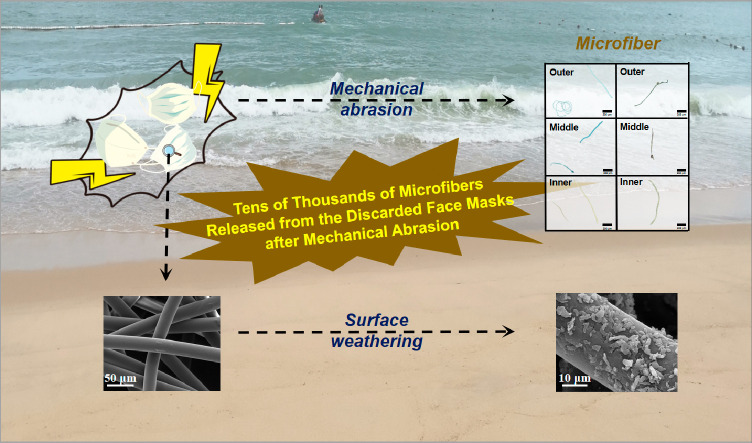
Abstract
While mechanical abrasion by water and sediment is a primary and critical step in weathering process, the upsurge of discarded face masks will undoubtedly become a potential source of micro-/nanofibers owing to the spread of novel coronavirus (COVID-19) pneumonia. However, effects of mechanical abrasion on discarded face masks have neither been seriously addressed nor understood. Therefore, we conducted a simulated experiment to explore abundance, size distribution and morphology of microfibers released from common, surgical and face filtering piece (FFP) masks after mechanical abrasion. Technologies such as Fourier transform infrared spectrometry, fluorescence microscopy, scanning electron microscopy, and confocal laser scanning microscopy were used. Results showed that the abundance of released microfibers followed order of surgical > common > FFP in both water and sediment environments, and the maximum abundance reached 272 ± 12.49 items per square centimeter of mask (items·cm−2) after sediment abrasion. Taking surgical mask for further investigation, the length of released fiber was observed to vary from 47.78 μm to 3.93 mm, and 72.41–89.58% of the total number of released microfibers fell in the range of 0.1–1 mm. However, microfibers with a very small length (1–100 μm) can occupy 0.09–13.59% of the total number of released fibers in sediment environment. The roughness of fiber surface after sediment abrasion was successively increased. Furthermore, the morphology analysis showed significant changes with countless cracks and many prominent protrusions on fiber surface after sediment abrasion. The cracks and protrusions may further accelerate mask decomposition, thereby potentially resulting in the adsorption of other contaminants and the release of self-containing chemicals. This study provides a valuable database of microfibers released from discarded face masks at the primary but critical stage, and further contributes knowledge on environmental impact of discarded personal protective equipment due to COVID-19.
Keywords: Face masks, Mechanical abrasion, Microfiber release, Confocal microscopy, COVID-19
Graphical abstract
1. Introduction
Novel coronavirus pneumonia (COVID-19) has been rapidly spreading worldwide since the beginning of 2020 (Murray et al., 2020). The World Health Organization (WHO) calls for strict interventions to prevent COVID-19 from further spread, if which, wearing a face mask is considered to be one of the best protective measures that can effectively block the spread of coronavirus by droplets from presymptomatic and asymptomatic individuals (Howard et al., 2020; Xu et al., 2020). This has led to a dramatic increase in global face mask production (up to 396.6%) by 2020 (Chua et al., 2020; Fadare and Okoffo, 2020). The daily capacity of masks in China has increased by 450%, from the 20 million at the beginning to 110 million by the end of February 2020 (Ren, 2020; Singh et al., 2020a). It was anticipated that even in the post-pandemic period, the compound annual growth rate (CAGR) of face masks could still be increased by 10.8% from 2020 to 2027 (Global Protective Face Masks Market (GPFMM), 2020). Such an extensive production of face masks will not only create a massive disruption of the upstream supply chain but also overwhelm the downstream waste management problems (Klemeš et al., 2020; Narendra et al., 2021; Singh et al., 2020b).
If the used face masks are not properly managed, they will eventually enter and persist in the environment (Huang and Morawska, 2019). At present, a large amount of mask debris has been observed on the coast of Hong Kong, the United States, France, and Mainland China (Yeh, 2020), while dozens of masks have also been found floating on the waves of the Mediterranean Sea (Roberts et al., 2020). The abandoned face masks are likely to be fragmented when subjected to weathering effects and then release small secondary particles that can be recognized as microplastics (1 μm–5 mm) or nanoplastics (<1 μm) (Peeken et al., 2018; Wu et al., 2020a). In addition, some microfibers can also be formed and trapped in the face masks when the thermoplastic fiber is extruded and blown by high velocity and temperature airflow during the manufacturing processes (Hutten, 2007; Li et al., 2021). Currently, micro/nanofibers, as the primary constituent of micro/nanoplastics, have been considered as emerging contaminants with needle-like shapes (Kutralam-Muniasamy et al., 2020; Liu et al., 2019). Compared to micro/nanoplastics with other shapes, the micro/nanofiber can more easily penetrate the biological membrane, leading to the dysregulation of tissues and organs(de Sá et al., 2018; Galloway and Lewis, 2016; Wu et al., 2020b, Wu et al., 2020c) and biomagnification through the food chain from plankton to the human colon (de Sá et al., 2018; Ibrahim et al., 2021).
Weathering has been regarded as the most critical process for the aging of carbonaceous polymers, including ultraviolet irradiation, temperature degradation, oxidative transformation and mechanical abrasion (Wu et al., 2019). Recent studies have shown that biotic and abiotic hydrolysis can gradually erose the surface of plastics, thereby reforming various reactive oxygen groups to accelerate the oxidative transformation of the micro/nanoplastic (Min et al., 2020). Nanoplastics, together with styrene monomers, were found to be released from coffee cups into nature after being subjected to ultraviolet irradiation (Lambert and Wagner, 2016). Mechanical abrasion caused by tidal currents, especially plastic wastes on beaches, has been widely recognized as the primary and critical process for the generation of micro/nanoplastics (Chubarenko et al., 2020; Song et al., 2017). The occurrence of the discarded face masks on the beaches indicates that mechanical abrasion by water and sediment may be critically involved in the weathering processes, especially during the formation of microfibers at an early stage. Several publications point out that the face mask as a potential source of microfibers can quickly intensify the already critical situation (Morgana et al., 2021; Saliu et al., 2021; Wang et al., 2021). The mechanical strength of masks decreased after ultraviolet irradiation (Wang et al., 2021), and then macro-, micro-, and even nanoplastics can be released from the face masks under different shear stress intensities (Morgana et al., 2021). However, the weathering effect of mechanical abrasion caused by the discarded face masks has not been seriously addressed and fully understood by the current reported studies. Particularly during the COVID-19 pandemic, the upsurge of face masks promoted the emergence of investigating such effects on the formation of micro/nanofibers at an early stage.
Therefore, in this study, the aging of face masks towards micro/nanofibers under mechanical abrasion by water and sediment was investigated. Face masks of different types and brands were applied, and the increase in microfiber abundance was evaluated over the abrasion time in water and sediment environments. The abundance, size and color were also described to explain the possible release behavior of microfibers from the surgical face masks. Furthermore, the surface morphologies of the masks were characterized to clarify the variation of surface roughness during the aging process. This study provides a comprehensive investigation of the release of microfibers from the discarded face masks under mechanical abrasion in different environments, and further contributes towards the expansion of valuable database in current studies on environmental burden caused by the dramatic increase in personal protective equipment due to the COVID-19 pandemic.
2. Materials and methods
2.1. Raw materials and sample preparation
As shown in Table S1 of the Supplementary Information (SI), three types of face masks generally used during the COVID-19 pandemic were selected for this research, including common masks, surgical masks, and face filtering piece masks (FFP; e.g. N95) (Greenhalgh et al., 2020). Three layers, namely the inner, middle and outer layers, consisted of the face masks mentioned above. To simulate a real environment, sediments were sampled from Soko Beaches, Hong Kong. The sediments were pretreated to remove the plastics according to the density separation and extraction reported by Wu et al. (2020b). The organic matter in the sediments was subsequently washed for three times with 18.2 MΩ deionized (DI) water (Millipore Co., USA) and combusted in a muffle furnace at 450 °C for 3 h. After cooling down, the particle size distribution (D50 = 562.25 μm) of the sediments was measured using a laser scattering technique (Mastersizer 3000, Malvern) (Fig. S1).
Mechanical abrasion experiments were conducted in both the water and sediment environments. Each layer of the face mask was separated and cut into one square centimeter, and then transferred into a glass tube (Fisher Scientific, USA). The glass tubes were then filled with 15 mL of DI water or the saturated sands (6 g) with 10 mL of DI water with no head space, to represent the water or sediment environmental conditions, respectively. After that, all tubes were rotated end-over-end at 60 rpm at different time intervals from 0 to 240 h. After agitation, the suspension under water condition was filtered directly through a 1 μm GF/C glass fiber (Whatman, UK). Meanwhile, the microfibers in the sediment environment were suspended in 500 mL of NaI (Sigma-Aldrich, USA) solution (1.8 g·cm−3) by magnetic stirring for 4 h to separate microfibers. After settling for another 24 h, the solution was filtered through a glass fiber membrane, and then the membranes with extracted microfibers were transferred into the glass culture dish for oven-drying at 60 °C for 3 h for further analysis. All experiments were conducted in triplicates. For more detailed information, please refer to the extraction procedures reported in our previous study (Wu et al., 2020b).
2.2. Sample characterization and analysis
Each layer of different mask types was measured using a Fourier transform infrared spectrometer (FT-IR) apparatus with attenuated total reflection (ATR) mode (Spotlight 200i, PerkinElmer, USA), and the spectra were recorded as six accumulations ranging from 400 to 4000 cm−1. According to the polymer library in the PerkinElmer database (around 16,000 reference spectra), PP polymer was used as the material for the three layers of the surgical masks, the middle and outer layer of the common masks, and the inner and outer layers of the N95 masks. The inner layers of the common masks and middle layer of the N95 masks were composed of high-density polyethylene (HDPE) (Table S1).
Fluorescence microscopy (DM2500, Leica, Germany) with 10× magnification was applied to determine the abundance, size and color of the microfibers. The unit of the released microfibers was recorded as the number of microfibers per square centimeter of the face masks. The measurement of microfiber abundance was repeated six times using ImageJ software according to the protocol reported by Erni-Cassola et al. (2017). The comparison of microfiber abundance between each layer was investigated using Statistical Product and Service Solution 16.0 (SPSS Inc., USA) with one-way analysis of variance (ANOVA), and the statistical significance was set at a p-value < 0.05 (Barberán et al., 2012).
The wear degree of the face masks was measured by confocal laser scanning microscopy (Nikon Eclipse Ti2, Japan) coupled with a Nikon Ti2-E inverted microscope platform. Small pieces of pristine and weathered face masks were cleaned and fixed with double-sided adhesive tape on. The samples were excited with the laser line at 491.2 nm and collected with a wavelength range of 494.0–531.0 nm. The scanning area was captured at 300 μm × 300 μm with a Z-step size spacing of 0.2 μm (50– 80 μm; controlled by Nikon NIS Elements AR software). The roughness was measured using ProflimOnline with surface analysis (Profilm Online, 2021). All the pristine and weathered face masks were fixed with carbon tape on the sample holder. The morphology with detailed element analysis was further analyzed using a scanning electron microscope (SEM; LEO1530, ZEISS, Germany) attached to an energy dispersive X-ray spectroscopy (EDS; ZEISS, Germany). The magnification of the observation was set at 0.2 k–30 k times by a secondary electron detector at 5 kV, while the mapping-mode EDS measurement was set at 20 kV. Prior to SEM-EDS, all samples were sputtered with a 3 nm layer of platinum using Leica coating system (Leica, Germany) to obtain a better conductivity.
2.3. Quality assurance and quality control
Some quality control processes were adopted throughout the experiments. All containers were cleaned at least 3 times with DI water. A clean glass dish with a glass fiber membrane inside was placed on the experimental bench to collect airborne microfibers. Cotton lab-coat, nitrile gloves and cotton mask were worn to avoid cross-contamination during the extraction process. Procedural blanks with two replicates were also analyzed to cross-check microplastic contamination. Less than 2% of the microplastics were observed in each blank, indicating that the laboratory environment was clean enough to conduct microfiber experiments (Lin et al., 2018).
3. Results and discussion
3.1. Microfiber release from face masks with different types
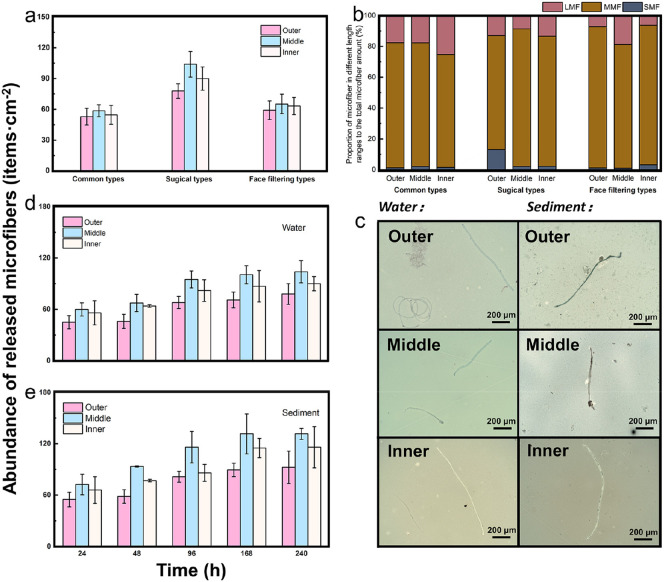
Fig. 1a illustrates the abundance of released microfibers from three types of face masks in water conditions; the total number was counted as 1909 items. Among them, the surgical face mask released the largest number of microfiber (mean ± SD: 272 ± 12.49 items·cm−2), comparing to the common face mask (165.7 ± 9.2 items·cm−2) and the FFP face mask (187.9 ± 9.45 items·cm−2). The abundance of the released microfiber varied among different layers of the three types of face masks, but the value can be ordered as middle layer > inner layer > outer layer for each type. The middle layer is mainly composed of 25 gsm (gram per square meter) of melt-blown fibers (0.5–10 μm) binding together insecurely through the melt blowing process (Table S1), while 20 gsm of the spunbonded non-woven fabrics were used for the inner and outer layers, resulting in the highest number of microfibers released from the middle layer (Fig. S2). For direct skin contact, the inner layer is relatively softer than the outer layer which is mainly designed according to the requirement for fluid repellency. Therefore, based on different functions, the inner layer with a velvet surface and loose structure is more likely to release microfibers in comparison with the outer layer (Du et al., 2020). This phenomenon was also observed in another study (Wang et al., 2021), which explained that the maximum load force of the middle layer (~3.5 N) is much lower than that of the outer and inner layers of the face masks. Thus, more microfibers can be released from the middle layer under the same weathering conditions.
Fig. 1.
The release of microfiber from different types and layers of the face masks. (a) Abundance and (b) size distribution of the microfibers released from the common, surgical and face filtering piece (FFP) masks. (c) The image, and (d, e) the abundance of the released microfiber from the outer, middle and inner layer of the surgical mask in both water and sediment environment. The time range for mechanical abrasion for the surgical mask is within the time range of 0–240 h, while the abundance of the microfiber in both water and sediment increased significantly in the first 96 h, and then gradually slowed down after approximately 168 h. On each time interval, the released microfiber abundance in sediment is higher than that in water (p-value < 0.05).
Mechanical abrasion can break the molecular chains of the polymer, resulting in a wide size range of the released microfibers. According to a previous study, microfibers can be classified as small (SMFs; 1–100 μm), medium (MMFs; 0.1–1 mm) and large (LMFs; 1–5 mm) by size (length) (Baldwin et al., 2016; Wu et al., 2020b). Therefore, based on this classification, the size distribution of the released microfibers in this study is summarized in Fig. 1b, which shows that the MMFs (0.1–1 mm) are predominant in every layer of all face masks, varying between 63.30 and 91.57%. The LMFs (1–5 mm) accounted for the second-highest proportion, ranging between 6.19% and 21.82%, followed by the SMFs (1.07–12.82%) with a size of 0.45–100 μm. It was noticed that some other studies also checked the microplastic release from the face masks, but with a larger size range (50–250 μm) caused by self-crosslinking under short-time UV weathering effects (Wang et al., 2021). Apart from the microfiber release from face masks, some other microplastics generated in the plastic product caused by physical effects in the water environment have also been reported. For example, the highest proportion of microplastics released from teabags is smaller than 0.15 mm (Hernandez et al., 2019). Du et al. (2020) also found that approximately 55–95.13% of microplastics with size <1 mm were released from take-out food containers after immersion in hot water. Meanwhile, Ranjan et al. (2021) reported a median value of 53.65 μm (~25,000 particles) from the paper cup after being contained in hot liquid (85–90 °C) for 15 min, which is smaller than that reported in our study. This discrepancy may be explained by the different shapes of the microplastic, and a longer size was observed for the fiber released from the face masks in comparison with the particles reported in other studies.
3.2. Microfiber release from surgical mask affected by brand, time, and environmental condition
The surgical mask, as the most extensively used one among the three types of face masks (Howard et al., 2020), was found to have the most serious microfiber release, as shown in Fig. 1. Therefore, a surgical mask with five different brands was selected and intensively examined for the release behavior of the microfibers affected by abrasion time and environmental conditions. Table S2 shows similar releasing behavior of the microfiber from the five brand masks, with abundances of 272–297.5 items·cm−2 (P value < 0.05) and order as middle layer > inner layer > outer layer. The results indicated that the influence of the production process of different brands was negligible for the surgical mask. Therefore, the data obtained in this study can represent the general release level of microfibers from surgical face masks.
Furthermore, the microfiber release from each layer of the surgical mask was evaluated, taking into consideration the environmental conditions (water or sediment) and time interval. Fig. 1c shows a photograph of the released fiber taken by a fluorescence microscope. It can be seen that the microfiber from the water environment is clean but that after sediment abrasion, it is entirely filled with sand particles. Fig. 1d–e further demonstrate the increasing tendency of the released microfibers in both water and sediment environments, that is the abundance increased significantly in the first 96 h, and then gradually slowed down after approximately 168 h. However, at each time interval, the abundance of the released microfiber in sediment was higher than that in water (P value < 0.05), with the discrepancy ranging from 4 items·cm−2 in the inner layer at 96 h to 31 items·cm−2 in the middle layer at 168 h. As shown in Fig. S3, a similar size distribution was observed for the microfibers obtained at each time interval. The MMFs (0.1–1 mm) accounted for the major component among the total amount (74.62–89.58% in water and 72.41–86.74% in sediment), followed by LMFs (7.64–22.54% in water and 9.71–23.2% in sediment) and SMFs (0–10.44% in water and 0.09–13.59% in sediment). After sediment abrasion, an obvious tendency was found, showing a decrease in the proportion of LMFs, but an obvious increase in SMFs over prolonged time for each layer of the surgical masks (Fig. S3). This phenomenon suggests that the SMFs can also be generated from the previously released larger microfibers in addition to directly originating from the raw surgical masks.
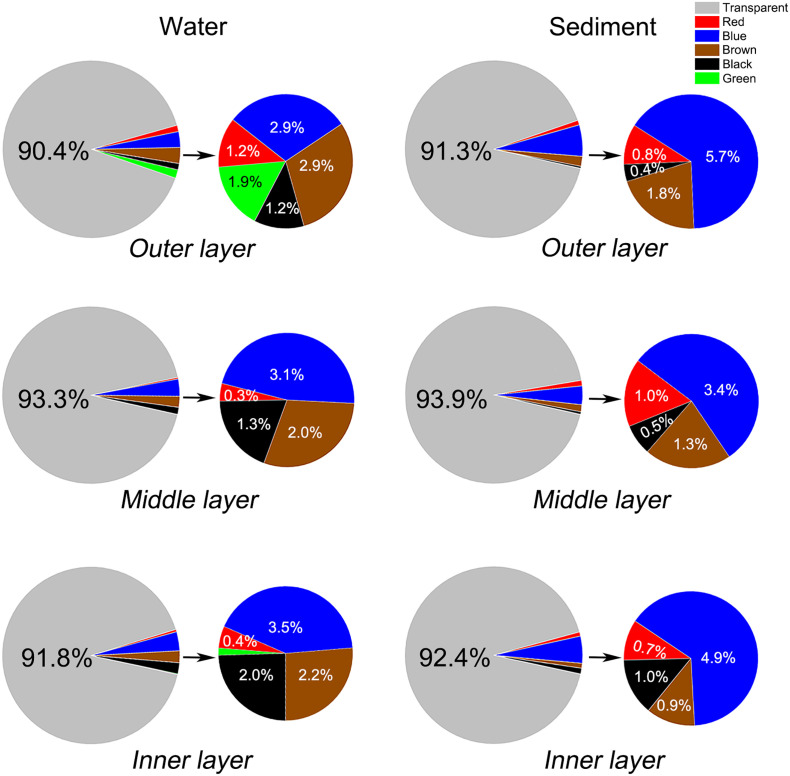
In addition, as an important characteristic that can potentially reflect the origin of microfibers (Martí et al., 2020), the color was also determined and classified into six groups: transparent, blue, red, black, green, and brown. The transparent, translucent and white microfibers were classified as transparent because it is difficult to distinguish them due to diffuse reflection (Asamoah et al., 2019). As shown in Fig. 2 , the transparent microfiber accounted for 90.37% for the outer layer, 93.31% for the middle layer, and 91.80% for the inner layer in the water environment, and 91.26%, 93.85%, and 92.41% for the outer, middle and inner layers, respectively, after the mechanical processes in the sediment. The predominance of the transparent microfiber is reasonable as the face masks are mainly composed of colorless fibers. The rest are colored items consisting of blue, red, black, green, and brown, which are mainly attributed to the release of some impurities during the manufacturing processes of the face masks.
Fig. 2.
Color distribution of the released microfiber from the outer, middle and inner layer of the surgical mask in water and sediment environment. Transparent is the predominant color, accounting for 90.37–93.31% in water and 91.26–93.85% in sediment, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Changes in morphology
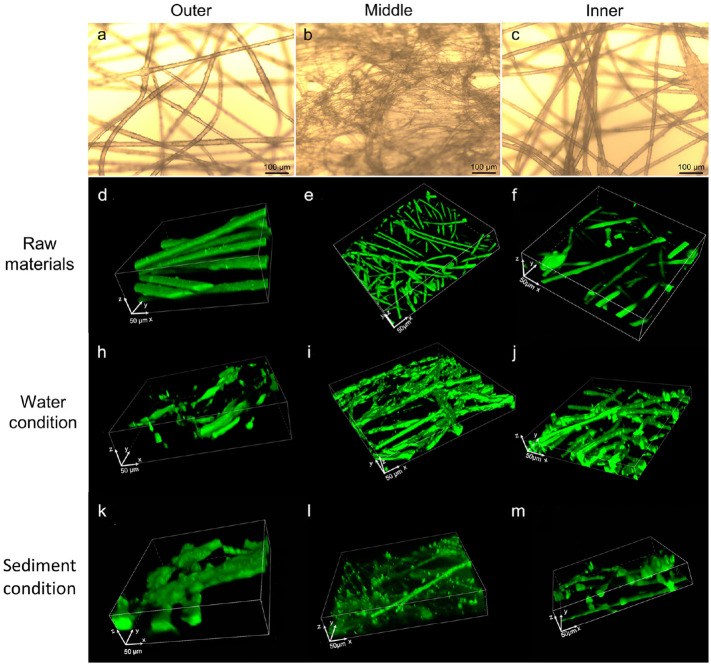
Changes in the surface texture are crucial considerations for evaluating the degree of weathering, especially after mechanical abrasion. In this study, two- or three-dimensional (2D or 3D) topographical images of the face masks before and after weathering processes were characterized using fluorescent and laser confocal microscopy, respectively (Fig. 3 ). The topography of the fibers from the raw mask was clearly observed, with the mean average diameter of 21.26 ± 6.08 μm (outer), 6.62 ± 1.58 μm (middle), and 18.58 ± 8.22 μm (inner). Subsequent to the abrasion processes, the topography changed dramatically (Fig. 3h–m); the average diameter of the fiber was changed to 21.75 ± 6.56 μm (outer), 7.81 ± 2.01 μm (middle), and 19.91 ± 9.36 μm (inner) in water condition and 21.21 ± 11.22 μm (outer), 7.52 ± 2.05 μm (middle), and 20.67 ± 11.21 μm (inner) in sediment condition, respectively. The obvious increase in the deviation of the fiber diameter of the face masks indicated that the roughness increased accordingly, owing to the mechanical abrasion. The surface roughness was then analyzed by measuring the average height of the peak above and below the test line drawn in the 2D topographical image (Fig. S4), with the calculation of arithmetic mean roughness (R a) and root mean square roughness (R q). The data in Table S3 shows that the R a and R q values are successively increased for all the mask fibers, including the pristine mask and the aged ones by water and sediment. Compared to water, the sediment caused an obviously larger increase in the fiber's roughness, because a higher Mohs hardness (~7) of the sediment can produce more energy during the abrasion to dissociate the C—C and C—H bonds of the molecular chain, and subsequently cause more severe mechanical abrasion (Ismail et al., 2020; Posch, 2011).
Fig. 3.
Florescent microcopy of the (a) outer, (b) middle and (c) inner layer of the pristine surgical face mask, and the confocal 3D micrographs (300 μm × 300 μm) of the outer, middle and inner layer of the pristine mask (d, e, f) and the mask after water (h, i, j) and sediment (k, l, m) abrasion.
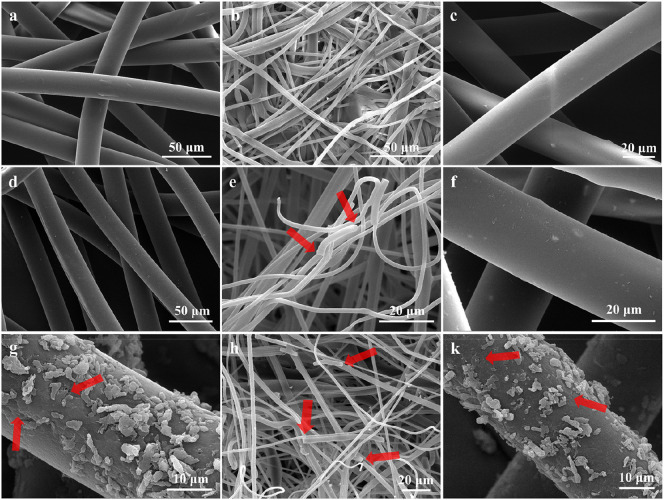
The SEM-EDS analysis was further applied for surface characterization, which confirmed much stronger wearing caused by sediment abrasion (Fig. 4 ). Fig. 4(a–c) show the smooth surface of the virgin mask fiber but with some tiny cracks with a flaky surface, which may be self-carried during production (Han and He, 2021). After abrasion in water environment, the surface of the mask fiber was also relatively smooth (Fig. 4d–f), but with microplastics attached due to the breakage of the fiber. However, when the mask was aged in a sediment environment, significant changes with large numbers of cracks were detected on the fiber of the face masks (Fig. 4g–i). In addition to cracks, there are many prominent protrusions attached to the fiber surface, which were confirmed as sediment particles (Si oxides as the main component) from the EDS results (Fig. S5). The protrusions will further decrease the surface energy of the fiber, and accelerate microbial colonization when the microfiber is prolonged in the natural environment (Pan et al., 2019; Rummel et al., 2019). The appearance of the cracks and the attachment of the protrusions indicated that the fiber undergoes heavy friction by the sediment, which changes the surface properties of the fiber and therefore alters the ability of contaminant adsorption on the fiber surface (Liu et al., 2020). Such enhanced surface changes would accelerate the weathering process, the adsorption of other contaminants, and the release of self-containing chemicals such as formaldehyde and bronopol in the mask fibers (Aerts et al., 2020; Donovan and Skotnicki-Grant, 2007). The uptake of contaminants induces health risks through the following pathways: particle toxicity, chemical toxicity and pathogen/parasite vectors (Vethaak and Leslie, 2016; Wu et al., 2020c). Therefore, the emerging concern caused by the release of fibers from the discarded face masks is that the transport of the contaminants may become much easier and faster than the other plastic products (e.g. plastic bottles and bags) because of their smaller size, lower strength and more elastic surfaces of the fibers (Xu and Ren, 2021). Recent studies have documented that the particles, additives, and pathogens on polymer materials can cause potential toxicity to organisms (Rist et al., 2018). Microfibers with needle-like shapes can more easily penetrate the cell membrane together with plastic additives, inducing energy homeostasis, oxidative stress, circulatory systems, immune system dysregulation, and neurological dysfunction (de Sá et al., 2018; Galloway and Lewis, 2016; Rist et al., 2018; Wu et al., 2020c). Although no literature has reported the propagation of the coronavirus through plastics, it should be seriously considered that plastics can be vectors for the transmission of pathogens (e.g. Halofolliculina) or bacteria (e.g. Vibrio), causing the skeletal eroding band disease in coral reefs (Goldstein et al., 2014; Ziajahromi et al., 2017), particularly during the pandemic.
Fig. 4.
Surface morphology of the outer, middle and inner layer of the original surgical mask (a, b, c) and the mask after water (d, e, f) and sediment (g, h, i) abrasion. The fiber surface of the original face mask was relatively smooth. After water abrasion, the fiber surface was still smooth but with increased cracks. A significant difference was observed for the fiber surface after sediment abrasion, with numerous cracks and protrusions.
4. Conclusions
This study provides exact data for the generation of microfibers and offers clear evidence for the severe destructive effects caused by mechanical abrasion. The results showed that the abundance of microfiber released from the surgical face masks can reach as high as 272.0 ± 12.49 items·cm−2. For a commonly used mask with dimensions of 20 cm × 10 cm, it can be estimated that after 240 h, approximately 54,400 ± 2498 items and 68,000 ± 4808 items can be released, attributed to water and sediment abrasion, respectively. More than 99% of microplastics are in the form of microfibers. Apart from the microfiber abundance, the dramatic increase in the surface roughness may decrease the surface energy and thereby accelerate microbial colonization, which can further accelerate the release of micro/nanofibers and other harmful chemicals during decomposition, embrittlement, and disintegration of the face masks. Therefore, this study can be of significant importance to understand the release behavior of microfibers from the face masks discarded in the natural environment, and to further contribute valuable information to the environmental transformation of plastics induced by weathering processes especially mechanical abrasion as the primary and critical step.
CRediT authorship contribution statement
Yuanyuan Tang and Zongwei Cai co-supervised the whole work with conceptualization and methodology. Pengfei Wu and Jiangpeng Li contributed equally to this work, by conducting the experiments, analyzing data and writing the draft manuscript. Xiao Lu helped with conducting experiments, analyzing data and revising the draft manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported financially by the National Natural Science Foundation of China (NSFC) (41977329, 22106130), the Natural Science Foundation of Guangdong Province (2021B1515020041), the Special Funds for the Cultivation of Guangdong College Students' Scientific and Technological Innovation (“Climbing Program” Special Funds pdjh2021c0038), the State Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control, and the Guangdong Provincial Key Laboratory of Soil and Groundwater Pollution Control. We also thank Mr. Yingzhe She (Thermo Fisher Scientific, Guangzhou, China) for technical support.
Editor: Jay Gan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.150458.
Appendix A. Supplementary data
Supplementary material
References
- Aerts O., Dendooven E., Foubert K., Stappers S., Ulicki M., Lambert J. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;88(2):172–173. doi: 10.1111/cod.13626. [DOI] [PubMed] [Google Scholar]
- Asamoah B.O., Kanyathare B., Roussey M., Peiponen K.E. A prototype of a portable optical sensor for the detection of transparent and translucent microplastics in freshwater. Chemosphere. 2019;231:161–167. doi: 10.1016/j.chemosphere.2019.05.114. [DOI] [PubMed] [Google Scholar]
- Baldwin A.K., Corsi S.R., Mason S.A. Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016;50:10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- Barberán A., Bates S.T., Casamayor E.O., Fierer N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012;6:343–351. doi: 10.1038/ismej.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y.C., Mao L., Wang S., Xue K., Yang L., Ye E., Zhang K., Cheong W.C.D., Tan Beng Hoon, Li Z., Tan Ban Hock, Loh X.J. Face masks in the new COVID-19 Normal: materials, testing, and perspectives. Research. 2020;2020:1–40. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubarenko I., Efimova I., Bagaeva M., Bagaev A., Isachenko I. On mechanical fragmentation of single-use plastics in the sea swash zone with different types of bottom sediments: insights from laboratory experiments. Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110726. [DOI] [PubMed] [Google Scholar]
- de Sá L.C., Oliveira M., Ribeiro F., Rocha T.L., Futter M.N. Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018;645:1029–1039. doi: 10.1016/j.scitotenv.2018.07.207. [DOI] [PubMed] [Google Scholar]
- Donovan J., Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18(1):40–44. doi: 10.2310/6620.2007.05003. [DOI] [PubMed] [Google Scholar]
- Du F., Cai H., Zhang Q., Chen Q., Shi H. Microplastics in take-out food containers. J. Hazard. Mater. 2020;399 doi: 10.1016/j.jhazmat.2020.122969. [DOI] [PubMed] [Google Scholar]
- Erni-Cassola G., Gibson M.I., Thompson R.C., Christie-Oleza J.A. Lost, but found with Nile red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 2017;51:13641–13648. doi: 10.1021/acs.est.7b04512. [DOI] [PubMed] [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T.S., Lewis C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2331–2333. doi: 10.1073/pnas.1600715113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Protective Face Masks Market (GPFMM) Global opportunity analysis and industry forecast (2019–2026): impact analysis of COVID-19 on protective face masks market. 2020. https://www.researchdive.com/covid-19-insights/359/protective-face-masks-market [WWW Document]. URL.
- Goldstein M.C., Carson H.S., Eriksen M. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar. Biol. 2014;161:1441–1453. doi: 10.1007/s00227-014-2432-8. [DOI] [Google Scholar]
- Greenhalgh T., Schmid M.B., Czypionka T., Bassler D., Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369:1–4. doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- Han J., He S. Need for assessing the inhalation of micro(nano)plastic debris shed from masks, respirators, and home-made face coverings during the COVID-19 pandemic. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L.M., Xu E.G., Larsson H.C.E., Tahara R., Maisuria V.B., Tufenkji N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019;53:12300–12310. doi: 10.1021/acs.est.9b02540. [DOI] [PubMed] [Google Scholar]
- Howard J., Huang A., Li Z., Tufekcim Z., Zdimale V., van der Westhuizen H.-M., von Delfto A., Price A., Fridman L., Tang L.-H., Tang V., Watson G.L., Baxs C.E., Shaikh R., Questier F., Hernandez D., Chu L.F., Ramirez C.M., Rimoin A.W. Face masks against COVID-19: an evidence review. Proc. Natl. Acad. Sci. U. S. A. 2020;1–8 doi: 10.20944/preprints202004.0203.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Morawska L. Face masks could raise pollution risks. Nat. Comment. 2019;574:29–30. doi: 10.1038/d41586-019-02938-1. [DOI] [PubMed] [Google Scholar]
- Hutten I.M. Processes for nonwoven filter media. Handb. Nonwoven Filter Media. 2007;195–244 doi: 10.1016/b978-185617441-1/50020-2. [DOI] [Google Scholar]
- Ibrahim Y.S., Tuan Anuar S., Azmi A.A., Wan Mohd Khalik W.M.A., Lehata S., Hamzah S.R., Ismail D., Ma Z.F., Dzulkarnaen A., Zakaria Z., Mustaffa N., Tuan Sharif S.E., Lee Y.Y. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5:116–121. doi: 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M.Y., Patanen M., Kauppinen S., Kosonen H., Ristolainen M., Hall S.A., Liimatainen H. Surface analysis of tissue paper using laser scanning confocal microscopy and micro-computed topography. Cellulose. 2020;27:8989–9003. doi: 10.1007/s10570-020-03399-w. [DOI] [Google Scholar]
- Klemeš J.J., Fan Y.Van, Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sust. Energ. Rev. 2020;127 doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Pérez-Guevara F., Elizalde-Martínez I., Shruti V.C. An overview of recent advances in micro/nano beads and microfibers research: critical assessment and promoting the less known. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.139991. [DOI] [PubMed] [Google Scholar]
- Lambert S., Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016;145:265–268. doi: 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhao X., Li Z., Song K. COVID-19: performance study of microplastic inhalation risk posed by wearing masks. J. Hazard. Mater. 2021;411:1–9. doi: 10.1016/j.jhazmat.2020.124955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Zuo L.Z., Peng J.P., Cai L.Q., Fok L., Yan Y., Li H.X., Xu X.R. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018;644:375–381. doi: 10.1016/j.scitotenv.2018.06.327. [DOI] [PubMed] [Google Scholar]
- Liu P., Qian L., Wang H., Zhan X., Lu K., Gu C., Gao S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019;53:3579–3588. doi: 10.1021/acs.est.9b00493. [DOI] [PubMed] [Google Scholar]
- Liu P., Lu K., Li J., Wu X., Qian L., Wang M., Gao S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121193. [DOI] [PubMed] [Google Scholar]
- Martí E., Martin C., Galli M., Echevarría F., Duarte C.M., Cózar A. The colors of the ocean plastics. Environ. Sci. Technol. 2020;54:6594–6601. doi: 10.1021/acs.est.9b06400. [DOI] [PubMed] [Google Scholar]
- Min K., Cuiffi J.D., Mathers R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-14538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgana S., Casentini B., Amalfitano S. Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J. Hazard. Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray O.M., Bisset J.M., Gilligan P.J., Hannan M.M., Murray J.G. Respirators and surgical facemasks for COVID-19: implications for MRI. Clin. Radiol. 2020;75:405–407. doi: 10.1016/j.crad.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra S., A O.O., Yuanyuan T. Medical waste: current challenges and future opportunities for sustainable management. Crit. Rev. Environ. Sci. Technol. 2021:1–23. doi: 10.1080/10643389.2021.1885325. [DOI] [Google Scholar]
- Pan Z., Guo H., Chen H., Wang S., Sun X., Zou Q., Zhang Y., Lin H., Cai S., Huang J. Microplastics in the northwestern Pacific: abundance, distribution, and characteristics. Sci. Total Environ. 2019;650:1913–1922. doi: 10.1016/j.scitotenv.2018.09.244. [DOI] [PubMed] [Google Scholar]
- Peeken I., Primpke S., Beyer B., Gütermann J., Katlein C., Krumpen T., Bergmann M., Hehemann L., Gerdts G. Arctic Sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch W. Polyolefins. Appl. Plast. Eng. Handb. 2011;23–48 doi: 10.1016/B978-1-4377-3514-7.10003-0. [DOI] [Google Scholar]
- Ranjan V.P., Joseph A., Goel S. Microplastics and other harmful substances released from disposable paper cups into hot water. J. Hazard. Mater. 2021;404 doi: 10.1016/j.jhazmat.2020.124118. [DOI] [PubMed] [Google Scholar]
- Ren Z. COVID-19: China boosts face mask production capacity by 450 per cent in a month, threatening a glut scenario [WWW Document] 2020. https://www.scmp.com/business/companies/article/3075289/china-boosts-face-mask-production-capacity-450-cent-month
- Rist S., Carney Almroth B., Hartmann N.B., Karlsson T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018;626:720–726. doi: 10.1016/j.scitotenv.2018.01.092. [DOI] [PubMed] [Google Scholar]
- Roberts K., Bowyer C., Kolstoe S., Fletcher S. Coronavirus face masks: an environmental disaster that might last generations [WWW Document] 2020. https://theconversation.com/coronavirus-face-masks-an-environmental-disaster-that-might-last-generations-144328
- Rummel C.D., Escher B.I., Sandblom O., Plassmann M.M., Arp H.P.H., Macleod M., Jahnke A. Effects of Leachates from UV-weathered microplastic in cell-based bioassays. Environ. Sci. Technol. 2019;53:9214–9223. doi: 10.1021/acs.est.9b02400. [DOI] [PubMed] [Google Scholar]
- Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ. Adv. 2021;4 doi: 10.1016/j.envadv.2021.100042. [DOI] [Google Scholar]
- Singh N., Tang Y., Ogunseitan O.A. Environmentally sustainable management of used personal protective equipment. Environ. Sci. Technol. 2020;10–12 doi: 10.1021/acs.est.0c03022. [DOI] [PubMed] [Google Scholar]
- Singh N., Tang Y., Zhang Z., Zheng C. 2020. COVID-19 Waste Management: Effective and Successful Measures in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.K., Hong S.H., Jang M., Han G.M., Jung S.W., Shim W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017;51:4368–4376. doi: 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- Vethaak A.D., Leslie H.A. Plastic debris is a human health issue. Environ. Sci. Technol. 2016;50:6825–6826. doi: 10.1021/acs.est.6b02569. [DOI] [PubMed] [Google Scholar]
- Wang Z., An C., Chen X., Lee K., Zhang B., Feng Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Cai Z., Jin H., Tang Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019;650:671–678. doi: 10.1016/j.scitotenv.2018.09.049. [DOI] [PubMed] [Google Scholar]
- Wu P., Tang Y., Cao G., Li J., Wang S., Chang X., Dang M., Jin H., Zheng C., Cai Z. Determination of environmental micro(nano)plastics by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry. Anal. Chem. 2020 doi: 10.1021/acs.analchem.0c01928. [DOI] [PubMed] [Google Scholar]
- Wu P., Tang Y., Dang M., Wang S., Jin H., Liu Y., Jing H., Zheng C., Yi S., Cai Z. Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.135187. [DOI] [PubMed] [Google Scholar]
- Wu P., Tang Y., Jin H., Song Y., Liu Y., Cai Z. Consequential fate of bisphenol-attached PVC microplastics in water and simulated intestinal fluids. Environ. Sci. Ecotechnol. 2020;2 doi: 10.1016/j.ese.2020.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E.G., Ren Z.J. Preventing Masks From Becoming the Next Plastic Problem. Vol. 15. 2021. pp. 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Yan C., Fu Q., Xiao K., Yu Y., Han D., Wang W., Cheng J. Possible environmental effects on the spread of COVID-19 in China. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Where did 5,500 tonnes of discarded face masks end up? [WWW Document] 2020. https://www.greenpeace.org/international/story/44629/where-did-5500-tonnes-of-discarded-face-masks-end-up/
- Ziajahromi S., Kumar A., Neale P.A., Leusch F.D.L. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: implications of single and mixture exposures. Environ. Sci. Technol. 2017;51:13397–13406. doi: 10.1021/acs.est.7b03574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material