Abstract
Recent data suggest that oral therapy can be effective for bone infections. We aim to assess the efficacy of an early switch to oral therapy ( weeks) compared to a non-early switch in bacterial native vertebral osteomyelitis. We conducted a cohort study at Mayo Clinic, Rochester (MN), between 2019–2021 combined with a systematic review, which queried multiple databases. Data were analyzed using a random-effects model. The cohort study included 139 patients: two received an early switch. Of 3708 citations, 13 studies were included in the final analysis. Meta-analysis demonstrated no difference in treatment failure (odds ratio 1.073, 95 % confidence interval 0.370–3.116), but many studies presented high risk of bias. Current evidence is insufficient to conclude the proportion of patients with failure or relapse is different in the two groups. High-quality studies are warranted before early switch can be routinely recommended.
1. Introduction
Vertebral osteomyelitis (VO) represents a small percentage of all osteomyelitis, accounting for 3 %–5 % of all cases (Issa et al., 2018). The incidence of VO is increasing, likely due to our enhanced ability to establish accurate diagnosis and the increasing prevalence of risk factors, such as aging population, diabetes mellitus, spinal surgery, and dialysis. VO can occur as a result of direct inoculation during surgery or contiguous spread from adjacent sites, but it is often the result of hematogenous seeding of adjacent disc space from a distant focus (Zimmerli, 2010). The blood supply of vertebral bodies is different from other bones, since it is based on cerebrospinal venous system, which is a large-capacity, valveless plexiform venous network in which flow is bidirectional and which provides a potential direct route for the spread of tumor, infections, or air emboli (Nathoo et al., 2011). This has important clinical considerations. The cornerstone of treatment is antimicrobial therapy. Unlike other sites of osteomyelitis, surgery is not a prerequisite for cure and is reserved for cases with spine instability, large epidural abscess formation, intractable back pain, or failure of medical treatment (Berbari et al., 2015).
Bacterial native vertebral osteomyelitis (NVO) is often the result of hematogenous seeding of bacteria to the end plate of the vertebral body. This entity is distinguished from hardware-associated, tubercular, brucellar, and fungal VO. These etiologies commend different epidemiology and treatment strategies (Hogan et al., 2019; Esmaeilnejad-Ganji and Esmaeilnejad-Ganji, 2019; Henry et al., 2017).
Parenteral antibiotics administered for 6 weeks is considered the standard of care for the treatment of most patients with bacterial NVO according to the IDSA guidelines (Berbari et al., 2015). However, we do recognize that oral therapy may be favored in selected patients and other regions around the world (Oh et al., 2019). A longer duration may be required for patients with certain specific risk factors, such as undrained abscess, multilevel disease, infection with methicillin-resistant Staphylococcus aureus (MRSA), and renal failure (Park et al., 2016).
Due to its inherent benefits and convenience, there is an increasing interest in using oral antimicrobial therapy as initial or as step-down treatment for various infectious diseases (Spellberg et al., 2020; Wald-Dickler et al., 2022; Mogle et al., 2019). Oral therapy can provide advantages, such as shortened length of hospital stay, fewer intravascular catheter-related adverse events, and lower costs. There is an abundance of emerging data on the efficacy and safety of oral antimicrobial therapy in bone and joint infections (Li et al., 2019; Azamgarhi et al., 2021), but data are scant for bacterial NVO.
We therefore performed a retrospective cohort study followed by a systematic review and meta-analysis to study differences in efficacy and safety between early switch and non-early switch to oral antibiotics for the treatment of bacterial NVO. This approach is based on published recommendations on incorporating unpublished health system data with systematic reviews to expand the evidence base and improve the strength of evidence, i.e. when data are sparse or limited (Lin et al., 2020).
2. Methods
2.1. Mayo Clinic retrospective study
After obtaining approval of the Institutional Review Board, we identified patients admitted with VO between 1 January 2019 and 31 December 2021, using administrative codes. We chose this timeframe to include more patients with an early switch to oral antibiotics, since the Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA) trial was published in January 2019 (Li et al., 2019).
We manually reviewed the charts. The criteria used to define bacterial NVO were (a) consistent imaging findings AND (b) microbiological growth in blood or spine tissue culture OR histological presence of inflammation OR strong suspicion based on symptoms (new or worsening back pain, fever, or neurological deficiencies), risk factors (recent episode of Staphylococcus aureus bloodstream infection, infective endocarditis, injection drug use, past surgical intervention in the spine, or immunocompromised status), and lab results (elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP)) in absence of another alternative diagnosis. A switch to oral antibiotics within 2 weeks from the start of the treatment was considered an early switch. A full course of oral therapy without a previous IV lead-in was considered an early switch. A full course of IV therapy or a switch after 2 weeks was considered a non-early switch.
We excluded children (age years), tubercular, brucellar, fungal infections, hardware-associated infections, and VO related to decubitus ulcers.
Data were gathered on demographics (age, sex, BMI), comorbidities, presence of a distant focus of infection, radiological features, treatment (type and duration of antibiotics, surgical management, implantation of hardware), and follow-up. We defined relapse as a new diagnosis of VO caused by the same organism after clinical and microbiological resolution of a previous episode within 6 months of the end of treatment and failure as a lack of response to initial therapy as reflected by an unplanned surgery or a start of a new cycle of antibiotic therapy.
2.2. Systematic review
2.2.1. Data sources and search strategies
A comprehensive search of several databases was performed on 3 February 2022. Date limits were set from 1985 forward. Animal studies were excluded. Results were limited to the English language. Databases searched were Ovid MEDLINE(R) 1946 to Present and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase 1974+, Ovid Cochrane Central Register of Controlled Trials 1991+, Ovid Cochrane Database of Systematic Reviews 2005+, Web of Science Core Collection via Clarivate Analytics (1975+), and Scopus via Elsevier (1988+). The search strategy was designed and conducted by a medical reference librarian with input from the investigators. Controlled vocabulary supplemented with keywords was used to search for studies describing VO and the use of oral versus parenteral antibiotics. The strategy listing all search terms and how they are combined is available in the Supplement (Fig. S2).
2.2.2. Study selection, data extraction, and quality assessment
Eligible studies had to have sufficient details to fulfill the criteria for bacterial NVO (as listed earlier) and enable the estimation of the proportion of patients with relapse and failure.
Two reviewers (Matteo Passerini and Julian Maamari) screened all titles and abstracts independently. Studies included at this level by either reviewer were included for full-text review.
The same pair of reviewers also screened full-text articles independently. Disagreements were resolved through discussion with a third reviewer (Elie F. Berbari). Study selection was managed using the Covidence platform. The same two reviewers extracted data independently including study design, year of study, demographics (age, gender), type and duration of parenteral antibiotic, type and duration of oral antibiotic, total duration of therapy, type of bacteria involved, presence of complications, presence of bacteremia, and effect estimates for outcomes of interest. Early switch was considered a switch to oral antibiotics within 2 weeks or a full course of oral antibiotics. Non-early switch was considered a switch after 14 d or a full course of IV therapy. Missing data were handled by contacting study authors to request needed information.
Risk of bias assessment was performed using the Cochrane Risk of Bias tool 2 for randomized clinical trials (RCTs) (Sterne et al., 2019), the Newcastle-Ottawa Scale for comparative observational studies (Stang, 2010), and a dedicated tool for single-arm non-comparative studies (Murad et al., 2018). Conflicts were resolved through discussion.
2.2.3. Evidence synthesis
For the retrospective cohort, dichotomous data were presented as numbers and percentages, while means and standard deviations or median and interquartile ranges were used to describe continuous variables. For the systematic review, data from our retrospective cohort were meta-analyzed with data from the other studies identified in the literature. We used the restricted maximum-likelihood random-effects model because heterogeneity of patients' characteristics and study settings was anticipated. Results of the comparative analysis (early switch vs. late switch) were expressed as an odds ratios (OR) and associated 95 % confidence intervals (CI). Results of the non-comparative series were expressed as the proportion of patients with relapse or failure, pooled using Freedman–Tukey transformation (Lin and Xu, 2020). Meta-analysis was conducted using OpenMeta[Analyst] (Wallace et al., 2012).
We assessed the certainty of evidence (CoE) using the GRADE approach. High initial certainty is assigned to the evidence derived from randomized controlled trials. Evidence from observational studies starts at low initial certainty. Then CoE is rated down based on risk of bias, inconsistency (i.e., heterogeneity), indirectness, imprecision, or publication bias. Consequently, CoE is judged as very low, low, moderate, or high (Guyatt et al., 2011).
The protocol of the systematic review is registered in PROSPERO (no. CRD42022308086).
3. Results
3.1. Mayo Clinic retrospective study
We identified 148 cases of confirmed bacterial NVO. In the non-early-switch group, two patients were lost to follow-up, and seven patients died within 4 weeks from the start of treatment. After reviewing the chart of these patients, we did not consider bacterial NVO as the cause of death. We assessed the outcome of failure and relapse among the remaining 139 patients with a median follow-up of 11.16 months (interquartile range (IQR) 3.8–21.6). The clinical characteristics are summarized in Table 1. Two patients were managed with an early switch to oral therapy. One was a 71-year-old man with methicillin-sensitive Staphylococcus aureus (MSSA) as the culprit organism; he underwent a L2–L5 laminectomy with history of postoperative wound dehiscence; 2 years after the surgical procedure, he developed back pain and recurrence of wound dehiscence, which was treated successfully with debridement and a 6-week oral cefadroxil without a previous IV course. The second patient was a 75-year-old man who slipped and fell and shortly after began to have some back pain without fever or neurological symptoms. A CT scan was obtained which showed some erosive changes at the L1–L2 disc space. A CT-guided biopsy was obtained, which demonstrated Pseudomonas aeruginosa. He was started on oral ciprofloxacin for a total duration of 12 weeks with clinical success. Neither of them had bacteremia, endocarditis, or a related abscess.
In the non-early-switch group ( ), we registered five cases of relapse and eight cases of failure (Table 1). Of the 13 patients, there were five cases of MSSA and two of MRSA, methicillin-resistant Staphylococcus epidermidis (MRSE), streptococcal infections, and culture-negative infections, each. Of the 13 patients, 10 had a concomitant bacteremia, 2 had endocarditis, and 8 presented with a related abscess, 2 of which were treated surgically. Of these 13 patients, 7 met the WHO definition of obesity (https://www.who.int/health-topics/obesity#tab=tab_1, last access: 1 August 2022).
Table 1.
Demographic and clinical characteristics of Mayo patients with bacterial NVO between 2019–2021.
| Early switch ( ) | Non-early switch ( ) | ||
|---|---|---|---|
| Female, (%) | 0 | 50 (36.5) | |
| Age, mean (SD) | 73 (2.82) | 64.63 (11.48) | |
| BMI, mean (SD) | 34.83 (16.19) | 30.10 (6.77) | |
| Comorbidities, (%) | 0 | 69 (50.4) | |
| Diabetes | 0 | 31 (22.6) | |
| Immunosuppression | 0 | 17 (12.4) | |
| Dialysis | 0 | 10 (7.3) | |
| Injection drug users | 0 | 2 (1.5) | |
| Active malignancy | 0 | 9 (6.6) | |
| Obesity | 1 (50) | 58 (42.3) | |
| Related abscess, (%) | 0 | 63 (46) | |
| Bacteremia, (%) | 0 | 79 (57.7) | |
| Endocarditis, (%) | 0 | 16 (11.7) | |
| Surgical-treated, (%) | 1 (50) | 33 (24) | |
| Hardware-implantation,
(%) |
0 |
15 (11) |
|
| Outcomes,
(%) | |||
| Relapse | 0 | 5 (3.6) | |
| Failure | 0 | 8 (5.8) | |
3.2. Systematic review and meta-analysis
The literature search results are presented in the PRISMA flow diagram in Fig. 1. A total of 14 studies (1078 patients with bacterial NVO) were included in this systematic review, including the present cohort (Table 2). We found one RCT (Li et al., 2015), six comparative observational studies (Babouee Flury et al., 2014; Lestin-Bernstein et al., 2018; Locke et al., 2014; Oh et al., 2019; Kim et al., 2019; Russo et al., 2020), and six non-comparative observational studies (Bettini et al., 2009; García Del Pozo et al., 2018; Guo et al., 2021; Livorsi et al., 2008; Azamgarhi et al., 2021; Sakeni and Al-Nimer, 2008), where the non-early-switch group represented the single arm. To satisfy our predetermined criteria, asking for additional information from some authors was necessary. We received the data requested for five of the studies included (Azamgarhi et al., 2021; García Del Pozo et al., 2018; Kim et al., 2019; Lestin-Bernstein et al., 2018; Li et al., 2019).
Figure 1.
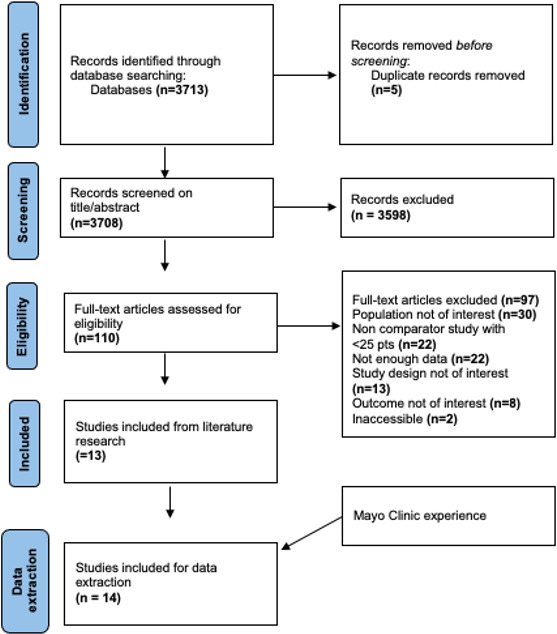
PRISMA flow diagram of the studies included.
Table 2.
Studies included in systematic review and meta-analysis.
| First author | Year | Design | Male | Age, years | Related | Bacteremia | Endocarditis | No. of pts |
||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | mean (SD) | abscess (%) | (%) | (%) | Early | Non-early | ||||
| Li | 2019 | RCT | 58.3 | 55 (12.6) | NR | NR | 0 | 18 | 18 | |
| Babouee Flury | 2014 | Comparative observational | NR | NR | NR | NR | 0 | 21 | 40 | |
| Lestin-Bernstein | 2018 | Comparative observational | 73.3 | 68.7 (11.5) | 79 | 46.7 | 6.7 | 15 | 30 | |
| Locke | 2014 | Comparative observational | NR | NR | NR | NR | NR | 9 | 30 | |
| Oh | 2019 | Comparative observational | NR | NR | NR | NR | 0 | 5 | 37 | |
| Kim | 2019 | Comparative observational | 63.6 | 64 (13) | 25 | 80 | 5,7 | 12 | 408 | |
| Russo | 2020 | Comparative observational | NR | NR | 0 | 40 | 8.3 | 5 | 55 | |
| Bettini | 2009 | Non-comparative observational | 62.5 | 51.9 (10) | 5.4 | 17.85 | NR | 0 | 56 | |
| Garcia del Pozo | 2018 | Non-comparative observational | 53.3 | 69.3 (12.28) | NR | NR | NR | 0 | 15 | |
| Guo | 2021 | Non-comparative observational | 77 | 55 (11) | NR | NR | NR | 0 | 76 | |
| Livorsi | 2008 | Non-comparative observational | 86 | 52.9 (6.5) | NR | 100 | NR | 0 | 35 | |
| Azamgarhi | 2021 | Non-comparative observational | 75 | 70 (5.22) | NR | NR | NR | 0 | 4 | |
| Sakeni | 2008 | Non-comparative observational | 62 | 38.2 (13.8) | NR | 12 | NR | 0 | 50 | |
3.2.1. Failure and relapse
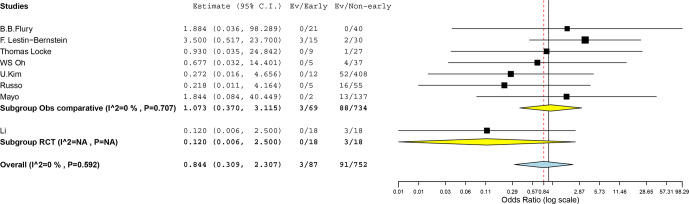
In the RCT we found cases of failure or relapse for the early-switch group and for the non-early switch (OR 0.120, CI 0.006–2.500). A meta-analysis of the seven observational comparative studies (including the Mayo retrospective study) did not have enough evidence to show a higher efficacy of one group (OR 1.073, 95 % CI 0.370–3.115). The heterogeneity between the studies was low ( %, ). Combining data from the RCT and the comparative observational studies further confirmed the inconclusiveness of the results given the wide confidence interval (OR 0.844, 95 % CI 0.309–2.307, %, ; Fig. 2).
Figure 2.
Meta-analysis of risk for failure and relapse between early switch and non-early switch to oral therapy for bacterial NVO (CI, confidence interval; Obs, observational, RCT, randomized clinical trial, Ev, events).
3.2.2. Failure or relapse in the non-early group
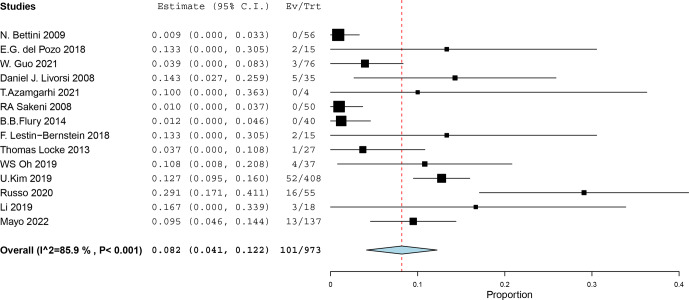
The proportion of patients with failure or relapse in the non-early-switch group among all the studies was 8.2 % (95 % CI 4.1 %–12.2 %; Fig. 3). The heterogeneity between the studies was high ( %, ).
Figure 3.
Proportion of patients with failure and relapse among all the studies including a non-early switch (Ev, events; Trt, group of treated patients with non-early switch).
3.2.3. Methodological quality of included studies
The methodological quality assessment of the studies included is shown in the Supplement (Table S1). The quality of the only RCT included in our review was considered high (Li et al., 2019). Regarding the quality of the observational comparative studies, four studies suffered from high risk of bias in the comparability domain due to differences in demographics, comorbidities, and clinical presentation between the patients in the two arms (Locke et al., 2014; Oh et al., 2019; Alshamsi et al., 2016; Russo et al., 2020). For the remaining three studies, including the Mayo Clinic cohort, there were not enough data to assess whether the two included cohorts are comparable (Babouee Flury et al., 2014; Lestin-Bernstein et al., 2018). The ascertainment of exposure and outcomes was low-risk overall. Regarding the observational non-comparative studies, reported details were not sufficient to draw inferences that can be used in decision making; hence the quality of these studies was deemed low (Bettini et al., 2009; García Del Pozo et al., 2018; Guo et al., 2021; Livorsi et al., 2008; Li et al., 2019; Sakeni and Al-Nimer, 2008).
3.2.4. Certainty in the evidence
The certainty in the reported outcomes of failure or relapse was assessed separately for the seven observational comparative studies and the RCT. The CoE stemming from the RCT was downrated by two levels (low CoE) due to severe imprecision; the optimal information size criteria were not met, and the CI overlaps both thresholds of considerable benefit and harm. The CoE from observational studies was very low due to the poor methodological quality of the studies included and severe imprecision (Supplement Fig. S1).
4. Discussion
Our study results were inconclusive to show a significant difference in the proportion of patients with failure or relapse between early and non-early switch to oral antibiotics for the treatment of bacterial NVO. The overall proportion of patients developing failure or relapse among the non-early treatment group was 8.2 % (95 % CI 4.1 %–12.2 %).
Our results are consistent with previous literature regarding treatment of osteomyelitis, but they provided a better focus on bacterial NVO. A Cochrane meta-analysis found no difference between oral and parenteral therapy in the rate of remission at the end of the therapy and at follow-up in the treatment of chronic osteomyelitis in adults (Conterno and Turchi, 2013). This meta-analysis compiled all anatomic sites of osteomyelitis; moreover, it was published in 2013, so it did not include some studies we included in our systematic review. A more recent meta-analysis comprising 1321 patients concluded that the overall treatment success was not significantly different between oral step-down therapy and IV-only antibiotic therapy for the treatment of bone infections (Wald-Dickler et al., 2022). However, in the studies included in this meta-analysis, VO was either excluded (Mader, 1990; Gomis, 1999; Euba et al., 2009), or the number of patients with this specific bone infection was too low to draw a significant conclusion (Li et al., 2019). In a French RCT published in 2015, the authors randomized the patients in two groups to assess whether 6 weeks of antibiotic treatment was non-inferior to 12 weeks in patients with bacterial NVO. In this study, 52 % of the patients received IV antibiotics for less than 14 d, reflecting the more widespread use of an early switch to oral therapy in some European institutions. Moreover, the proportion of patients with treatment failure at 1 year of follow-up was not significantly different between patients treated with IV therapy for less than 1 week ( , 13 %) and for more than 1 week ( , 7 %; ) (Bernard et al., 2015). Since we used a different cutoff of 2 weeks to define the early switch to oral therapy, we could not include this study in our meta-analysis.
The belief that bone is a difficult site to penetrate with antibiotics and that the parenteral route achieves more effective concentration could explain the hesitation to use oral therapy for bone infections. However, the assumption that parenteral therapy is more effective in treating bone infections is not supported by strong evidence. Moreover, some studies demonstrated that many oral antibiotics have excellent oral bioavailability and can reach concentrations above the minimum inhibitory concentration (MIC) in the bone, similar to parenteral antimicrobial therapy (Spellberg and Lipsky, 2012; Kutscha-Lissberg et al., 2003; Fong et al., 1986).
On the other side, bone and joint infections comprise a broad range of diseases, including different sites of infection, different complications, different treatments, and different etiological microorganisms. Vertebral tissue has a unique vascular system compared to other bones. For this reason, guidelines specific to NVO were published (Berbari et al., 2015). Thus, particular attention should be paid to the application of results of studies regarding other types of osteomyelitis to NVO. This could explain the low number of patients in the early-switch group in the Mayo Clinic cohort, on data collected from 2019 to 2021, after the publication of the OVIVA trial (Li et al., 2019).
Since this systematic review did not provide a conclusive result, further studies are needed to assess the efficacy of an early switch to oral therapy for patients with bacterial NVO. A possible design for a future study could be a pragmatic trial (Iversen et al., 2019; Ford and Norrie, 2016) designed to show the real-world effectiveness of the intervention in different presentations of bacterial NVO. Some patients with bacterial NVO can reasonably benefit from parenteral therapy at the beginning given the clinical instability, concomitant bacteremia or endocarditis, and difficulty absorbing oral medication. Thus, in this hypothetical trial, we propose to randomize the patients to switch to oral or prolonged parenteral therapy when recently published criteria are met: (a) clinical stability (hemodynamically and no spinal instability), (b) adequate source control, (c) likelihood to absorb oral medications, (d) an available oral regimen used in published studies to cover the pathogen, (e) no psychosocial reasons that preclude the safe use of oral therapy, and (f) any other concomitant infection which requires a prolonged course of intravenous antibiotic therapy (Spellberg et al., 2022).
To the best of our knowledge, our study provides the best available evidence regarding oral therapy for bacterial NVO.
However, it has several limitations. First, most of the articles included in this review were retrospective series, with a relatively low number of patients in the early-switch group. Second, the comparability of the two groups of patients among most of the studies was at high risk of bias; there is the possibility that in many studies the patients receiving an early switch were “less severe” than the patients with a non-early switch. Third, some studies included only specific types of bacterial NVO. The OVIVA trial excluded patients with Staphylococcus aureus bacteremia on presentation or within the last month and patients with endocarditis (Li et al., 2015). These two groups represent a significant proportion of patients with bacterial NVO, as was also demonstrated by our retrospective study, where we found 31.65 % of patients with concomitant S. aureus bacteremia and 11.5 % of patients with endocarditis. Livorsi et al. (2008) only included patients with concomitant S. aureus bacteremia, and Russo et al. (2020) only included patients with enterococcal or staphylococcal bacterial NVO. Fourth, we were not able to perform any subgroup analysis given the paucity of data. Fifth, to assess the appropriateness of a pharmacological intervention, safety is an important outcome. We were not able to perform a meta-analysis for adverse events since most of the studies did not report this data. In the OVIVA trial, there were four cases of line complications and one case of Clostridium difficile infection in the non-early-switch group. No such complications were seen in the early-switch group. Sixth, given the low number of studies included, we did not perform a statistical analysis to assess the risk of publication bias.
In conclusion, considering the very wide confidence interval and the high risk of bias in non-randomized data, there is insufficient evidence to conclude that the proportion of patients with failure or relapse is different in the early-switch group compared to the non-early-switch group to oral antibiotics for the treatment of bacterial NVO. Therefore, the meta-analysis with the existing data is too limited to draw conclusions for clinical practice. Future studies specifically addressing bacterial NVO are needed to increase the evidence.
Supplement
Acknowledgements
Special thanks are expressed to Massimo Coen for introducing Matteo Passerini to the field of orthopedic infection.
Disclaimer
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Matteo Passerini, Email: matteo.passerini1@gmail.com.
Elie F. Berbari, Email: berbari.elie@mayo.edu.
Data availability
Data are available upon request.
Author contributions
MP, MHM, and EFB were responsible for study conception and design. MP, JM, and LCH extracted the data from the retrospective cohort and from the literature. MP, TN, and MHM analyzed the data. MP, TN, MHM, and EFB were responsible for the interpretation of the data. All authors reviewed the results and approved the final version of the paper.
Competing interests
At least one of the (co-)authors is a member of the editorial board of . The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Ethical statement
The study was carried out in accordance with the applicable legislation, including review by an accredited research ethics committee (22-001001).
Review statement
This paper was edited by Marjan Wouthuyzen-Bakker and reviewed by two anonymous referees.
References
- Alshamsi F, Belley-Cote E, Cook D, Almenawer SA, Alqahtani Z, Perri D, Thabane L, Al-Omari A, Lewis K, Guyatt G, Alhazzani W. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20:120. doi: 10.1186/s13054-016-1305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azamgarhi T, Shah A, Warren S. Clinical Experience of Implementing Oral Versus Intravenous Antibiotics (OVIVA) in a Specialist Orthopedic Hospital. Clin Infect Dis. 2021;73:e2582–e2588. doi: 10.1093/cid/ciaa985. [DOI] [PubMed] [Google Scholar]
- Babouee Flury B, Elzi L, Kolbe M, Frei R, Weisser M, Schären S, Widmer AF, Battegay M. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis. 2014;14:226. doi: 10.1186/1471-2334-14-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, Petermann GW, Osmon DR. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adultsa. Clin Infect Dis. 2015;61:e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, Belmatoug N, Lesprit P, Bru JP, Therby A, Bouhour D, Dénes E, Debard A, Chirouze C, Fèvre K, Dupon M, Aegerter P, Mulleman D. Duration of Treatment for Spondylodiscitis (DTS) study group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–882. doi: 10.1016/S0140-6736(14)61233-2. [DOI] [PubMed] [Google Scholar]
- Bettini N, Girardo M, Dema E, Cervellati S. Evaluation of conservative treatment of non specific spondylodiscitis. Eur Spine J. 2009;18:143–150. doi: 10.1007/s00586-009-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conterno LO, Turchi MD. Antibiotics for treating chronic osteomyelitis in adults. Cochrane database Syst Rev. 2013;9:CD004439. doi: 10.1002/14651858.CD004439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: A review. World J Orthop. 2019;10:54–62. doi: 10.5312/wjo.v10.i2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euba G, Murillo O, Fernández-Sabé N, Mascaró J, Cabo J, Pérez A, Tubau F, Verdaguer R, Gudiol F, Ariza J. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother. 2009;53:2672–2676. doi: 10.1128/AAC.01504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong IW, Ledbetter WH, Vandenbroucke AC, Simbul M, Rahm V. Ciprofloxacin concentrations in bone and muscle after oral dosing. Antimicrob Agents Chemother. 1986;29:405–408. doi: 10.1128/AAC.29.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- García Del Pozo E, Collazos J, Cartón JA, Camporro D, Asensi V. Bacterial osteomyelitis: microbiological, clinical, therapeutic, and evolutive characteristics of 344 episodes. Rev Esp Quimioter. 2018;31:217–225. [PMC free article] [PubMed] [Google Scholar]
- Gomis M. Oral ofloxacin versus parenteral imipenem-cilastatin in the treatment of osteomyelitis. Rev Esp Quimioter. 1999;12:244–249. [PubMed] [Google Scholar]
- Guo W, Wang M, Chen G, Chen K-H, Wan Y, Chen B, Zou X, Peng X. Early surgery with antibiotic medication was effective and efficient in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord. 2021;22:288. doi: 10.1186/s12891-021-04155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Henry MW, Miller AO, Walsh TJ, Brause BD. Fungal Musculoskeletal Infections. Infect Dis Clin North Am. 2017;31:353–368. doi: 10.1016/j.idc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Hogan JI, Hurtado RM, Nelson SB. Mycobacterial Musculoskeletal Infections. Thorac Surg Clin. 2019;29:85–94. doi: 10.1016/j.thorsurg.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, Emami A. The Epidemiology of Vertebral Osteomyelitis in the United States From 1998 to 2013. Clin Spine Surg. 2018;31:E102–E108. doi: 10.1097/BSD.0000000000000597. [DOI] [PubMed] [Google Scholar]
- Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Høfsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosbøll EL, Rosenvinge F, Schønheyder HC, Køber L, Torp-Pedersen C, Helweg-Larsen J, Tønder N, Moser C, Bundgaard H. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med. 2019;380:415–424. doi: 10.1056/NEJMoa1808312. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Bae JY, Kim S-E, Kim C-J, Kang S-J, Jang H-C, Jung SI, Song K-H, Kim ES, Kim HB, Park WB, Kim NJ, Park K-H. Comparison of pyogenic postoperative and native vertebral osteomyelitis. Spine J. 2019;19:880–887. doi: 10.1016/j.spinee.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Kutscha-Lissberg F, Hebler U, Muhr G, Köller M. Linezolid penetration into bone and joint tissues infected with methicillin-resistant staphylococci. Antimicrob Agents Chemother. 2003;47:3964–3966. doi: 10.1128/AAC.47.12.3964-3966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestin-Bernstein F, Tietke M, Briedigkeit L, Heese O. Diagnostics and antibiotic therapy for spondylodiscitis. J Med Microbiol. 2018;67:757–768. doi: 10.1099/jmm.0.000703. [DOI] [PubMed] [Google Scholar]
- Li HK, Scarborough M, Zambellas R, Cooper C, Rombach I, Walker AS, Lipsky BA, Briggs A, Seaton A, Atkins B, Woodhouse A, Berendt A, Byren I, Angus B, Pandit H, Stubbs D, McNally M, Thwaites G, Bejon P. Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial. Trials. 2015;16:583. doi: 10.1186/s13063-015-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-K, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, Lipsky BA, Hughes HC, Bose D, Kümin M, Scarborough C, Matthews PC, Brent AJ, Lomas J, Gundle R, Rogers M, Taylor A, Angus B, Byren I, Berendt AR, Warren S, Fitzgerald FE, Mack DJF, Hopkins S, Folb J, Reynolds HE, Moore E, Marshall J, Jenkins N, Moran CE, Woodhouse AF, Stafford S, Seaton RA, Vallance C, Hemsley CJ, Bisnauthsing K, Sandoe JAT, Aggarwal I, Ellis SC, Bunn DJ, Sutherland RK, Barlow G, Cooper C, Geue C, McMeekin N, Briggs AH, Sendi P, Khatamzas E, Wangrangsimakul T, Wong THN, Barrett LK, Alvand A, Old CF, Bostock J, Paul J, Cooke G, Thwaites GE, Bejon P, Scarborough M. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380:425–436. doi: 10.1056/NEJMoa1710926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Murad MH, Leas B, Treadwell JR, Chou R, Ivlev I, Kansagara D. A Narrative Review and Proposed Framework for Using Health System Data with Systematic Reviews to Support Decision-making. J Gen Intern Med. 2020;35:1830–1835. doi: 10.1007/s11606-020-05783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Heal Sci Reports. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livorsi DJ, Daver NG, Atmar RL, Shelburne SA, White AC, Musher DM. Outcomes of treatment for hematogenous Staphylococcus aureus vertebral osteomyelitis in the MRSA ERA. J Infect. 2008;57:128–131. doi: 10.1016/j.jinf.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Locke T, Kell ME, Bhattacharyya D, Cole AA, Chapman ALN. Spontaneous methicillin-sensitive Staphylococcus aureus spondylodiscitis-Short course antibiotic therapy may be adequate: Evidence from a single centre cohort. J Infect Public Health. 2014;7:44–49. doi: 10.1016/j.jiph.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Mader J. Oral ciprofloxacin compared with standard parenteral antibiotic therapy for chronic osteomyelitis in adultsNo Title. J Bone Jt Surg. 1990;72:104–110. [PubMed] [Google Scholar]
- Mogle BT, Beccari MV, Steele JM, Fazili T, Kufel WD. Clinical considerations for oral beta-lactams as step-down therapy for Enterobacteriaceae bloodstream infections. Expert Opin Pharmacother. 2019;20:903–907. doi: 10.1080/14656566.2019.1594774. [DOI] [PubMed] [Google Scholar]
- Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ evidence-based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathoo N, Caris EC, Wiener JA, Mendel E. History of the Vertebral Venous Plexus and the Significant Contributions of Breschet and Batson. Neurosurgery. 2011;69:1007–1014. doi: 10.1227/NEU.0b013e3182274865. [DOI] [PubMed] [Google Scholar]
- Oh WS, Moon C, Chung JW, Choo EJ, Kwak YG, Kim SH, Ryu SY, Park SY, Kim BN. Antibiotic Treatment of Vertebral Osteomyelitis caused by Methicillin-Susceptible Staphylococcus aureus: A Focus on the Use of Oral -lactams. Infect Chemother. 2019;51:284–294. doi: 10.3947/ic.2019.51.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-H, Cho O-H, Lee JH, Park JS, Ryu KN, Park SY, Lee Y-M, Chong YP, Kim S-H, Lee S-O, Choi S-H, Bae I-G, Kim YS, Woo JH, Lee MS. Optimal Duration of Antibiotic Therapy in Patients With Hematogenous Vertebral Osteomyelitis at Low Risk and High Risk of Recurrence. Clin Infect Dis. 2016;62:1262–1269. doi: 10.1093/cid/ciw098. [DOI] [PubMed] [Google Scholar]
- Russo A, Ceccarelli G, Bellelli V, Bianchi L, Marincola Cattaneo F, Gregori F, Palmarini V, Marotta N, Landi A, Cuzzolino A, Stefanini M, Aureli A, Mastroianni CM, Venditti M, d'Ettorre G, Sabetta F. Efficacy of Daptomycin-Containing Regimen for Treatment of Staphylococcal or Enterococcal Vertebral Osteomyelitis: A Prospective Clinical Experience. Antibiot (Basel, Switzerland) 2020;9:889. doi: 10.3390/antibiotics9120889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakeni RA, Al-Nimer MSM. Infectious discitis in adults: 9 years experience from Al-Yarmouk Teaching Hospital in Baghdad, Iraq. Int J Rheum Dis. 2008;11:175–180. doi: 10.1111/j.1756-185X.2008.00354.x. [DOI] [Google Scholar]
- Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Chambers HF, Musher DM, Walsh TL, Bayer AS. Evaluation of a Paradigm Shift From Intravenous Antibiotics to Oral Step-Down Therapy for the Treatment of Infective Endocarditis. JAMA Intern Med. 2020;180:769. doi: 10.1001/jamainternmed.2020.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Aggrey G, Brennan MB, Footer B, Forrest G, Hamilton F, Minejima E, Moore J, Ahn J, Angarone M, Centor RM, Cherabuddi K, Curran J, Davar K, Davis J, Dong MQ, Ghanem B, Hutcheon D, Jent P, Kang M, Lee R, McDonald EG, Morris AM, Reece R, Schwartz IS, So M, Tong S, Tucker C, Wald-Dickler N, Weinstein EJ, Williams R, Yen C, Zhou S, Lee TC, Baden R, Bedard-Dallare S, Beltran C, Blythe M, Brass E, Chi S, Coffey C, Cowart M, Diaz A, Dwyer J, Jordan Villegas A, Khan E, Martinez J, Mattappallil A, Meshkaty N, Patel A, Pullen M, Rajan S, Saxinger L, Tirupathi R, Trivedi J, Vilchez-Molina G, Werge D, Werge D. Use of Novel Strategies to Develop Guidelines for Management of Pyogenic Osteomyelitis in Adults. JAMA Netw Open. 2022;5:e2211321. doi: 10.1001/jamanetworkopen.2022.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Wald-Dickler N, Holtom PD, Phillips MC, Centor RM, Lee RA, Baden R, Spellberg B. Oral Is the New IV. Challenging Decades of Blood and Bone Infection Dogma: A Systematic Review. Am J Med. 2022;135:369–379. doi: 10.1016/j.amjmed.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: as a Computational Back-End. J Stat Softw. 2012;49:1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.