Abstract
Acute respiratory distress syndrome (ARDS) is the most common form of acute severe hypoxemic respiratory failure in the critically ill with a hospital mortality of 40%. Alveolar inflammation is one of the hallmarks for this disease. β-Glucans are polysaccharides isolated from a variety of natural sources including mushrooms, with documented immune modulating properties. To investigate the immunomodulatory activity of β-glucans and their potential as a treatment for ARDS, we isolated and measured glucan-rich polysaccharides from seven species of mushrooms. We used three models of in-vitro injury in THP-1 macrophages, Peripheral blood mononuclear cells (CD14+) (PMBCs) isolated from healthy volunteers and lung epithelial cell lines. We observed variance between β-glucan content in extracts isolated from seven mushroom species. The extracts with the highest β-glucan content found was Lentinus edodes which contained 70% w/w and Hypsizygus tessellatus which contained 80% w/w with low levels of α-glucan. The extracts had the ability to induce secretion of up to 4000 pg/mL of the inflammatory cytokine IL-6, and up to 5000 pg/mL and 500 pg/mL of the anti-inflammatory cytokines IL-22 and IL-10, respectively, at a concentration of 1 mg/mL in THP-1 macrophages. In the presence of cytokine injury, IL-8 was reduced from 15,000 pg/mL to as low as 10,000 pg/mL in THP-1 macrophages. After insult with LPS, phagocytosis dropped from 70–90% to as low 10% in CD14+ PBMCs. After LPS insult CCL8 relative gene expression was reduced, and IL-10 relative gene expression increased from 50 to 250-fold in THP-1 macrophages. In lung epithelial cells, both A549 and BEAS-2B after IL-1β insult, IL-8 levels dropped from 10,000 pg/mL to as low as 6000 pg/mL. TNF-α levels dropped 10-fold from 100 pg/mL to just below 10 pg/mL. These results demonstrate the therapeutic potential of β-glucans in inflammatory lung conditions. Findings also advance bio-based research that connects green innovation with One Health applications for the betterment of society.
Keywords: β-Glucans, THP-1 macrophages, Lung injury, ARDS, Medicinal mushrooms, One-health
Graphical abstract

1. Introduction
Acute respiratory distress syndrome (ARDS), is the most common form of acute severe hypoxemic respiratory failure in the critically ill (Rezoagli et al., 2017). The syndrome is defined by: acute onset of hypoxemia (PaO2:FiO2 ratio <300) and bilateral pulmonary opacities not explained by cardiac failure or fluid overload (Bellani et al., 2016). ARDS is a diffuse inflammatory reaction and can be characterised by an explosive acute inflammatory response in lung parenchyma (Crimi and Slutsky, 2004), impairing the principal function of gas exchange, which can lead to hypoxaemia. Treatment is mainly focused on clinical management as there remains no effective direct pharmacological therapy for this condition (Rezoagli et al., 2019). There is an urgent need for treatment as mortality and morbidity are unacceptably high at 40% (Horie et al., 2020). Furthermore, infection by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to further incidences of COVID-19-related ARDS, which is associated with 70% of fatal cases (Rezoagli et al., 2021; Li et al., 2020; Zhou et al., 2020). In both ARDS and in COVID-19-induced ARDS there is a marked increase in serum levels of inflammatory cytokines and chemokines, which is a major contributor to disease severity and ultimately death (G. Chen et al., 2020; X. Chen et al., 2020; Huang et al., 2020; Mehta et al., 2020; Qin et al., 2020). The pathophysiology of ARDS is associated with numerous target immune cells including macrophages. The lung microenvironment during injury determines the functional phenotype of macrophages, which can promote either wound healing or inflammation (Nanchal and Truwit, 2018). Thus, in developing potential therapeutics it is important to understand the potential effects of these therapeutics on lung tissue and on macrophages. An ideal treatment for this condition would aim at reducing the effects of the proinflammatory cascade and would seek to maximize the anti-inflammatory immunomodulatory response (Zambelli et al., 2021).
β-Glucans are defined as complex polysaccharides that are found in an abundance of sources including fungi, yeast, grain, bacteria, and algae (Murphy et al., 2021). β-Glucans can be classified structurally as either 1,3 1,4-linked or 1,3 1,6-linked, which is dependent on their source (Cui et al., 2011; Pogue et al., 2021; Murphy et al., 2020). These molecules are widely marketed as biologically active molecules (bioactives) (Wang et al., 2017a). There are over 200 clinical trials registered for their use for a range of applications. There are also licenced drugs containing β-glucans on the market since 1980 in Japan, for the treatment of cancer (Novak and Vetvicka, 2008; Takeshita et al., 1991; Yang et al., 2019). β-Glucans as pharmaceutical agents have also been authorised in several countries, including the United States of America, Canada, Finland, Sweden, China and Korea (van Steenwijk et al., 2021). The diverse functional effects of these molecules include alteration of lipid and glucose metabolism, cholesterol reduction, obesity regulation and reduction of cardiovascular and diabetic risk, modulating the gut microbiome, altering lipid and glucose metabolism and beneficial effects on gastrointestinal conditions such as irritable bowel syndrome (Drozdowski et al., 2010; Maki et al., 2003; McRorie and McKeown, 2017; Sima et al., 2018; Tiwari and Cummins, 2011). β-Glucans, specifically from non-cereal sources, are widely documented for their immunomodulatory properties, with the ability to stimulate the immune response and initiate inflammatory properties, and to promote resistance to infections (Ooi and Liu, 2012). (Bohn and BeMiller, 1995). Mushroom-derived β-glucans are the most potent immune modulators (Borchers et al., 1999; Ooi and Liu, 2012; Lorenzen and Anke, 1998; Ooi and Liu, 1999; Tzianabos, 2000; Wasser and Weis, 1999). Moreover, they have demonstrated therapeutic effects in alleviating infective respiratory conditions (Fuller et al., 2012; Jesenak et al., 2013; Yamauchi et al., 2008). They have also been documented to reduce pro-inflammatory cytokines, increase anti-inflammatory cytokines, increased formation of antioxidants as well reduction of inflammatory cells in preclinical lung injury models (Bedirli et al., 2007; Jedinak et al., 2011; Johnson et al., 2009; Kofuji et al., 2012; Soltys and Quinn, 1999; Yamada et al., 2007). These beneficial effects can also be seen in clinical trials. When patients were administered β-glucans for the prevention of nosocomial pneumonia and sepsis, the treatment group compared to the control group had lower incidences of pneumonia as well as a lower mortality rate (De Felippe et al., 1993).
We have previously investigated the effects of a commercial β-glucan and an in-house extract of β-glucans from the mushroom Lentinus edodes (Masterson et al., 2020; Murphy et al., 2020, Murphy et al., 2020, Murphy et al., 2020). Specifically, Murphy et al. (2019) showed that β-glucans from the same mushroom, one isolated by hot water extraction and one sourced commercially had different effects, namely reduction in inflammatory cytokines, reduction in phagocytic activity of macrophages after LPS insult and reduction of inflammatory response in in-vitro lung cells. Thus, to continue this work and understand the potential immunomodulatory properties of other mushroom β-glucans as a potential treatment for inflammatory lung conditions like ARDS we decided to replicate the assays and include additional test parameters. In the current study, we first extracted and measured β-glucans from seven species of mushroom to determine BRM variance among species by applying them to a monocytic cell line and an in-vitro lung injury model. Second, we isolated CD14+ monocytes from healthy volunteers and exposed the cells to the extracts, then measured phagocytic activity. Third, we simulated an injurious environment on two types of alveolar cell lines using IL-1β and measured cytokine expression. Finally, we extended this assay to a monocytic cell line, which was inflamed with different insults (LPS and cytomix). It has recently emerged that macrophages are reduced and equally as inflamed as lung cells during COVID-19 infection. Therefore, after injury we measured cytokine release, gene expression, and phagocytosis of these cells to determine immune-modulatory potential in an inflammatory micro-environment.
2. Materials & methods
Commercial Lentinan (CLE) was sourced from Carbosynth (FL33321, Compton, Berkshire, UK). Fruiting bodies of mushrooms were kindly gifted by Garryhinch Wood Exotics Ltd. Garryhinch, Portarlington, Co Offaly, Ireland. The fruiting body of Agaricus blazeii was kindly gifted by Professor Leo van Griensven, Wageningen University, The Netherlands. Other species of mushroom included; Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
2.1. β-Glucan extraction
To extract β-glucans from the fruiting bodies of the mushrooms, the method used previously by Murphy et al. (2019) was employed. Briefly – the fruiting bodies were washed and dried. The samples were blended into a fine powder. Roughly, 100 g of dried blended biomass was placed in 1 Litre of water and autoclaved. After autoclaving the polysaccharides were precipitated from supernatant using 100% Ethanol. Precipitates were dried and solubilised in PBS for analysis.
2.2. β-Glucan quantification
Extracts were analysed for 1-3 1-6 β-glucan content using the Megazyme yeast and mushroom kit (K-YBGL) (Megazyme Ltd., Bray, Co. Wicklow, Ireland). Assays were carried out according to manufacturer's instructions. After milling, samples were placed in 12 M H2SO4 at −4 °C for 2 h to solubilize the β-glucans. Samples were then hydrolysed in 2 M H2SO4 at 100 °C for a further 2 h. Any remaining β-glucan fragments were quantitatively hydrolysed to glucose using a mixture of exo-1,3-β-glucanase and β-glucosidase which gives a measurement of total β-glucan content after substrate addition. The α-glucan content of the sample was determined by hydrolysing specifically to d-glucose and d-fructose. Glucose was measured with amyloglucosidase and invertase using a glucose oxidase peroxidase GOPOD reagent. β-Glucan content was determined by the difference between the two measurements.
2.3. Blood donor cell collection
Blood sample collection was approved by the Athlone Institute of Technology Ethics Committee. Blood samples were obtained from healthy volunteers for isolation of immune cells. A total of 15 mL was collected from each donor. Individual cells were isolated from 5 mL aliquots of collected blood. Samples were magnetically labelled with whole blood microbeads (Miltenyi Biotec, Germany) to isolate cells based on specific surface molecules according to the manufacturer's instructions, using the autoMACS separator (Miltenyi Biotec).
2.4. Cell culture
A549 cells (used at passage 90), BEAS-2B cells (used at passage 10), and THP-1 monocyte cells (used at passage 20), were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal calf serum (Sigma-Aldrich), 1% penicillin G (100 U/mL) and streptomycin (100 μg/mL) solution (Sigma-Aldrich), at 37 °C a 5% CO2 environment. For differentiation into macrophages, THP-1 monocyte cells were treated with phorbol 12-myristate 13-acetate (PMA) for differentiation into THP-1 macrophages. (Peprotech EC, London, UK), at a concentration of 100 ng/mL, for 48 h.
2.5. CD14+ PBMCs
CD14+ cells were positively isolated based on the surface molecule CD14, which is primarily found on monocytes (Shin et al., 2019). Isolated cells were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal calf serum (Sigma-Aldrich), 1% penicillin G (100 U/mL)/streptomycin (100 μg/mL) solution (Sigma-Aldrich) and 50 ng/mL of macrophage colony stimulating growth factor (MCSGF) (RnD Systems MN, USA) at 37 °C in a 5% CO2 environment.
2.6. Cell injury and β-glucan treatment
All cell types were treated with 1 mg/mL of β-glucan in PBS based on other published work (Jung et al., 2007; Murphy et al., 2020, Murphy et al., 2020, Murphy et al., 2020; Sari et al., 2020; Sivinski et al., 2020). For injury assays THP-1 PMA differentiated macrophage cells were seeded at a density of 4 × 105 cells/well in 96-well plates, and 24 h later were injured with two different types of insult: either LPS (100 ng/mL) (Sigma) or cytomix (TNF-α, IFN-Υ, IL-1β), at 25 ng/mL (Immunotools), in RPMI supplemented with 1% penicillin/streptomycin. After 24 h cells were washed three times in PBS and treated with 1 mg/mL of extracts for a further 24 h before analysis. Pulmonary alveolar type II A549 cells were seeded at a density of 4 × 105 cells/well in 96 well plates. After 24 h cells were injured with 1 ng/mL of IL-1β (Peprotech, Rocky Hill, NJ) in RPMI supplemented with 1% penicillin/streptomycin.
2.7. Enzyme linked immunosorbent assay (ELISA)
A human Duoset sandwich ELISA kit (RnD Systems MN, USA) was used to measure cytokine levels in the medium after β-glucan exposure. All ELISA assays were performed according to the manufacturer's instructions. Results were expressed either in pg/mL or in ng/mL.
2.8. Phagocytosis assays
To determine Phagocytic activity, THP-1 macrophages (PMA differentiated) and CD14+ cells were seeded into 96-well plates at 4 × 105 cells/well. After 24 h, cells were either injured or treated with PBS. After a further 2 h, cells were treated with β-glucan extracts. After a further 24 h cells were washed with PBS and incubated with Alexa Fluor 488-conjugated E.coli (K-12 strain) Bioparticles (E13231; Life Technologies) for 2 h, after which cells were washed three times with PBS to remove residual particles before resuspension in FACS flow buffer and measured for fluorescent particles by flow cytometry (Miltenyi Biotec, Germany).
2.9. RNA extraction
For RNA extraction from THP-1 macrophage cells, Media was removed, the cells were washed 3× with PBS, and RNA was extracted using the Purelink RNA Mini kit (Thermo-Fisher), according to the manufacturer's instructions. RNA was analysed using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, DE, USA) to determine RNA concentrations and A260/A280 ratios.
2.10. cDNA synthesis and real-time PCR
cDNA was prepared for the replicate samples using the SensiFAST cDNA Synthesis kit (Bioline), according to the manufacturer's instructions. RNA input for all samples was normalized to the sample, so that 325.5 ng of total RNA were used in each reaction. Real-time quantitative PCR was performed using pre-designed TaqMan Gene Expression Assays for the respective genes, together with the TaqMan Gene Expression Master Mix (Thermo-Fisher). The transcripts examined were: TLR2, IL-10, CCL8, CLEC-7a and MCSGF. Reactions were carried out on the LightCycler 96 equipment (Roche), using the GAPDH transcript as endogenous control. Relative gene expression was calculated using the 2^-ddCq method.
2.11. Statistical analysis
Continuous data were expressed as mean and standard error of the mean (SEM). Differences of continuous variables between species of mushrooms and PBS and injury (i.e. LPS or Cytomix or IL-1 β) were assessed by one-way analysis of variance for independent measures. Post-hoc comparisons were investigated by controlling the False Discovery Rate using the two-stage step-up method of Benjamini, Krieger and Yekutieli test. Statistical significance was considered with a p-value < 0.05 (two-sided). Statistical analyses were performed using STATA/MP 16.0 for Windows (StataCorp LLC, College Station, TX 77845, USA) and GraphPad Prism 8 for Windows (Version 8.0.2, FraphPad Software, Inc.).
3. Results
3.1. β-Glucans quantification
β-glucans were extracted from seven species of mushrooms as previously described (Murphy et al., 2020, Murphy et al., 2020, Murphy et al., 2020). After extraction and isolation, the Megazyme 1,3 1,6 kit was used to determine the concentration of α- and β-glucans.
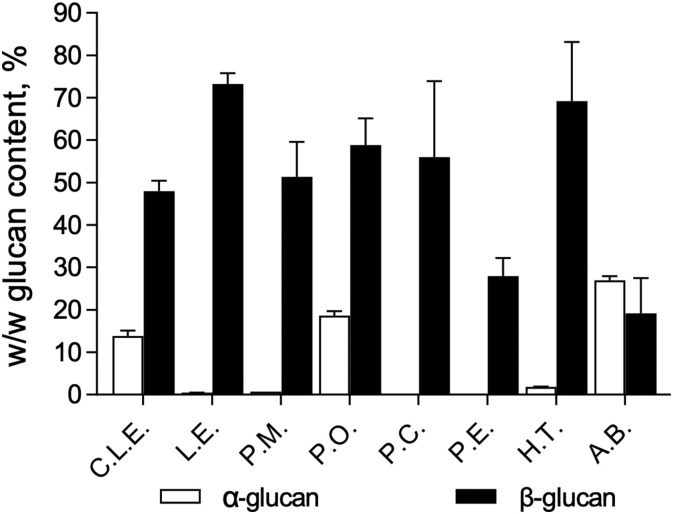
There was variance between species as can be seen in the relative concentrations of β-glucan and α-glucan displayed in Fig. 1 . P.E appeared to have the purest concentration of β-glucan compared to other extracts. A.B and P.O appeared to have high levels of contaminating α-glucan present. H.T yielded the highest concentration of β-glucan with little contaminating α-glucan. H.T yielded the highest concentration of β-glucan with little contaminating α-glucan.
Fig. 1.
The percentage w/w α-glucan and β-glucan content in mushroom extracts using Megazyme. Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
3.2. Effect of β-glucans on macrophages
3.2.1. The direct effect of β-glucans on THP-1 macrophages
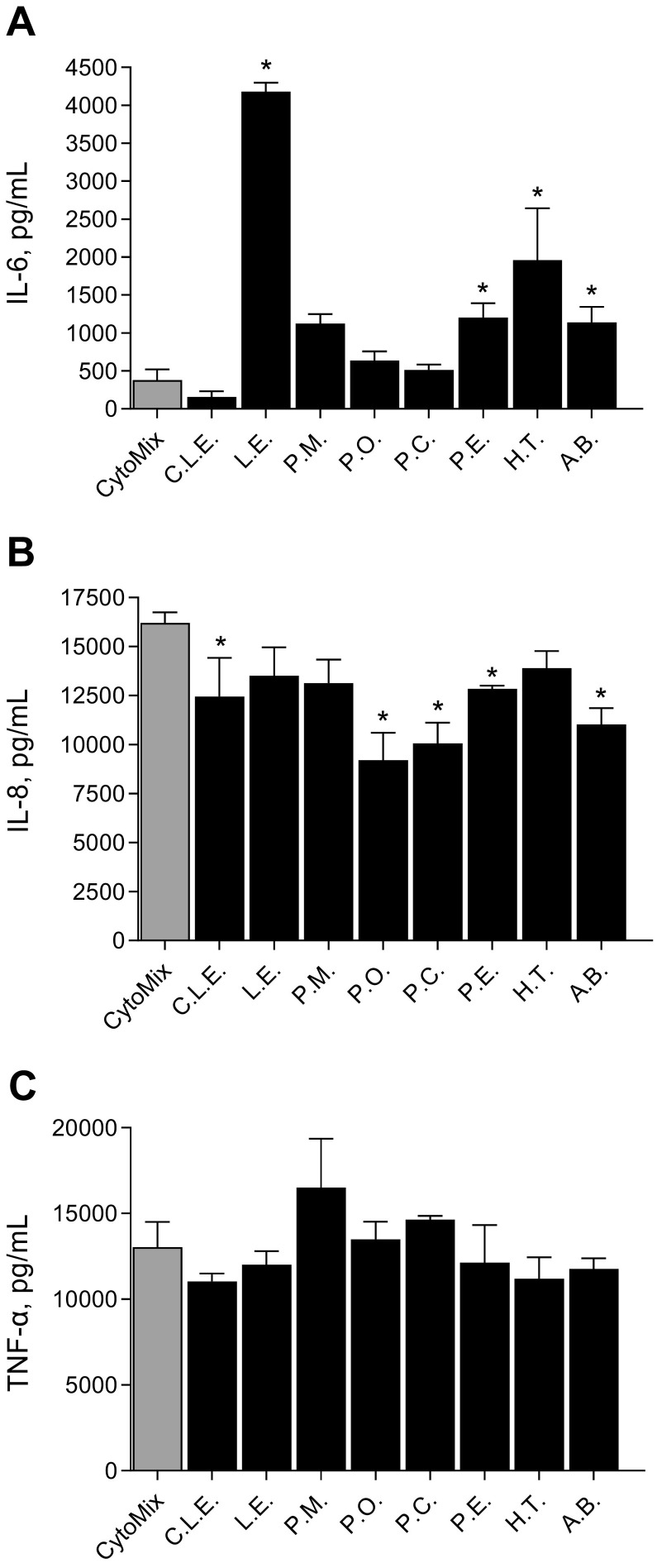
To understand the effects of β-glucan extracts on macrophages, THP-1 macrophages were treated with each of the extracts and cytokine secretion levels were measured by an ELISA. Results show (Fig. 2 ) that each extract had a different effect on the cytokine release profile from THP-1 macrophages. Extracts had the potential to increase secretion of both inflammatory cytokines (IL-6, IL-8, TNF-α) and anti-inflammatory cytokines (IL-10 and IL-22). CLE is a commercial source of β-glucan, and its extraction method is unknown; all other extracts were processed as described in the methods Section 2.1. Although the extracts have different effects on the cytokine secretion profile, the pattern was generally similar except for CLE. CLE induced lower secretion levels of IL-6 (Panel A), IL-22 (Panel C), and IL-10 (Panel E), compared to the other extracts and lower levels of IL-2 (Panel F) compared to the PBS control. The remaining extracts increased IL-6, TNF-α (Panel B) and IL-10 secretion and maintained IL-22 and IL-2 compared to PBS control. CLE and some of the extracts (L.E, C.L.E and P.C) appeared to increase the secretion of the chemokine IL-8 compared to control (Panel D).
Fig. 2.
The effect of the β-glucan extracts on cytokine expression in THP-1 macrophages (PMA differentiated) measured using ELISA. Panel A; IL-6, Panel B; TNF-α, Panel C; IL-22, Panel D; IL-8, Panel E; IL-10 Panel F; IL-2. p < 0.05 versus PBS. Cells were treated with 1 mg/mL of extracts for 24 h before cytokine analysis. Phosphate buffer saline (PBS), Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
3.2.2. Effect of β-glucans on phagocytosis by THP-1 and CD14+ PBMC macrophages
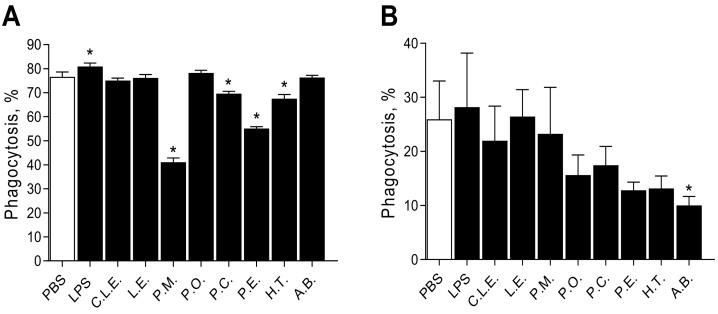
To compare the effects of β-glucan extracts on a macrophage cell line (THP-1) and on fresh PBMCs (CD14+), cells were treated with extracts, and after 24 h phagocytic activity was measured and displayed in Fig. 3 . Results varied: Panel A shows the effects on THP-1 macrophages; LPS significantly increased phagocytosis relative to untreated cells. Four extracts (P.M, P.C, P. E and H.T) were able to significantly reduce phagocytosis, with PM showing by far the greatest reduction. CD14+ PBMCs (Fig. 3, Panel B) showed varying responses to the extracts in terms of phagocytosis, as expected due to donor variability. The extract A.B significantly reduced the phagocytic activity, although the overall percentage phagocytosis was low in these cells. The extracts showed a tendency toward reduction in PBMCs, however, the results do not show significance, potentially due to donor variability.
Fig. 3.
The effects of the β-glucan extracts on percentage phagocytosis measured using flow cytometry analysis of uptake of Alexa Fluor 488-conjugated E.coli (K-12 strain) Bioparticles. THP-1 macrophages were treated with 1 mg/mL of extracts for 24 h before phagocytic analysis. Panel A; THP-1 macrophages; Panel B; CD14+ primary macrophages. Phosphate buffer saline (PBS), Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
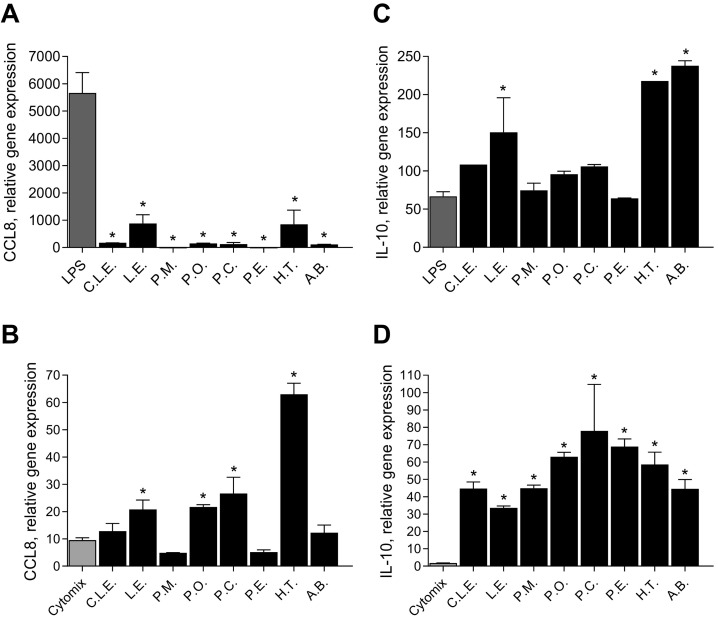
To gain a further understanding of the mechanism, expression levels of four genes were measured. Two genes were related to cytokine/chemokine response: IL-10 (Fig. 4 ; Panel B), and CCL8 (IL-8; Fig. 4, Panel D). Two genes corresponded to cell surface ligands associated with β-glucan recognition: toll-like receptor 2 TLR-2 (Fig. 4, Panel A), and dectin-1 (CLEC7a, Fig. 4, Panel C).
Fig. 4.
The effect of the β-glucan extracts on gene expression levels of THP-1 macrophages (PMA differentiated), relative to PBS-treated cells (expression level = 1.0). Panel A; TLR2, Panel B; IL-10, Panel C; CLEC7a, Panel D; CCL8. Differences in relative gene expression *p < 0.05 versus PBS; #p < 0.05 versus LPS. Cells were treated with 1 mg/mL of extracts for 24 h before analysis of gene expression levels. Phosphate buffer saline (PBS), Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
Expression of TLR2 in THP-1 macrophages showed no significant difference in induction between LPS and the β-glucans. However, compared to PBS control, P.O induced expression of TLR2. All the extracts significantly inhibited the relative gene expression levels of CLEC7a compared to both PBS control and LPS in THP-1 macrophages. IL-10 expression showed no significant increase in gene expression compared to controls except for with P.M. The extracts did not significantly induce the expression of CCL8 compared to PBS controls and induction was significantly lower compared to LPS.
To determine the effect of β-glucans on macrophages after injury, THP-1 macrophages were injured with Cytomix (IL-1β, TNF-α & IFN-γ), and then treated with β-glucan extracts (Fig. 5 ). ELISA assay results show the β-glucan extracts from L.E, P.E, H.T and A.B significantly increased the secretion of IL-6 after insult (Panel A). P.O and P.C did not have the same induction profile as when directly treated with injury (Fig. 2 Panel A), which induced ~2000 pg/mL secretory levels of IL-6. After insult with cytomix P.O and P.C induced ~1000 pg/mL secretory levels of IL-6. IL-8 secretion was increased after cytomix treatment alone (Fig. 5, Panel B). However, the strongest inducers of IL-8 secretion with direct treatment were L.E, C.L.E and P.C (Fig. 2 Panel D), all of which significantly reduced the secretion of this inflammatory chemokine after injury except for LE which was not significant. There was no significant effect of β-glucan treatments on TNF-α secretion after cytomix insult (Fig. 5, Panel C).
Fig. 5.
The effects of the β-glucan extracts on THP-1 macrophages (PMA differentiated) after cytokine insult measured using ELISA. Panel A; IL-6, Panel B; IL-8, Panel C; TNF-α. *p < 0.05 versus cytomix. Cells were treated with cytomix (TNF-α, IFN-Υ, IL-1β), at 25 ng/mL for 24 h, after which they were washed with PBS and treated with extracts (1 mg/mL) for 24 h before cytokine analysis. Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
Both THP-1 macrophages and PBMCs were analysed for phagocytic activity (Fig. 6 ) after injury with LPS. The THP-1 macrophages after injury (Fig. 6, Panel A) had a very similar response to those with β-glucan extracts alone (Fig. 3 Panel A) compared with treatment after injury. When CD14+ cells were treated with β-glucan extracts, only A.B significantly reduced phagocytosis (Fig. 3 Panel B). However, when administered after LPS, all β-glucan extracts reduced percentage phagocytosis (Fig. 6, panel B). Panel C displays the phagocytosis percentage of THP-1 macrophages after cytomix insult; C.L.E, P.M, P.O, P.C and P.E reduced the phagocytic activity after insult and treatment.
Fig. 6.
The effects of the β-glucan extracts on THP-1 macrophages (PMA differentiated) after cytokine insult or LPS insult. Percentage phagocytosis measured using flow cytometry analysis of uptake of Alexa Fluor 488-conjugated E. coli (K-12 strain) Bioparticles. Panel A; THP-1 macrophages (PMA differentiated) treated with LPS, Panel B; CD14+ PBMCs treated with LPS. Panel C; THP-1 macrophages (PMA differentiated) treated with cytomix. *p < 0.05 versus Cytomix or LPS. Cells were treated with cytomix (TNF-α, IFN- Υ, IL-1β), at 25 ng/mL or LPS for 24 h after which they were washed with PBS and treated with extracts (1 mg/mL) for 24 h before phagocytic activity analysis. Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazeii (A.B).
Inflammatory gene expression and anti-inflammatory gene expression markers were analysed after both types of injury, as displayed in Fig. 7 . The inflammatory marker CCL8 was measured after LPS injury (Panel A) and cytomix (Panel B). In the presence of LPS, CCL8 relative gene expression was significantly reduced after treatment with all extracts. In the presence of cytomix insult, L.E, P.O, P.C and H.T all increased the relative gene expression of CCL8. The anti-inflammatory marker IL-10 was measured after LPS injury (Panel C) and cytomix (Panel D). After LPS insult L.E, H.T and A.B all significantly increased the expression of IL-10 gene compared to injury alone. All the extracts increased the expression of IL-10 compared to injury alone.
Fig. 7.
The effects of the β-glucan extracts on THP-1 macrophages (PMA differentiated) after LPS (Panels A, C) or Cytomix (Panels B, D) injury relative to PBS-treated cells (expression level = 1.0). Panels A and B show IL-8 expression after β-glucan treatments, relative to insult; Panels C and D show IL-10 expression after β-glucan treatments, relative to insult. *p < 0.05 versus LPS /Cytomix. THP-1 macrophages (PMA differentiated) were treated with cytomix (TNF-α, IFN- Υ, IL-1β), at 25 ng/mL or LPS for 24 h after which they were washed with PBS and treated with extracts (1 mg/mL) for 24 h before gene expression analysis. Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazei (A.B).
3.3. Effect of β-glucans in an in-vitro lung injury model
In previous work carried out by this group, we established that β-glucan extracts (L.E) have the potential to reduce inflammation in alveolar A549 cell lines (Murphy et al., 2019). To further expand on this work, the same assays were repeated with another alveolar cell line BEAS-2B, using six new extracts.
3.3.1. A549 cells
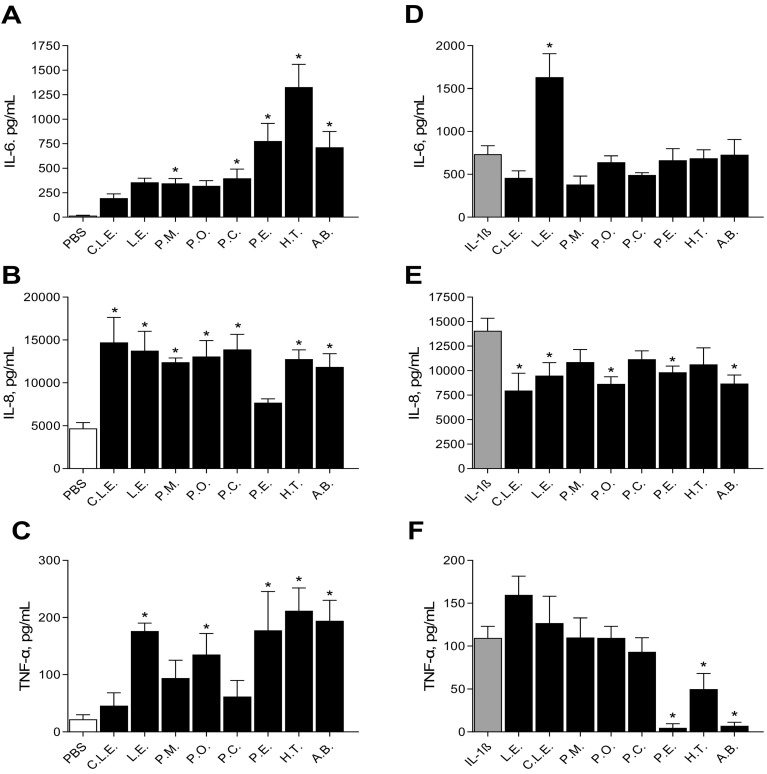
To determine the direct effects of the β-glucan extracts on lung cells, extracts were incubated with A549 cells for 24 h and supernatant was measured for cytokines. Results are displayed in Fig. 8 . Panel A shows the release of IL-6 after treatment; P.M, P.A, P.E, H.T and A.B all significantly induce the secretion of IL-6 compared to PBS control. Panel B shows the secretion of IL-8. All the extracts induced the secretion of IL-8 from A549 cells with respect to PBS control except for P.E. When A549 cells were treated with the extracts, TNF-α secretion was increased with L.E, P.O, P.E, H.T and A.B with respect to PBS control (Panel C).
Fig. 8.
The effect of the β-glucan extracts on cytokine expression in A549 cells measured using ELISA. Panel A; IL-6, Panel B; IL-8, Panel C; TNF-α. The effect of the β-glucan extracts on cytokine expression in BEAS-2B cells after IL-1β insult, measured using ELISA. Panel D; IL-6, Panel E; IL-8, Panel F; TNF-α. p < 0.05 versus PBS or IL-1β. Uninjured cells were treated with extracts (1 mg/mL) for 24 h before cytokine analysis. Cells were treated with IL-1β at 1 ng/mL for 24 h after which they were washed with PBS and treated with extracts (1 mg/mL) for 24 h before cytokine analysis. Phosphate buffer saline (PBS), Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazei (A.B).
A549 cells were then treated with IL-1β to induce a cytokine injury. Subsequently the cells were treated with the β-glucans extracts and the cytokine analysis was repeated, as displayed in Fig. 8. Panel D shows that C.LE., P.M and P.A slightly reduced IL-6 secretion (though not significantly), after insult except for L.E which increases secretion with respect to injury alone. Panel E shows that after insult L.E, C.L.E, P.O, P.E and A.B reduce the section of IL-8, which is an opposite response to when cells are treated in the absence of injury (Fig. 8; Panel B). The extracts P.E, H.T and A.B significantly reduced TNF-α secretion after IL-1β insult (Panel F) which is again an opposite response to when the cells are treated alone with extracts (Panel C).
3.3.2. BEAS-2B
To understand if the β-glucan extracts would have a similar effect in another lung epithelial cell line, BEAS-2B cells were treated with the β-glucans extracts. The supernatant was then measured for cytokine secretion. Results are displayed in Fig. 9 . All extracts induced the secretion of IL-6 (Panel A), and IL-8 (Panel B) with respect to PBS control. Panel C shows the secretion of TNF-α after treatment, C.L.E increased secretion but the other extracts had no effect.
Fig. 9.
The effect of the β-glucan extracts on cytokine expression in BEAS-2B cells measured using ELISA. Panel A; IL-6, Panel B; IL-8, Panel C; TNF-α. The effect of the β-glucan extracts on cytokine expression in BEAS-2B cells after IL-1β insult, measured using ELISA. Panel D; IL-6, Panel E; IL-8, Panel F; TNF-α. p < 0.05 versus PBS or IL-1β. Uninjured cells were treated with extracts (1 mg/mL) for 24 h before cytokine analysis. Cells were treated with IL-1β at 1 ng/mL for 24 h after which they were washed with PBS and treated with extracts (1 mg/mL) for 24 h before cytokine analysis. Phosphate buffer saline (PBS), Commercial Lentinan (C.L.E.), Lentinus edodes (L.E), Pholiota microspora (P.M), Pleurotus ostreatus (P.O), Pleurotus citrinopileatus (P.C), Pleurotus eryngii (P.E), Hypsizygus tessellatus (H.T) and Agaricus blazei (A.B).
Like the A549 cells, BEAS-2B cells were then treated with IL-1β to induce a cytokine injury; cells were then treated with the β-glucans extracts and the cytokine analysis was repeated, as displayed in Fig. 9. There was no effect on IL-6 secretion (Panel D). Panel E shows that after insult C.L.E, P.A, P.E, H.T and A.B reduced the section of IL-8 which is an opposite response to when cells are treated in the absence of injury (Fig. 9; Panel B). The extracts P.E, H.T and A.B significantly reduced TNF-α secretion after IL-1β insult (Fig. 9; Panel F) however these levels were the same in the absence of injury (Fig. 9; Panel C) suggesting expression levels are maintained in the presence of injury.
4. Discussion
ARDS-associated lung injury develops a state which is marked by an increase in serum levels of inflammatory chemokines and cytokines; this is a major contributor to disease severity and ultimately death (G. Chen et al., 2020; X. Chen et al., 2020; Huang et al., 2020; Mehta et al., 2020; Qin et al., 2020). There is currently a vast literature relating to the immunomodulatory effects of β-glucan (Rao et al., 2020), and there is a huge level of enthusiasm regarding their therapeutic potential. Thus with this in mind, there are three aims to this work. Firstly, to understand if β-glucans extracted in the same way from different species of mushroom contained the same levels of β-glucan content and if these extracts had the same effect on a key player in cellular immunity and response – macrophages. The second aim was to determine if the samples could elicit a response or prime immune cells, and whether there would be a difference in priming effects. Our final aim was to determine whether there may be the potential for the extracts to be used in hyperinflammatory conditions such as ARDS. To understand this, two types of in-vitro models were used – injured macrophages and lung injury models. This approach will potentially facilitate a greater understanding into the biological variance of these compounds and realising their therapeutic potential.
It is recognised in the literature that β-glucans from different sources can exert different biological effects, and that different extraction methods may help to optimize performance. Our experiments were performed using the same extraction method on all seven mushroom species. As such, we can compare the immunomodulatory effects of the β-glucan extracts by the same extraction procedure. Further studies may examine the effects of altering extraction parameters on β-glucan activity. Furthermore, future structural analyses would allow us to deepen the structure-function relationship. Further studies may examine the effects of altering extraction parameters on β-glucan activity. Furthermore, future structural analyses would allow us to deepen the structure-function relationship. The diverse mechanisms of action of β-glucans is unknown. There are differences in the effects of β-glucans that can be observed between similar preparations from the same species or source. The cellular pathways that are activated after recognition are also not fully understood. β-Glucans appear to be recognised as pathogen associated molecular patterns (PAMPs) and modulate immune function via this pathway (Brown and Gordon, 2005; Borchers et al., 1999). However, the exact mechanism by which β-glucans suppress inflammatory cytokines and induce anti-inflammatory cytokines are complex, and incompletely understood. With this in mind, previous work by this group, Murphy et al., 2019 investigated the differential effects of two β-glucan extracts in an in-vitro lung injury model and in an in-vivo model of pulmonary sepsis (Masterson et al., 2020). Once, determined fungal β-glucans had immune-modulatory effects in lung injury pre-clinical models, the next advancement is to highlight other potential fungal derived β-glucans with immune-modulatory activity. Once identified, future studies will investigate the structure-activity relationship to gain an understanding of how these molecules elicit their effects and the pathways associated with these effects.
The Key findings of this work include - There is a variance in the levels of β-glucan between mushroom species extracted in the same way. This study found that Lentinus edodes and Hypsizygus tessellatus had the highest levels of β-glucan content when measured using the Megazyme assay. Most extracts had the ability to induce both pro and anti-inflammatory cytokines individually at a concentration of 1 mg/mL in THP-1 macrophages. In the presence of a paracrine insult of a cocktail of cytokines; IL-8 was reduced in THP-1 macrophages. Also observed was a reduction in phagocytosis in THP-1 macrophages and CD14+ macrophages in the presence and absence of injury. After LPS insult, CCL8 relative gene expression was reduced, and IL-10 gene expression was increased in THP-1 macrophages. In lung epithelial cells, the extracts had the ability to reduce two cytokines (IL-8 and TNF-α) which are heavily correlated to pathogenesis of inflammation in the presence of IL-1β.
4.1. β-Glucans quantification in mushroom species
Hot water extracts were prepared from seven species of mushroom, and β-glucan content was determined. Results show that although extracts were isolated by the same method, each species yielded different levels of α- and β-glucans. Although there is some evidence to suggest that α-glucans can have immune-modulating properties (Masuda et al., 2017; Okamoto et al., 2007). There is substantially more evidence to suggest that the β-glucan molecule is the immune-stimulating compound found in mushrooms. These results highlight the variability between β-glucan contents in the different mushroom species. Two other studies using the same analysis procedure found variance among mushroom species (McCleary and Draga, 2016; Sari et al., 2017). Other studies have found that α- and starch glucans are usually of low abundance in cultivated mushrooms (Bak et al., 2014; Sari et al., 2017; Synytsya et al., 2008).
4.2. Effects of β-glucans on macrophages
Macrophages have the potential to intensify inflammation or exhibit regulatory repair activity during injury (Wynn and Barron, 2010).
As well as variance in content there is also evidential variance in response, which is most evident in Fig. 2 Panel A, measurement of IL-6. P.M and P.O have similar levels of β-glucan content (Fig. 1), yet P.M induced THP-1 macrophages to produce nearly double the amount of IL-6 in comparison to P.O, according to the ELISA assay. This could be correlated to the higher levels of α-glucan, in the P.O sample or to structural variances between β-glucans from different species. However, the high amount of α-glucan present in A.B sample does not hinder its activity in stimulating IL-6 secretion.
Variance can also be seen in phagocytic activity (Fig. 3, Panel A), where some samples (P.M, P.C and H.T) reduced phagocytosis in THP-1 macrophages. A.B reduced phagocytic activity in the donor PBMCs. Other extracts had no effect on phagocytic index. The THP-1 macrophages were differentiated using PMA, and the PBMCs were differentiated using MCSGF. Thus, as they should have a high phagocytic potential in this assay, it is interesting that some of the samples appeared to reduce this. This ability is potentially useful especially in conditions where macrophages are hypersensitive, and phagocytosis is uncontrolled.
Previous research has also shown that varied sources and structures lead to a varied biological response (Bohn and BeMiller, 1995; Bose et al., 2014; Demleitner et al., 1992; Driscoll et al., 2009; Goodridge et al., 2009; Volman et al., 2008; Wang et al., 2017b). As such, our results are in agreement with the literature in that β-glucan from different mushroom sources can induce varied responses. Further investigation into these correlations may identify optimized β-glucan sources for treatment of different pathological conditions.
Dectin-1 is a type II membrane receptor, which is documented as one of the principal receptors for β-glucans (Baert et al., 2015). TLR 2, 4 and 6 co-bind to dectin-1 after β-glucan recognition (Guo et al., 2015), modulating and contributing to cell responses including the release of pro and anti-inflammatory cytokines and phagocytic activity (Kanjan et al., 2017). The results of the present study showed a low- to absent expression for the gene dectin-1 receptor (CLEC7a). This could be for two reasons; a limitation of this study was that the samples were taken at 24 h when the gene could be (temporarily) switched off. Secondly, dectin-1 does not recognise all β-glucans equally; studies have shown that dectin-1 reacts differently based on structural determinants such as side-branching and size of the molecule (Adams et al., 2008). No gene expression could also be correlated to inhibition of CLEC7a, which could be correlated to a negative feedback effect. This result warrants a further timeline study to understand this mechanism.
Nonetheless these results demonstrate that the β-glucan samples are recognised by macrophages of a cell line lineage and from fresh PBMCs. This recognition can induce the secretion of both pro- and anti-inflammatory cytokines, reduce phagocytic activity, and alter gene expression levels reducing pro-inflammatory chemokines and increasing the secretion of the anti-inflammatory marker IL-10. Extracts increased secretion of both inflammatory cytokines (IL-6, IL-8, TNF-α) and anti-inflammatory cytokines (IL- 10 and IL-22). M1 macrophage polarization is associated with the secretion of pro-inflammatory cytokines: IL-1β, IL-6, and TNF-α (Bouhlel et al., 2007). M2 macrophage polarization is associated with the secretion of anti-inflammatory cytokines IL-10 (Arora et al., 2018; Wang et al., 2014). As the β-glucan extracts induce the secretion of both, it is possible that they stimulate the cells into a mixed population of M1/M2 macrophages. The commercial sample C.L.E had a different effect on the cells. L.E and C.L.E are isolated from the same mushroom species, again showing the great variances between β-glucan samples which can be dependent on cultivation, seasonal variation as well as extraction procedure. Taken together these results show the potential of β-glucans from mushrooms to behave as biological response modifiers.
To understand the immunomodulatory effects of β-glucan in an inflammatory M1 phenotype-inducing environment two types of insult were used. Firstly, LPS which stimulates macrophages toward an M1 phenotype (Zheng et al., 2013) and secondly a cocktail of cytokines (cytomix) was used to stimulate an inflammatory environment (Farley et al., 2009). After insult β-glucan samples were added to determine if the effects of the insult could be tempered.
After treatment with cytomix, some of the β-glucan extracts increased the secretion of IL-6. However, after insult, some of the extracts (P.O and P.C) induced less secretion of IL-6, compared with β-glucan alone, thus suggesting that the immune response is reduced in the presence of an injuring agent (cytomix) (Fig. 5 Panel a). One interesting finding in this study is the reduction of phagocytosis of PBMCs after LPS insult. All β-glucan extracts reduced the phagocytic index in the presence of LPS to just under half of the activity of positive controls. This result demonstrates the potential of β-glucans to modulate macrophage activity as these cells are from healthy volunteers. There is donor variation in these samples which is to be expected; future studies would investigate this effect in larger groups of healthy volunteers. The β-glucans also reduced phagocytosis after cytomix insult.
Impressively, the β-glucan samples reduced IL-8 gene expression levels after LPS injury and increased the gene expression levels of IL-10. This demonstrates an intracellular shift from an inflammatory phenotype to an anti-inflammatory phenotype in the presence of LPS. Although IL-8 was not reduced in the presence of cytomix, IL-10 was increased, again demonstrating a shift to a more anti-inflammatory response.
During SARS-CoV-2 macrophages communicate with target cells through chemokines and phagocytic signaling (Qi et al., 2020). Macrophages respond to initial infection as a result of the inflammatory cytokines secreted by type II alveolar cells which include IL-1β, IL-6 and TNF-α (Denney and Ho, 2018). When aiming to reduce the response of macrophages in inflammatory conditions, it is also important to target the alveolar cells at the centre of the injury.
4.3. Effect of β-glucans in an in-vitro lung injury model
Cytokines and chemokines have an important role in immunity as well as in immune pathology as a dysregulated response has the potential to cause extensive tissue and organ damage, especially in the lungs (Pedersen and Ho, 2020). As SARS-Cov-2 infection is associated with the production of inflammatory cytokines we investigated the effects β-glucans would have in an inflammatory environment by measuring cytokine production after IL-1β insult on two types of alveolar cell lines; A549 and BEAS-2b. When A549 (Fig. 8) and BEAS-2B cells (Fig. 9) were treated with the β-glucan extracts, all inflammatory cytokines were elevated. However, in the presence of inflammatory insults, some of the inflammatory cytokines were reduced significantly. Fig. 8, Fig. 9 Panel E and F, shows that the extracts had the ability to reduce IL-8 and TNF-α in both A549 cells and BEAS-2B. In reducing the cytokine expression and inflammation of lung tissue the inflammatory process can be avoided and blood gas transfer potentially unaffected or minimally affected. After injury, when the invading pathogen is eliminated, large numbers of inflammatory monocytes and macrophages can be recruited to the distal alveolar space because of chemokine gradients, this can also exceed the total number of resident macrophages (Davies et al., 2013; Galli et al., 2011).
As epithelial cells are the main source of anti-viral responses in the first 24–48 h window after infection, this is an important result. Important signals are transmitted to innate immune cells which are translated to adaptive immune responses (Geller and Yan, 2020). By firstly priming these cells with bioactives such as β-glucans to respond to infection, innate cells are recruited, and a memory is created for prevention of a secondary infection. More importantly, if the cells are primed, the hyper inflammatory reaction might not occur as cells are modulated by the β-glucans.
As the infection is more lung-centred than multi-organ-centred (McGonagle et al., 2020).
In-vitro lung epithelial cells represent a good model to determine potential targets. This study has shown that β-glucan extracts from mushrooms can reduce inflammatory responses in models of in-vitro lung injury.
5. Conclusions
There is a growing awareness of therapies directed to modulate the immune response in many pathological contexts. Medicinal mushrooms, which contain the complex β-glucans sugars have been used to treat an array of conditions for centuries including inflammatory conditions. Previously, we have demonstrated that β-glucans from the same mushroom isolated by different methods have differential immune-modulation abilities in an in-vitro model as well as in an in-vivo preclinical model. Following on from this, the current work has demonstrated the potential of β-glucans as immunomodulators with dual functions, firstly as immune priming agents that may bolster the capacity of the body to maintain homeostasis in the face of infectious and other challenges, and secondly to temper the immune response following infection, thus helping to avoid the serious sequelae associated with immune hyper-inflammatory response in immune and epithelial cells in inflammatory lung conditions such as ARDS. Future work will investigate relationship between the structure of β-glucans and mechanistic effects at cell and molecular levels. The main findings of this research also strongly align with emergence of green innovation for OneHealth applications (Rowan and Galanakis, 2020).
CRediT authorship contribution statement
Emma Murphy: conceptualization, methodology, data curation, visualization, formal analysis, writing original draft, review and editing. Emanuele Rezoagli: conceptualization, methodology, data curation, visualization, formal analysis, writing original draft, review and editing. Robert Pogue: methodology, data curation, formal analysis, review and editing. Bianca Simonassi-Paiva: methodology, formal analysis, review and editing. Ismin Izwani Zainol Abidin: methodology, formal analysis, review and editing. Gustavo Waltzer Fehrenbach: methodology, formal analysis, review and editing. Emer O'Neil: methodology. John Laffey: supervision, funding acquisition, conceptualization, review and editing. Ian Major: methodology, supervision, review and editing. Neil Rowan: funding acquisition, project administration, conceptualization, supervision, methodology, review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Editor: Lotfi Aleya
References
- Adams E.L., et al. Differential high-affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008;325(1):115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- Arora S., et al. Immunobiology. Elsevier GmbH; 2018. Macrophages: their role, activation and polarization in pulmonary diseases; pp. 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert K., Sonck E., et al. Cell type-specific differences in β-glucan recognition and signalling in porcine innate immune cells. Dev. Comp. Immunol. 2015;48(1):192–203. doi: 10.1016/j.dci.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Bak W.C., et al. Determination of glucan contents in the fruiting bodies and mycelia of lentinula edodes cultivars. Mycobiology. 2014:301–304. doi: 10.5941/MYCO.2014.42.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedirli A., et al. Beta-glucan attenuates inflammatory cytokine release and prevents acute lung injury in an experimental model of sepsis. Shock. 2007;27(4):397–401. doi: 10.1097/01.shk.0000245030.24235.f1. [DOI] [PubMed] [Google Scholar]
- Bellani G., et al. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition! Crit. Care. 2016 doi: 10.1186/s13054-016-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn J.A., BeMiller J.N. (1→3)-β-d-glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr. Polym. 1995;28(1):3–14. doi: 10.1016/0144-8617(95)00076-3. [DOI] [Google Scholar]
- Borchers A.T., et al. Exp. Biol. Med. 1999. Mushrooms, tumors, and immunity; pp. 281–293. [DOI] [PubMed] [Google Scholar]
- Bose N., et al. Differential regulation of oxidative burst by distinct β-glucan- binding receptors and signaling pathways in human peripheral blood mononuclear cells. Glycobiology. 2014;24(4):379–391. doi: 10.1093/glycob/cwu005. [DOI] [PubMed] [Google Scholar]
- Bouhlel M.A., et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. Cell Press. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- Chen G., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., et al. Inflammation Research. Springer Science and Business Media Deutschland GmbH; 2020. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome; pp. 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimi E., Slutsky A.S. Inflammation and the acute respiratory distress syndrome. Best Pract. Res. Clin. Anaesthesiol. 2004;18(3):477–492. doi: 10.1016/j.bpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Cui S.W., Wang Q., Zhang M. 1(3) Multidisciplinary Digital Publishing Institute; 2011. β-Glucans; pp. 319–345. (RSC Polymer Chemistry Series). [DOI] [Google Scholar]
- Davies L.C., et al. Tissue-resident macrophages. Nature Immunology. 2013:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demleitner S., Kraus J., Franz G. Synthesis and antitumour activity of sulfoalkyl derivatives of curdlan and lichenan. Carbohydr. Res. 1992;226(2):247–252. doi: 10.1016/0008-6215(92)84072-Z. [DOI] [PubMed] [Google Scholar]
- De Felippe J., et al. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1-3 polyglucose (glucan) Surg. Gynecol. Obstet. 1993;177(4):383–388. [PubMed] [Google Scholar]
- Denney L., Ho L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018:218–233. doi: 10.1016/j.bj.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., et al. Therapeutic potential of various β-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol. Ther. 2009;8(3):218–225. doi: 10.4161/cbt.8.3.7337. [DOI] [PubMed] [Google Scholar]
- Drozdowski L.A., et al. β-Glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010;21(8):695–701. doi: 10.1016/j.jnutbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley K.S., Wang L., Mehta S. Septic pulmonary microvascular endothelial cell injury: role of alveolar macrophage NADPH oxidase. 2009;296(3):480–488. doi: 10.1152/AJPLUNG.90201.2008. https://doi.org/10.1152/ajplung.90201.2008. American Physiological Society. [DOI] [PubMed] [Google Scholar]
- Fuller R., et al. Influence of yeast-derived 1,3/1,6 glucopolysaccharide on circulating cytokines and chemokines with respect to upper respiratory tract infections. Nutrition. 2012;28(6):665–669. doi: 10.1016/j.nut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Galli S.J., Borregaard N., Wynn T.A. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A., Yan J. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge H.S., Wolf A.J., Underhill D.M. Β-glucan recognition by the innate immune system. Immunol. Rev. 2009;230(1):38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., et al. Leptospiral lipopolysaccharide stimulates the expression of toll-like receptor 2 and cytokines in pig fibroblasts. Anim. Sci. J. 2015;86(2):238–244. doi: 10.1111/asj.12254. [DOI] [PubMed] [Google Scholar]
- Horie S., et al. Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Med. 2020;46(12):2265–2283. doi: 10.1007/s00134-020-06141-z. Springer Science and Business Media Deutschland GmbH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedinak A., et al. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011;10(1) doi: 10.1186/1475-2891-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesenak M., et al. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013;15(2):395–399. doi: 10.1016/j.intimp.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Johnson E., et al. Effect of an extract based on the medicinal mushroom agaricus blazei murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 2009;69(3):242–250. doi: 10.1111/j.1365-3083.2008.02218.x. [DOI] [PubMed] [Google Scholar]
- Jung H.K., et al. Production and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115. Biotechnol. Bioprocess Eng. 2007;12(6):713–719. [Google Scholar]
- Kanjan P., et al. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods. 2017;37:433–440. doi: 10.1016/j.jff.2017.07.061. [DOI] [Google Scholar]
- Kofuji K., et al. Antioxidant activity of β -glucan. ISRN Pharmaceutics. 2012;2012:1–5. doi: 10.5402/2012/125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen K., Anke T. Basidiomycetes as a source for new bioactive natural products. Curr. Org. Chem. 1998;2:329–364. [Google Scholar]
- Maki K.C., et al. Food products containing free tall oil-based phytosterols and oat β-glucan lower serum total and LDL cholesterol in hypercholesterolemic adults. J. Nutr. 2003;133(3):808–813. doi: 10.1093/jn/133.3.808. [DOI] [PubMed] [Google Scholar]
- Masterson C.H., et al. Purified β-glucans from the Shiitake mushroom ameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020;71(4):405–412. doi: 10.1111/lam.13358. Blackwell Publishing Ltd. [DOI] [PubMed] [Google Scholar]
- Masuda Y., et al. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary B.V., Draga A. Measurement of β-glucan in mushrooms and mycelial products. J. AOAC Int. 2016;99(2):364–373. doi: 10.5740/jaoacint.15-0289. [DOI] [PubMed] [Google Scholar]
- McGonagle D., et al. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRorie J.W., McKeown N.M. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017;117(2):251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Mehta P., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.J., et al. 2019. Immunomodulation properties of a novel β-glucan extract from the mushroom lentinus edodes in an in-vitro lung injury model. pp. A2114–A2114. [DOI] [Google Scholar]
- Murphy Emma J., Masterson C., et al. β-glucan extracts from the same edible shiitake mushroom lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects — implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Emma J., et al. β-glucan metabolic and immunomodulatory properties and potential for clinical application. Journal of fungi (Basel, Switzerland). MDPI AG. 2020;6(4):1–33. doi: 10.3390/jof6040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Emma J., Rezoagli E., et al. Β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi. 2020;6(4):1–33. doi: 10.3390/jof6040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Emma J., et al. β-Glucans. Encyclopedia. 2021;1(3):831–847. doi: 10.3390/encyclopedia1030064. 2021. [DOI] [Google Scholar]
- Nanchal R.S., Truwit J.D. Recent advances in understanding and treating acute respiratory distress syndrome [version 1; referees: 2 approved] F1000Research. 2018 doi: 10.12688/f1000research.15493.1. F1000 Research Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M., Vetvicka V. β-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- Okamoto S., et al. Inflammatory immune responses by water-insoluble α-glucans. J. Dent. Res. 2007;86(3):242–248. doi: 10.1177/154405910708600309. [DOI] [PubMed] [Google Scholar]
- Ooi V.E.C., Liu F. A review of pharmacological activities of mushroom polysaccharides. Int. J. Med. Mushrooms. 1999;1(3):195–206. doi: 10.1615/intjmedmushrooms.v1.i3.10. [DOI] [Google Scholar]
- Ooi V.E.C., Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2012;7(7):715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Investig. 2020:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue R., et al. Vol. 21. Elsevier BV; 2021. Exploiting immunomodulatory properties of β-glucans derived from natural products for improving health and sustainability in aquaculture-farmed organisms: concise review of existing knowledge, innovation and future opportunities; p. 100248. (Curr. Opin. Environ. Sci. Health). [DOI] [Google Scholar]
- Qi F., et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K.S., et al. Role of immune dysregulation in increased mortality among a specific subset of COVID-19 patients and immune-enhancement strategies for combatting through nutritional supplements. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezoagli E., Fumagalli R., Bellani G. Ann. Transl. Med. 2017 Jul;5(14):282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezoagli E., et al. The safety and efficiency of addressing ards using stem cell therapies in clinical trials. Stem Cell-Based Therapy for Lung Disease. 2019:219–238. doi: 10.1007/978-3-030-29403-8_12. [DOI] [Google Scholar]
- Rezoagli E., et al. Development of a critical care response - experiences from Italy during the coronavirus disease 2019 pandemic. Anesthesiol. Clin. 2021;39(2):265–284. doi: 10.1016/j.anclin.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan N.J., Galanakis C.M. Unlocking challenges and opportunities presented by COVID-19 pandemic for cross-cutting disruption in agri-food and green deal innovations: Quo Vadis? Sci. Total Environ. 2020;748 doi: 10.1016/j.scitotenv.2020.141362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari M., et al. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017;216:45–51. doi: 10.1016/j.foodchem.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Sari M., et al. The birch bracket medicinal mushroom, Fomitopsis betulina (Agaricomycetes)-bioactive source for beta-glucan fraction with tumor cell migration blocking ability. Int. J. Med. Mushrooms. 2020;22(1):1–13. doi: 10.1615/IntJMedMushrooms.2019033291. [DOI] [PubMed] [Google Scholar]
- Shin J. Il, et al. Inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J. Autoimmun. 2019 doi: 10.1016/j.jaut.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Sima P., Vannucci L., Vetvicka V. β-glucans and cholesterol (review) Int. J. Mol. Med. 2018:1799–1808. doi: 10.3892/ijmm.2018.3411. Spandidos Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivinski S.E., et al. Development of an in vitro macrophage screening system on the immunomodulating effects of feed components. J. Anim. Sci. Biotechnol. 2020;11(1) doi: 10.1186/s40104-020-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys J., Quinn M.T. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with β-(1,6)-branched β-(1,3)-glucan. Infect. Immun. 1999;67(1):244–252. doi: 10.1128/iai.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synytsya A., et al. Mushrooms of genus pleurotus as a source of dietary fibres and glucans for food supplements. Czech J. Food Sci. 2008;26(6):441–446. doi: 10.17221/1361-cjfs. [DOI] [Google Scholar]
- Takeshita K., et al. Diversity of complement activation by lentinan, an antitumor polysaccharide, in gastric cancer patients. Nippon Geka Gakkai zasshi. 1991;92(1):5–11. [PubMed] [Google Scholar]
- Tiwari U., Cummins E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. 2011;27(10):1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Tzianabos A.O. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin. Microbiol. Rev. 2000:523–533. doi: 10.1128/CMR.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenwijk H.P., Bast A., de Boer A. Immunomodulating effects of fungal beta-glucans: from traditional use to medicine. Nutrients. 2021;13(4) doi: 10.3390/nu13041333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman J.J., Ramakers J.D., Plat J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008;94(2):276–284. doi: 10.1016/j.physbeh.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014:614. doi: 10.3389/fimmu.2014.00614. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang F., Xu Z., Ding Z. Bioactive mushroom polysaccharides: a review on monosaccharide composition, biosynthesis and regulation. Molecules. 2017;22(6):955. doi: 10.3390/molecules22060955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., et al. β-Glucans: relationships between modification, conformation and functional activities. Molecules. 2017;22(2) doi: 10.3390/molecules22020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P., Weis A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspectives (review) Int. J. Med. Mushrooms. 1999;1(1):31–62. doi: 10.1615/intjmedmushrooms.v1.i1.30. [DOI] [PubMed] [Google Scholar]
- Wynn T.A., Barron L. Seminars in Liver Disease. 2010. Macrophages: master regulators of inflammation and fibrosis; pp. 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J., et al. Alleviation of seasonal allergic symptoms with superfine β-1,3-glucan: a randomized study. J. Allergy Clin. Immunol. 2007;119(5):1119–1126. doi: 10.1016/j.jaci.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Yamauchi E., et al. Contribution of lung fibroblast migration in the fibrotic process of airway remodeling in asthma. Allergol. Int. 2008;57(1):73–78. doi: 10.2332/allergolint.O-06-481. [DOI] [PubMed] [Google Scholar]
- Yang D., Zhou Z., Zhang L. Prog. Mol. Biol. Transl. Sci. 2019. An overview of fungal glycan-based therapeutics; pp. 135–163. [DOI] [PubMed] [Google Scholar]
- Zheng X.F., et al. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PloS one. 2013;8(5) doi: 10.1371/JOURNAL.PONE.0063967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambelli V, et al. Hexarelin modulates lung mechanics, inflammation, and fibrosis in acute lung injury. Drug Target Insights. 2021 doi: 10.33393/dti.2021.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]