits appropriately, a linear shape if ρ is equal to 1, a concave shape if ρ is >1, and a convex shape if ρ is <1. Root Mean Square Error (RMSE) was calculated from the software to know the model's goodness of fit to the death rate. The RMSE values closer to 0 and adjusted R2 values close to 1.0 indicate the model's better fitness. The model has been used to fit for survival kinetics of both old and new banknotes.
2.10. Statistical analysis
To determine the prevalence and stability of SARS-CoV-2 RNA on banknotes, we repeated all experiments three times. In the stability experiment on banknotes, we transformed CT values as 1/CT ∗ 100 and plotted with respective time (h) by using Microsoft Excel 2010 Add-in GInaFiT 1.6 (Geeraerd et al., 2005) (https://cit.kuleuven.be/biotec/software/GinaFit). We calculated root mean square error (RMSE) using the software, and values closer to 0 indicate a better fit to the model. The initial virus concentrations and analysis of Weibull parameters by carrying out ANOVA using SAS software (version 9.4 SAS Institute Inc., Cary, NC, USA) for a completely randomized design. The effect has been considered significant (p < 0.05); Duncan's multiple range test accomplished separation of the means.
3. Result and discussion
3.1. Prevalence of SARS-CoV2 on banknotes
We collected a total of 425 (n) banknotes from 56 (N) entities over three months. The entity includes pharmacies, ticket vendors/collectors, and drivers of local and inter-city transports, various shops, and restaurants (Table A1). We have collected 7.58 banknotes on average (minimum two and maximum of 19 banknotes per entity, data not shown) among the entity. In total, we found 7.29% (31/425) banknote samples were positive for SARS-CoV-2 RNA assessed by real-time one-step RT-PCR method. The entity of local transport (N = 11, n = 84) includes banknotes from drivers of three-wheelers (e.g., auto-rickshaw and mechanically driven three-wheeler), which accounted for the highest prevalence (14.28%) among the entities. In a case, we found seven (out of 19) SARS-CoV-2 RNA positive banknotes from a particular auto-rickshaw driver. This event contributed to the overall higher prevalence rates among the banknotes collected from local transports.
On the contrary, banknotes sampled from the ticket vendors and - collectors at inter-city transport (bus) were negative for the viral RNA. During the study, the intercity transport authority ensured that the passengers wearing masks maintained social distancing (carrying 50% of total capacity) with personal hygiene. We detected SARS-CoV-2 RNA on around 8 to 10% of banknote samples collected from restaurants & food shops (N = 5, n = 38) and grocery shops (N = 13, n = 106). Table A1 enlisted detailed results. The study was designed and started when the number of SARS-CoV-2 infected confirmed cases was at the peak (end of June 2020) in the country. The weekly cases of new SARS-CoV-2 infection were around 21 K in July and declined periodically to about 5 K at the end of September 2020 (Fig. A1).
In this study, we collected banknote in a random basis which included higher denomination (1000 TK, 500 TK, 100 TK) (n = 22), medium denomination (50 TK and 20 TK) (n = 143), and lower denomination (10 TK, 5 TK, 2 TK) (n = 260) banknotes. Although the sample distribution was not uniform, we found a high prevalence in the higher denomination (13.63%, 3/22) compared to medium (4.89%; 7/143) and lower denomination banknotes (8.07%; 21/260). The higher denomination banknotes usually have fewer transaction frequencies and cleaner than middle or lower denominations. The higher prevalence in this group was not predicted but could be due to the larger surface area. However, we found that the new clean banknote supports more stability or recovery of SARS-CoV-2 RNA than the older one (discussed later). The samples were not similar in terms of monthly distribution, but the percentage of the positive cases among samples was homogenous (Table A5). However, the samples were collected only for three months; hence Levene's test could not be performed to calculate the homogeneity of variance.
All the samples collected from the environment were not related to the hospital or isolation center for SARS-CoV-2 patients. The presence of SARS-CoV-2 RNA on the circulating banknotes indicates the frequent movement of infected patients, either asymptomatic or with mild symptoms. Milder symptomatic patients may have a higher viral load (low CT value) and can shed the virus (Jan et al., 2020). A recent survey on 2157 human subjects in Bangladesh found out that 16.3% do not wear masks, and 24.6% do not avoid crowds, even having milder SARS-CoV-2 like symptoms (Hossain et al., 2020). Detection of viral RNA does not ensure the presence of infective viral particles but, at the same time, does not exclude the possibility of having it.
3.2. SARS-CoV-2 gene sequencing
Randomly selected banknote samples, tested positive for SARS-CoV-2 RNA in real-time RT-PCR method, were also amplified for different segments of other genes by conventional PCR method, and PCR products of 24 representative samples were sequenced. Sequence data evaluated that all the samples contained SARS-CoV-2 virus belongs to GR clade strains. The result signifies this clade's dominant presence in Bangladesh, as described in another study (Alam et al., 2020). In Bangladesh, 80% (401/501) of the viral strains are of GR clade as per GISAID sequence information, whereas 34% (106,454/309,040) of the viruses are of the clade worldwide. Notably, we did not find any long amplified products, i.e. 850 bp targeted amplicon spanning RBD region, for each of the samples, that states the lacking of intactness of the viral genome on the banknote, thus infectivity of the virus.
3.3. Stability kinetics of spiked samples
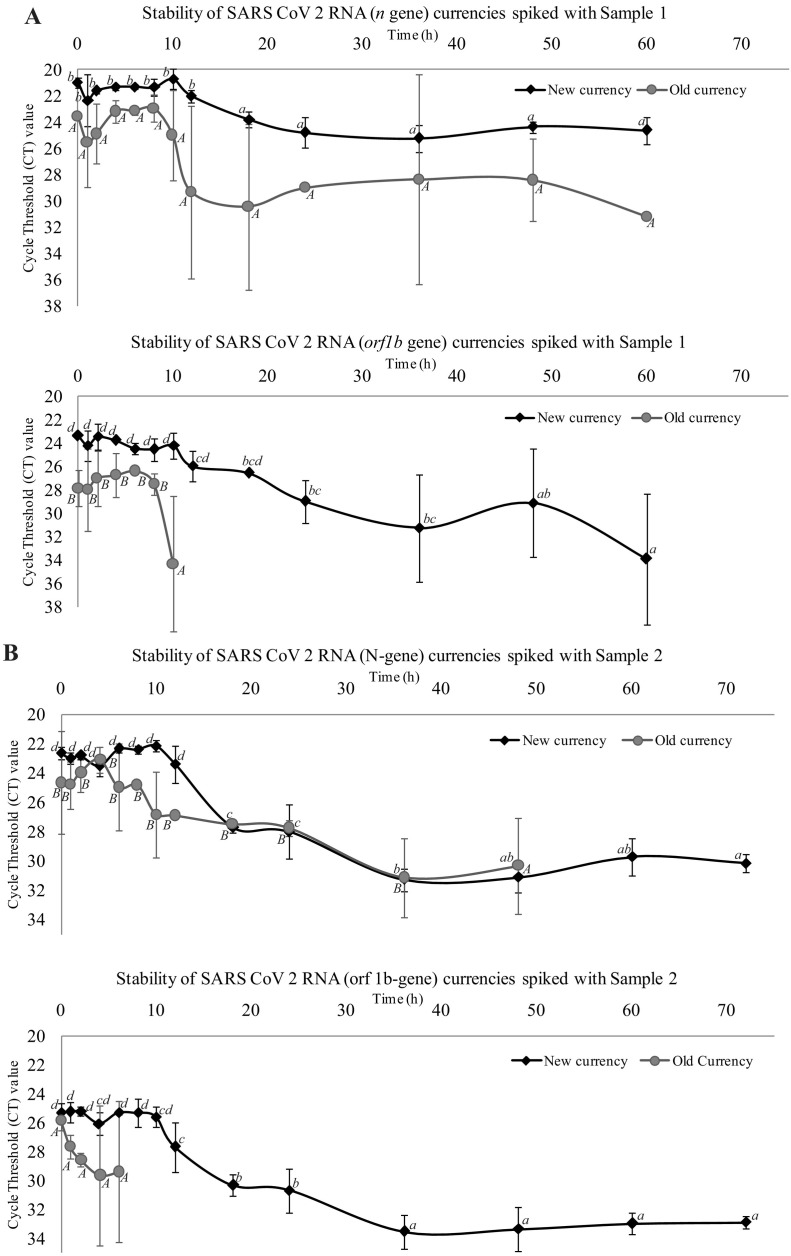
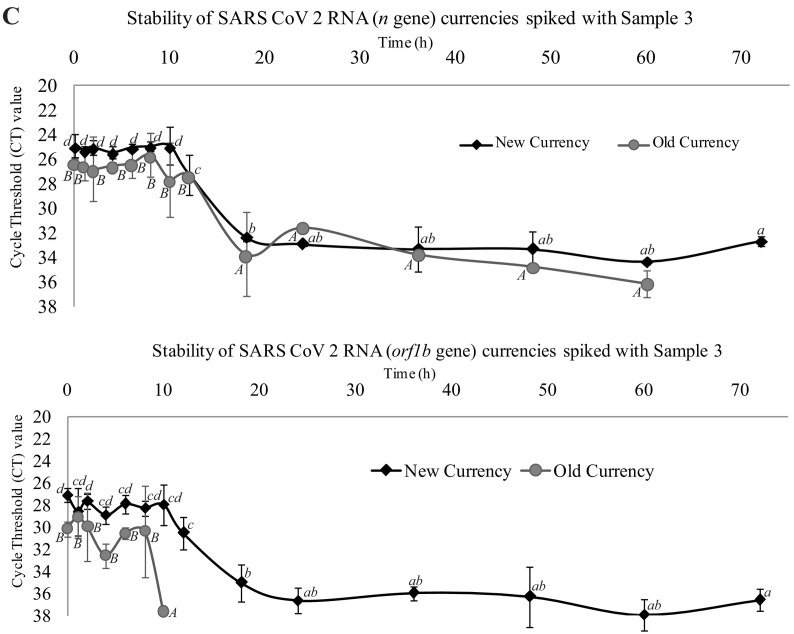
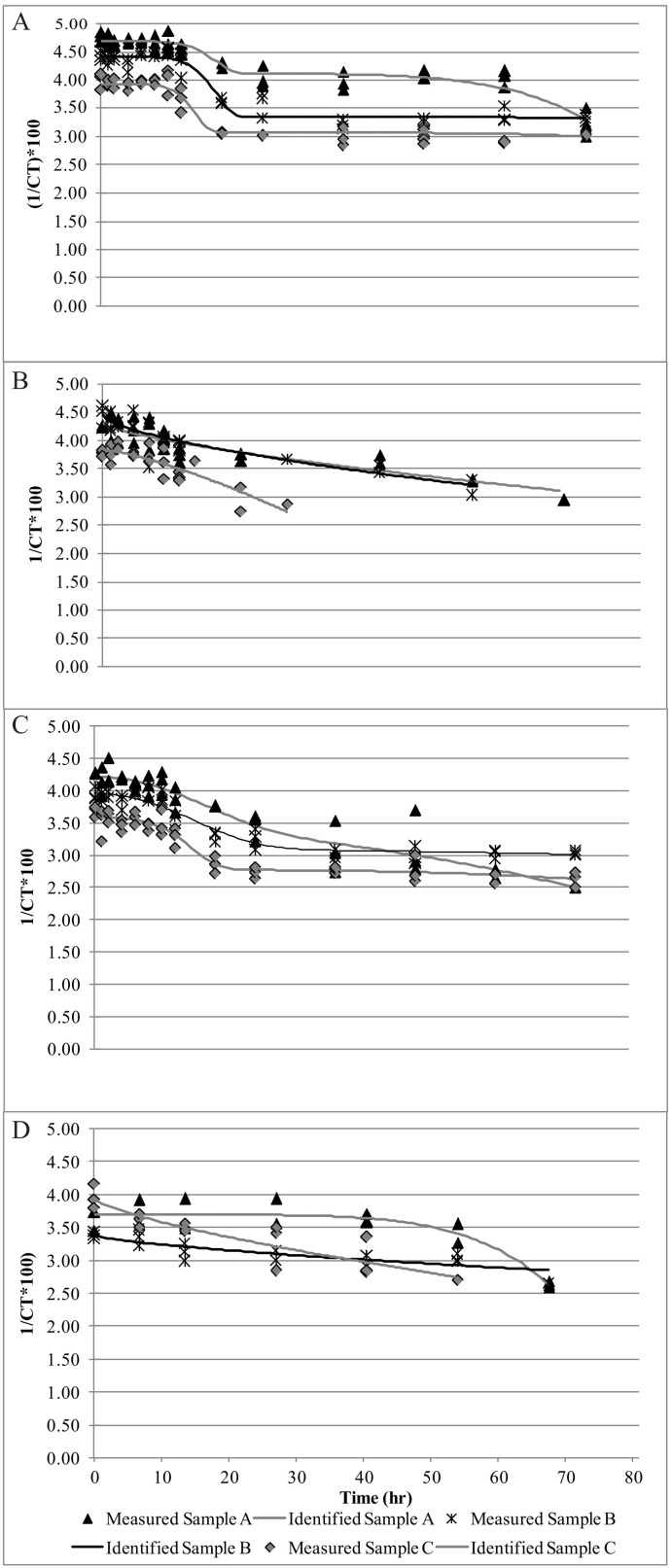
The survival kinetics of spiked samples of the present study on new and old banknotes were determined using Microsoft Excel 2010 Add-in GInaFiT 1.6 (Geeraerd et al., 2005) (https://cit.kuleuven.be/biotec/software/GinaFit). The results revealed that the overall survival of SARS-CoV-2 on new banknotes was higher compared to older banknotes. The n gene stability was higher compared to the orf gene (Fig. 1 ).The graphs show the stability of SARS-CoV-2 n gene to time (h) for new banknotes (Fig. 2A), n-gene for old banknotes (Fig. 2B), orf gene for new banknotes (Fig. 2C), and orf gene for old banknotes (Fig. 2D). For n gene in new banknotes, all three virus samples survived up to 60 h but absent after 72 h (Fig. 2A). The results also revealed that the values stabilize to 10 h for the new banknotes and gradually decrease to 60 h. The n gene stability was less for the old banknotes, and the value was zero after 60 h (Fig. 2B). For old banknotes, except one sample, the other two did not show any stability for the initial 10 h and later hours. The graph shows a sharp decrease in the case of old banknotes (Fig. 2B). All the samples were stable up to 60 h for the orf gene (Fig. 2C), whereas only 10 h for old banknotes (Fig. 2D). The graph is a concave type in new banknotes, while in old banknotes, it is a convex type. Therefore, we hypothesize that SARS-CoV-2 in old banknotes are less stable. Our results of stability of SARS-CoV-2 on new banknotes show similarity with the findings of Kampf et al. (2020).
Fig. 1.
Stability of SARS-CoV-2 on banknotes spiked with crude samples. Line graphs representing the changes in CT values of ‘n’ and ‘orf1b’ genes on new and old banknotes (non-issuable) spiked with Sample 1 (panel A), 2 (panel B) and 3 (panel C) at different time intervals (starting from zero to 72 h). Values are the mean (with standard deviation as error bars) of three independent replica experiments. Within each variable, values with the same letter are not significantly different according to Duncan's multiple range test (P = 0.05); lower case letters are used for mean values of experiments on new currencies and upper case letters for experiments old banknotes.
Fig. 2.
SARS-CoV-2 stability on spiked banknotes and fitness to modified Weibull model. SARS-CoV-2 RNA stability on banknotes spiked with RT-PCR tested positive diluted human nasopharyngeal swab samples on new and old banknotes. The spiked banknotes are incubated at room temperature, and stability of RNA detected at different times. The points represent the mean ± square error of mean (SEM) of 3 independent experiments, and vertical bars indicate the duration of time to keep the samples. Curves were fit to a modified Weibull model using GInaFiT 1.6 software: (A) curves for n gene of new banknotes of samples 1, 2, and 3; (B) curves for orf1b gene of new banknotes of samples 1, 2, and 3; (C) curves for n gene of old banknotes of samples 1, 2, and 3; (D) curves for orf1b gene of old banknotes of samples 1, 2, and 3.
The researchers found stability for SARS-CoV on paper surfaces. Riddell et al. (2020) found that the virus survived less than seven days on banknotes at 30 °C and one day at 40 °C. Our laboratory temperature was around 35 °C during the experimental conditions. Even though we used the samples from the patient's nasopharyngeal swab, whereas the authors used the virus grown in the Vero cell line, our study's results agreed with Riddell et al. (2020) and Kampf et al. (2020). Reports say that SARS-CoV-2 stays infective in various inanimate objects (e.g., metal, plastics, etc.) for 2 h to 9 days (Kampf et al., 2020). In another study, coronavirus exposed to various metals (copper or copper alloy) harms the virus, irreversibly damaged the intactness of the virus and RNA (Warnes et al., 2015). Temperature and relative humidity can negatively affect the stability of SARS-CoV-2 and reduce the transmissibility over time (Demongeot et al., 2020), which may be right for other respiratory enveloped virus, but this may be still inconclusive (Ma et al., 2020; To et al., 2021), more research needs to be performed for the SARS-CoV-2.
3.4. Mixed Weibull kinetics of SARS-CoV-2 RNA stability on spiked banknotes
Table A6 shows the Weibull model parameters and goodness-of-fit values of news and old banknotes spiked with SARS-CoV-2. The Weibull model accurately predicted with both n gene and orf gene, with adjusted correlation coefficients (R2) of ≥0.8.0. Estimated RMSE was <0.3, meaning that the Weibull model was a good fit for all the new and old banknotes' survival curves. As in the Weibull model, α, δ1, p, and δ2 influence the data's curves and fitness. The analysis shows that α parameters were significantly different (p ≤ 0.05) according to ANOVA, which means that subpopulations 1 and 2 for new were significantly different. We found that for samples 1, 2, and 3 for n gene and orf gene of new banknotes, differences in α, δ1, δ1, p, and δ2 were not significant (p > 0.05). The same parameters were non-significant (p ≥ 0.05) for old banknotes. In most of the cases of old banknotes, the p values were ˂1.0 means the convex curve for the old banknotes (Table A6). As the virus was most unstable, for old banknotes, the curves were fitted by Mafart et al. (2002), not by double Weibull of Coroller et al. (2006). Suman et al. (2020) reviewed the stability of SARS-CoV-2 on different surfaces, and none of the surfaces shows more than 72 h survival time. Even the authors demonstrated that infection capability is linear; however, their graphs show initial stability and then gradual decrease of the virus. Kwon et al. (2020) analyzed the virus on different surfaces and found a linear reduction of the virus. Riddell et al. (2020) also examined the survival curve with linear regression, but at 20 °C the graphs seem nonlinear.
3.5. FESEM observation of banknotes
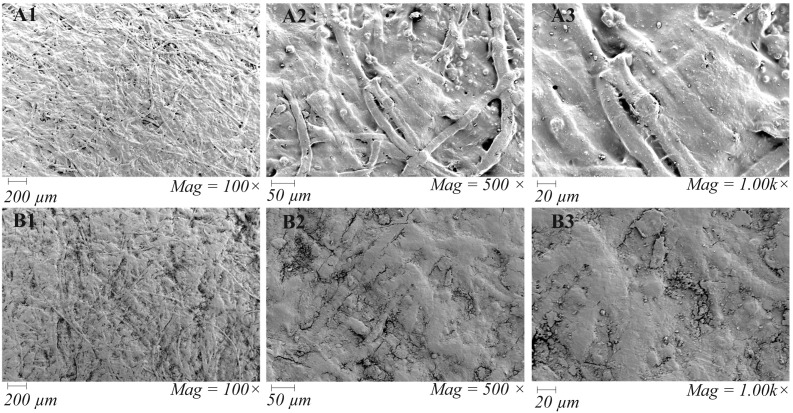
The observation stipulates the possibility for the presence of exogenous RNases sourced from sweat or dirt on banknotes. We could not assess the contaminating viruses' infectivity; however, the dirtier and old banknotes are less likely to support the stability of SARS-CoV-2 RNA. The scanning electron micrograph (Fig. 3 ) revealed that a loose layer of dirt almost covered the old banknotes (before spike with SARS-CoV-2 positive human sample), which possibly provide rooms for the predator microbes and harbor the exogenous RNases. On the other hand, the new banknotes were observed to have a more fibrous and compact texture and seemed more absorbent.
Fig. 3.
Field Emission Scanning Electron Micrograph of New (A1 to A3) and Old (B1 to B3) Bangladeshi Banknotes (representative fields). Emission and scanning parameters are: ‘electron high tension’ (EHT) = 5.0 kV, Signal A = secondary electron detector (SE2) at different magnifications. The arrow-a has pointed to show the paper banknote's fibrous structure, and arrow-b points loosely attached dirt particles on the banknote.
3.6. Limitations of the study
Due to the strict lockdown conditions, we could not collect samples at the early stages of the SARS-CoV-2 pandemic in Bangladesh, and sampling was limited to only two districts. We had no operational biosafety-level-3 laboratory (BSL 3) in our region and could not perform experiments in the cell line to isolate the virus or test its infectivity.
4. Conclusions
The presence of SARS-CoV-2 RNA in various environmental samples and surfaces profoundly affects viral epidemiology and infection. In our research, we found a significant portion of Bangladeshi banknotes contaminated with either virus or RNA. Fomites like banknote mediated transmission could exaggerate the overall situation and posed a risk for susceptible individuals. Implementing personal hygiene, avoiding touching unnecessary surfaces, washing hands after handling banknotes, and an effective decontamination strategy might prevent the fomite-mediated SARS-CoV-2 community transmission.
CRediT authorship contribution statement
S. Akter conceived the idea, performed the experiment, analyzed the data, and helped write the draft manuscript. P.C. Roy performed the experiment, analyzed the data, and wrote the draft manuscript. A. Ferdaus collected the entire samples and, with the help of S. Nigar, extracted RNA for RT-PCR assay. H. Ibnat and A.S.M. Rubayet Ul Alam performed the experiment involved in the polymerase chain reaction, operation, analysis, and deposition of the gene sequences. Iqbal Kabir Jahid and M. Anwar Hossain contributed significantly to the research design, supervision, result interpretation, statistical analyses, and manuscript improvement to its finished version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgements
We researched under the fund allocated from Jashore University of Science and Technology through the University Grants Commission (UGC) of Bangladesh. Special thanks to the Directorate General of Health Services, Ministry of Health & Family Welfare, Bangladesh, for approving the Genome Centre of our institution to provide real-time RT-PCR diagnostic service in the national COVID-19 response strategies. Thanks to Sonali Bank Limited, Corporate Branch, Jashore, Bangladesh, for providing nonissuable banknotes to support the research.
Editor: Ewa Korzeniewska
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.146133.
Appendix A. Supplementary data
Supplementary data containing additional tables (Table A1 through A6) and figures (Fig. A1 through A2) to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.146133
References
- Alam, A.S.M.R.U., Islam, M.R., Rahman, M.S., Islam, O.K., Hossain, M.A., 2020. Understanding the possible origin and genotyping of the first Bangladeshi SARS-CoV-2 strain. J. Med. Virol. n/a. doi: 10.1002/jmv.26115. [DOI] [PMC free article] [PubMed]
- Buzrul S., Alpas H. Modeling inactivation kinetics of food borne pathogens at a constant temperature. LWT Food Sci. Technol. 2007;40:632–637. doi: 10.1016/j.lwt.2006.02.019. [DOI] [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroller L., Leguerinel I., Mettler E., Savy N., Mafart P. General model, based on two mixed weibull distributions of bacterial resistance, for describing various shapes of inactivation curves. Appl. Environ. Microbiol. 2006;72:6493–6502. doi: 10.1128/AEM.00876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demongeot J., Flet-Berliac Y., Seligmann H. Temperature decreases spread parameters of the new covid-19 case dynamics. Biology (Basel) 2020;9(94) doi: 10.3390/biology9050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E.M., Coffey A., O’Mahony J.M. Investigation into the prevalence, persistence and antibiotic resistance profiles of staphylococci isolated from euro currency. J. Appl. Microbiol. 2013;115:565–571. doi: 10.1111/jam.12247. [DOI] [PubMed] [Google Scholar]
- Geeraerd A.H., Valdramidis V.P., Van Impe J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005;102:95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- GISAID, 2020. Global initiative on sharing all influenza data [WWW document]. Clade lineage Nomencl. Aids genomic Epidemiol. Stud. Act. hCoV-19 viruses. Munich.
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Viruses, C.S.G. of the I.C. on T. of The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiko A., Abdata K., Muktar Y., Woyesa M., Mohammed A. Contamination of Ethiopian paper currency notes from various food handlers with E. coli. Springerplus. 2016;5:1065. doi: 10.1186/s40064-016-2742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.A., Jahid M.I.K., Hossain K.M.A., Walton L.M., Uddin Z., Haque M.O., Kabir M.F., Arafat S.M.Y., Sakel M., Faruqui R., Hossain Z. Knowledge, attitudes, and fear of COVID-19 during the rapid rise period in Bangladesh. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.T., Alam A.S.M.R.U., Sakib N., Hasan M.S., Chakrovarty T., Tawyabur M., Islam O.K., Al-Emran H.M., Jahid I.K., Hossain M.A. A rapid and cost-effective multiplex ARMS-PCR method for the simultaneous genotyping of the circulating SARS-CoV-2 phylogenetic clades. medRxiv. 2020 doi: 10.1101/2020.10.08.20209692. 2020.10.08.20209692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.R., Hoque M.S., Rahman M.S., Alam A.S.M.R.U., Akther M., Puspo J.A., Akter S., Sultana M., Crandall K.A., Hossain M.A. Genome-wide analysis of SARS-CoV-2 virus strains circulating worldwide implicates heterogeneity. Sci. Rep. 2020;10:14004. doi: 10.1038/s41598-020-70812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, O.K., Al-Emran, H.M., Hasan, M.S., Anwar, A., Jahid, M.I.K., Hossain, M.A., 2020b. Emergence of European and north American mutant variants of SARS-CoV-2 in South-East Asia. Transbound. Emerg. Dis. 10.1111/tbed.13748. doi: 10.1111/tbed.13748. [DOI] [PMC free article] [PubMed]
- Jahid I.K., Lee N.-Y., Kim A., Ha S.-D. Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J. Food Prot. 2013;76:239–247. doi: 10.4315/0362-028X.JFP-12-321. [DOI] [PubMed] [Google Scholar]
- Jalali S., Kohli S., Latka C., Bhatia S., Vellarikal S.K., Sivasubbu S., Scaria V., Ramachandran S. Screening currency notes for microbial pathogens and antibiotic resistance genes using a shotgun metagenomic approach. PLoS One. 2015;10:e0128711. doi: 10.1371/journal.pone.0128711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan I., Chen K., Sayan M., Uprety P., Laumbach R.J., Ennis R.D., Haffty B.G. Prevalence of surface contamination with SARS-CoV-2 in a radiation oncology clinic. JAMA Oncol. 2020;6:1632–1634. doi: 10.1001/jamaoncol.2020.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Gaudreault N.N., Richt J.A. Environmental stability of SARS-CoV-2 on different types of surfaces under indoor and seasonal climate conditions. bioRxiv. 2020 doi: 10.1101/2020.08.30.274241. 2020.08.30.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.H., Fan Y.Z., Jiang L., Wang H.B. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol. Infect. 2020;148:e154. doi: 10.1017/S0950268820001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafart P., Couvert O., Gaillard S., Leguerinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002;72:107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- Maritz J.M., Sullivan S.A., Prill R.J., Aksoy E., Scheid P., Carlton J.M. Filthy lucre: a metagenomic pilot study of microbes found on circulating currency in new York City. PLoS One. 2017;12:e0175527. doi: 10.1371/journal.pone.0175527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nextstrain Genomic Epidemiology of Novel Coronavirus - Global Subsampling [WWW Document]. URL. 2020. https://nextstrain.org/ncov
- Pal R., Bhadada S.K. Cash, currency and COVID-19. Postgrad. Med. J. 2020;96:427–428. doi: 10.1136/postgradmedj-2020-138006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg. Infect. Dis. J. 2020;26:2256. doi: 10.3201/eid2609.201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini, K., Castrica, M., Menchetti, L., Maggi, L., Negroni, L., Orfeo, N. V, Pizzoccheri, A., Stocco, M., Muttini, S., Balzaretti, C.M., 2020. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 742, 140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia, J.L., Rivera, D.N., Herrera, V.L., Morwitzer, M.J., Creager, H.M., Santarpia, G.W., Crown, K.K., Brett-Major, D.M., Schnaubelt, E.R., Broadhurst, M.J., Lawler, J. V, Reid, S.P., Lowe, J.J., 2020. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 10, 12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman R., Javaid M., Haleem A., Vaishya R., Bahl S., Nandan D. Sustainability of coronavirus on different surfaces. J. Clin. Exp. Hepatol. 2020;10:386–390. doi: 10.1016/j.jceh.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Vogel G., Wunderli W., Suter P., Witschi M., Koch D., Tapparel C., Kaiser L. Survival of influenza virus on banknotes. Appl. Environ. Microbiol. 2008;74:3002–3007. doi: 10.1128/AEM.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T., Zhang K., Maguire B., Terebessy E., Fong I., Parikh S., Zhu J. Correlation of ambient temperature and COVID-19 incidence in Canada. Sci. Total Environ. 2021;750:141484. doi: 10.1016/j.scitotenv.2020.141484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boekel Martinus A.J.S. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002;74(1):139–159. doi: 10.1016/S0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriesekoop F., Russell C., Alvarez-Mayorga B., Aidoo K., Yuan Q., Scannell A., Beumer R.R., Jiang X., Barro N., Otokunefor K., Smith-Arnold C., Heap A., Chen J., Iturriage M.H., Hazeleger W., DeSlandes J., Kinley B., Wilson K., Menz G. Dirty money: an investigation into the hygiene status of some of the world’s currencies as obtained from food outlets. Foodborne Pathog. Dis. 2010;7:1497–1502. doi: 10.1089/fpd.2010.0606. [DOI] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the coronavirus disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6 doi: 10.1128/mBio.01697-15. e01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2021. Corona virus disease (COVID-19) situation report [WWW Document]. January 10. URL https://www.who.int/emergencies/diseases/novel-coronavirus-2019/.
- Xie M., Chen Q. Insight into 2019 novel coronavirus - an updated interim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J., Li Y.M., Poon, Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet (London, England) 2003;362:1353–1358. doi: 10.1016/s0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data containing additional tables (Table A1 through A6) and figures (Fig. A1 through A2) to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.146133