Abstract
Decades of research have highlighted the importance of lateral parietal cortex (LPC) across a myriad of cognitive domains. Yet, the underlying function of LPC remains unclear. Two domains that have emphasized LPC involvement are semantic memory and episodic memory retrieval. From each domain, sophisticated functional models have been proposed, as well as the more domain-general assumption that LPC is engaged by any form of internally directed cognition (episodic/semantic retrieval being examples). Here we used a combination of functional magnetic resonance imaging, functional connectivity, and diffusion tensor imaging white-matter connectivity to show that (i) ventral LPC (angular gyrus [AG]) was positively engaged during episodic retrieval but disengaged during semantic memory retrieval and (ii) activity negatively varied with task difficulty in the semantic task whereas episodic activation was independent of difficulty. In contrast, dorsal LPC (intraparietal sulcus) showed domain general activation that was positively correlated with task difficulty. Finally, (iii) a dorsal–ventral and anterior–posterior gradient of functional and structural connectivity was found across the AG (e.g. mid-AG connected with episodic retrieval). We propose a unifying model in which LPC as a whole might share a common underlying neurocomputation (multimodal buffering) with variations in the emergent cognitive functions across subregions arising from differences in the underlying connectivity.
Keywords: angular gyrus, episodic, fMRI, parietal, semantic
Introduction
Several decades of neuropsychological and neuroimaging research have highlighted the importance of lateral parietal cortex (LPC) across a myriad of cognitive domains (Corbetta and Shulman 2002; Wagner et al. 2005; Binder et al. 2009; Cabeza et al. 2012; Humphreys and Lambon Ralph 2015; Sestieri et al. 2017). It also forms a core part of the default mode network (DMN), a network that often deactivates during task performance (Raichle et al. 2001; Buckner et al. 2008; Humphreys et al. 2015) Yet, despite the prominence of this region in basic and clinical research, the underlying function of LPC remains unclear. Part of this confusion may reflect that cognitive neuroscience research is often focused and organized by the cognitive domain of interest. As a result, multiple cognitive domains have been associated with the LPC with minimal cross-talk between these separate literature and resultant cognitive neuroscience theories (with a few notable exceptions: Corbetta and Shulman 2002; Cabeza et al. 2012; Humphreys and Lambon Ralph 2015; Rugg and King 2018; Renoult et al. 2019). While these theories are often sophisticated and are based on a wealth of domain-specific data, they fail to explain both the wide variety of functions that appear to recruit this region and, thus, what the core underpinning neurocomputations might be. There are two different answers to this fundamental question (Humphreys and Lambon Ralph 2015; Humphreys, Lambon Ralph, et al. 2020b): (i) a form of “neuromarquetry” in which LPC contains numerous subregions each recruited by different tasks and serves distinct underlying cognitive functions and (ii) multiple cognitive activities rely, in common, upon a small number of underlying computations that arise from the LPC and its patterns of connectivity to the wider neural network (Caspers et al. 2008, 2011; Uddin et al. 2010; Cloutman et al. 2013). Given that the LPC is an anatomically heterogeneous region in terms of cytoarchitecture and functional/structural connectivity across LPC (Caspers et al. 2008, 2011; Uddin et al. 2010; Mars et al. 2011; Cloutman et al. 2013), at least some variability in cognitive function across LPC might be expected. Fathoming the nature of LPC neurocomputations (and by extension other higher cortical regions) will require a sophisticated approach. Rather than focus solely upon an individual cognitive domain, it will be necessary to (i) combine data across multiple higher cortical functions and (ii) consider how varying functional input might modulate the activation pattern across tasks.
The current study therefore had 2 primary aims. First, to determine the underlying LPC function by directly comparing 2 cognitive domains (semantic and episodic memory) that are traditionally associated with the LPC, as well as the more domain-general hypothesis that LPC is engaged by any form of internally directed cognition (episodic and semantic retrieval both being examples of this process). Despite being associated with the LPC (and, in particular, the angular gyrus [AG]) throughout long neuropsychological and functional neuroimaging literature, these domains have rarely been directly compared in the same group of participants (and those that have been conducted have suffered from some important limitations). The second aim was to determine the extent to which variations in the expressed cognitive functions across LPC subregions directly reflect their varying input. This was achieved by directly mapping the correspondence between task functional magnetic resonance imaging (fMRI) data, and both functional and diffusion tensor imaging (DTI) white-matter connectivity measures.
Domain-specific theories
Many LPC theories and datasets have focused on individual cognitive domains. The semantic hypothesis has a very long history and tradition in both neuropsychology and from the start of functional neuroimaging. According to this proposal, the ventral LPC (vLPC), specifically the AG, acts as a semantic hub, which stores multimodal semantic information (Geschwind 1972; Binder et al. 2009), in a similar manner to the anterior temporal lobe (ATL) (Lambon Ralph et al. 2017). The observations of concrete > abstract and words > nonword differences in the AG found in individual studies and in formal meta-analyses form a cornerstone of evidence for this proposal. In contrast, numerous studies have also observed AG responses during episodic retrieval, with activation typically correlating with the vividness of the memory retrieved. This has led to the suggestion that LPC might act as some form of episodic buffer (Wagner et al. 2005; Vilberg and Rugg 2008). The fact that these 2 very different components of human long-term memory seem to involve the same brain LPC region has received little attention despite the very large and growing number of studies on each one. In fact, the situation for episodic and semantic memory is a worked example of the broader challenge—namely that many different cognitive domains have been implicated and do overlap in the LPC, yet only a few explanations have been offered for this multidomain maelstrom (Humphreys and Lambon Ralph 2015; Rugg and King 2018; Renoult et al. 2019; Humphreys, Lambon Ralph, et al. 2020b).
Domain-general theories
A handful of research groups have noted this confluence of multiple cognitive functions in the LPC. The resultant theories argue that some LPC processes might be domain-general in nature and represent more fundamental neurocomputations that are required by multiple cognitive activities (Corbetta and Shulman 2002; Walsh 2003; Cabeza et al. 2012;Fedorenko et al. 2013; Humphreys and Lambon Ralph 2015, 2017). For instance, there is good evidence to suggest that a common region within dorsal LPC (intraparietal sulcus [IPS]) forms part of a “multiple demand network” and is recruited as part of a frontoparietal network for executive processing (Fedorenko et al. 2013; Humphreys and Lambon Ralph 2015, 2017). Going further, several theories suggest that dorsal LPC (dLPC) within the IPS and vLPC serve counterpointed functions that are utilized across cognitive domains. The dLPC has been implicated in any task that requires top-down attentional control, whereas ventral areas are automatically recruited for bottom-up attention (Corbetta and Shulman 2002) Relatedly, dLPC is involved in tasks that require externally directed attention, whereas vLPC is active during internally directed attention, as is the case when retrieving semantic and/or episodic memories, as well as other processes such as future planning or self-projection (Buckner et al. 2008; Andrews-Hanna 2012). Since these internally directed processes are not required during most fMRI tasks, the vLPC is deactivated, hence its involvement in the DMN.
Parietal unified connectivity-biased computation model
The Parietal Unified Connectivity-biased Computation (PUCC) model takes a cross-domain perspective of LPC function. A central underlying idea is that the expressed cognitive function of each region will reflect the product of its local neurocomputation and its input/output connections (because the connections constrain what forms of information the neurocomputation acts upon). Thus, PUCC is based on 2 key assumptions. First, the local neurocomputation is considered to be constant across the wider LPC and provides the basis for online, multisensory buffering across modalities (as well as multimodal combinations) of input. A multimodal convergent buffer is important for bringing together multiple inputs in order to process time-extended behaviors, such as remembering an episodic event, narrative speech comprehension, or sequential object use (Geschwind 1965; Damasio 1989; Botvinick and Plaut 2004, 2006). A number of prominent parallel distributed processing computational models have shown that the addition of recurrent feedback loops allows a model to “buffer” verbal or nonverbal spatiotemporal input (Elman nets: Elman 1990) in support of time-extended verbal and nonverbal behaviors (McClelland et al. 1989; Botvinick and Plaut 2004; Ueno et al. 2011). A “buffering-type” function is consistent and indeed part inspired by more domain-specific buffer models of LPC function (Baddeley 2000; Wagner et al. 2005; Vilberg and Rugg 2008), as well as a “working-memory” type system in dLPC (Pessoa et al. 2002; Humphreys and Lambon Ralph 2015).
The second key assumption of PUCC is that while the local neurocomputation may be constant across LPC, the “expressed” task contribution of each LPC subregion will be influenced by its long-range connections. Thus, even on an assumption that the local buffering computation might be the same throughout the LPC, the types and forms of information being buffered will reflect the inputs and outputs to each subregion. This tenet is observed in various implemented computational models, which have shown that the involvement of a processing unit to each cognitive activity is molded both by its local computation and its connectivity to different input/output information sources (“connectivity-constrained cognition—C3”: [28–30]). In terms of underlying architecture, anatomical evidence suggests that there are variations in cytoarchitecture and functional/structural connectivity across LPC (Caspers et al. 2008, 2011; Uddin et al. 2010; Cloutman et al. 2013). For instance, the dLPC is known to connect with the frontal executive network, whereas vLPC connects with a distributed set of regions associated with the DMN, saliency network, language network, etc. (Vincent et al. 2008; Spreng et al. 2010; Uddin et al. 2010; Power et al. 2011; Lee et al. 2012; Cloutman et al. 2013; Power and Petersen 2013; Yeo et al. 2013). Consequently, at least some variability in cognitive function across LPC might be expected.
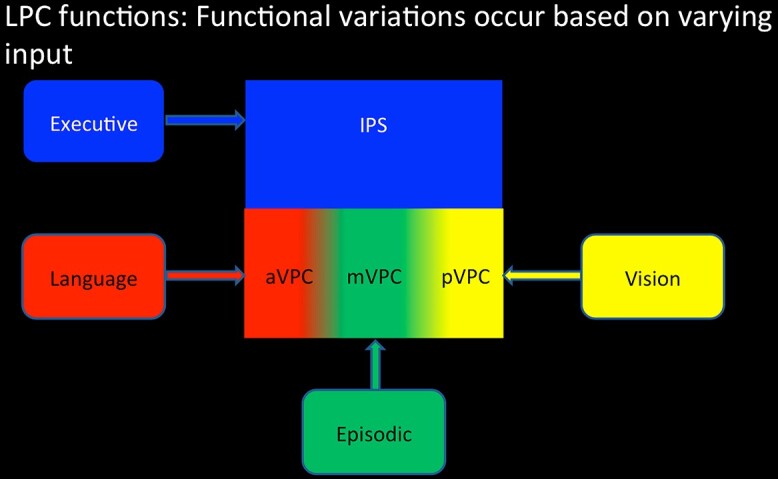
Therefore, according to PUCC, while LPC as a whole might share a common underlying neurocomputation (e.g. multimodal buffering), variations in task activation across subregions will arise due to differences in the underlying input (e.g. visual, verbal, spatial, executive, etc.) (Humphreys and Lambon Ralph 2015). Indeed, we have previously shown that the profile of functional activation varies across LPC and that this pattern directly maps onto variations in functional connectivity using task-based and resting-state functional connectivity measures. Specifically, using ICA, we demonstrated separable LPC functional connectivity networks, with each LPC subregion showing varying functional preference in a sentence, picture, and number sequence task. First, consistent with existing evidence LPC (Caspers et al. 2008, 2011; Uddin et al. 2010; Mars et al. 2011; Cloutman et al. 2013), dorsal areas (dorsal PGa/IPS) demonstrated functional connectivity with the frontal executive network, whereas vLPC varied in an anterior–posterior direction with central vLPC (mid PGp) connecting with the DMN, anterior vLPC (ventral PGa) connecting with the frontotemporal language network, and posterior vLPC (posterior PGp) connecting with the occipitoparietal visuospatial network. As PUCC would predict, these variations in functional connectivity were mirrored in terms of task activation profile. Dorsal LPC demonstrated a domain-general response, with equally strong positive activation for sentence, picture, and number domains relative to rest. Whereas ventral areas varied along an anterior–posterior axis: Specifically, the central AG (mid PGp), which functionally connected with the DMN, was equally deactivated by all 3 fMRI domains relative to rest; the anterior region that connected with the frontotemporal language system showed positive activation only for the sentence task; and the posterior region was part of the visual/SPL network and hence only responded to the picture sequences. Nevertheless, while this result is consistent with the predictions of the PUCC model, functional connectivity does not necessarily reflect the true underlying structural connectivity. Thus, one of the key aims of the current study was to examine the extent to which variations in activation patterns could arise from underlying structural variations white-matter connectivity.
Technical issues/recommendations for LPC research
Previous studies have demonstrated that, in order to explore LPC functions and reveal interpretable findings, it is necessary to take certain factors into account within the design and analysis of any study:
The direction of activation relative to rest
Given the involvement of LPC in the DMN, it is of critical importance to consider whether a task positively or negatively engages the LPC relative to rest. While many tasks generate deactivation in the AG, this is not always the case and the handful of activities that do positively engage the AG might be crucial sources of evidence about its true contribution (Humphreys and Lambon Ralph 2015). Contrasts between a cognitive task of interest versus an active control condition are ambiguous because the difference could result from (i) greater positive activation for the task or (ii) greater “deactivation” for the control. This issue becomes even more important when considering the impact of task difficulty on activation and deactivation in this region (see next). A straightforward expectation applied to almost all brain regions is that if a task critically requires the LPC then the LPC should be strongly engaged by that task. Indeed, this is the pattern observed in the ATL where semantic tasks are known to positively engage the ATL relative to rest, whereas nonsemantic tasks do not modulate/deactivate ATL (Humphreys et al. 2015). Perhaps one of the major motivations for considering task (de) activation relative to “rest” is that “rest” can be used as a common constant reference point across tasks. This is particularly important when conducting cross-domain comparisons. For instance, when one is examining a single cognitive domain, it is possible to use a domain-specific baseline, i.e. a contrast a task that places strong demands on the particular cognitive system versus a task with lower demands (e.g. remember > know in an episodic memory tasks, or words > nonwords in a semantic memory task). Since the same is not possible across cognitive domains, rest acts a common constant for cross-domain comparisons, even if the true cognitive interpretation of “rest” is unclear.
Task difficulty
Task difficulty is important in 2 different ways. First, task difficulty correlates positively with activation in dLPC (dorsal AG/IPS) but negatively with the level of activation within vLPC, or put in a different way, the level of deactivation in vLPC (mid-AG) is positively related to task difficulty. Indeed, the dLPC and vLPC have often been shown to be anticorrelated in resting-state data (Fox et al. 2009; Chai et al. 2012; Keller et al. 2013; Humphreys and Lambon Ralph 2017). Secondly, task-difficulty deactivations need to be accounted for when interpreting differences in vLPC areas. A “positive” difference can be obtained in the AG simply by comparing easy > hard task conditions even for tasks that are entirely nonsemantic, nonlinguistic, and nonepisodic (Humphreys and Lambon Ralph 2017). One major limitation in the evidence for the semantic hypothesis is that apparent semantic fMRI effects could be explained by a difficulty confound (e.g. word > nonword, concrete > abstract). Indeed, it is known that the level of deactivation correlates with task difficulty (Harrison et al. 2011; Gilbert et al. 2012; Humphreys and Lambon Ralph 2017), and it has been shown that one can both eliminate the difference between semantic and nonsemantic tasks when task difficulty is controlled (Humphreys and Lambon Ralph 2017) and, more compellingly, flip the typical “semantic” effects (e.g. words > nonwords, concrete > abstract) by reversing the difficulty of the tasks or stimuli (Pexman et al. 2007; Graves et al. 2017).
The importance of within-study comparisons
Reviews and formal meta-analyses of existing fMRI data have clearly identified overlapping areas of activations within the LPC (Humphreys and Lambon Ralph 2015). While highly suggestive of domain-general computations in these regions, one needs within-participant comparisons to test these hypotheses further. Without such evidence, 2 alternative interpretations are possible: (i) True overlap across tasks implicating the region in a common neurocomputation (Corbetta and Shulman 2002; Walsh 2003; Cabeza et al. 2012; Fedorenko et al. 2013; Humphreys and Lambon Ralph 2015, 2017) or (ii) small-scale variability in function across the LPC which is blurred by cross-study comparisons or meta-analyses (Dehaene et al. 2003).
The current study
The current study had 2 goals: (i) to compare alternative theories of LPC function directly within the same group of participants in an fMRI study and (ii) to examine the extent to which variations in the emergent task-activation patterns could arise from the underlying functional and structural connectivity across the LPC, as predicted by the PUCC model.
The first goal was addressed in experiment 1: Following some of the dominant proposals about LPC function (reviewed above), in an fMRI study, we manipulated internally versus externally directed attention and episodic retrieval versus semantic retrieval. Importantly, we considered the direction of activation/deactivation versus rest as well as the extent to which the results can be explained in terms of variations in task difficulty. There were 4 conditions, 2 involving internally directed attention (semantic or episodic retrieval) and 2 involving externally directed visual attention (real-world object decision or scrambled pattern decision). In each task, the participant was presented with word triads including a target word in the center of the screen and 2 words below. In the semantic task, the participants indicated which item was semantically related to the target (e.g. moose: antlers or feathers). In the episodic task, the participants selected the feature that best matched the target items. The target items were verbal labels that corresponded to color photographs that were viewed prior to the scan (e.g. bucket: green or red). Vividness ratings were made after each trial. In the object-decision tasks, participants responded to a question about a picture presented on the screen (chair: blue red), and in the control task, they indicated the direction of a scrambled picture that was shifted to the left or right side of the screen (see Fig. 1). LPC activation was also contrasted with the activation in a known semantic region within the ATL.
Fig. 1.
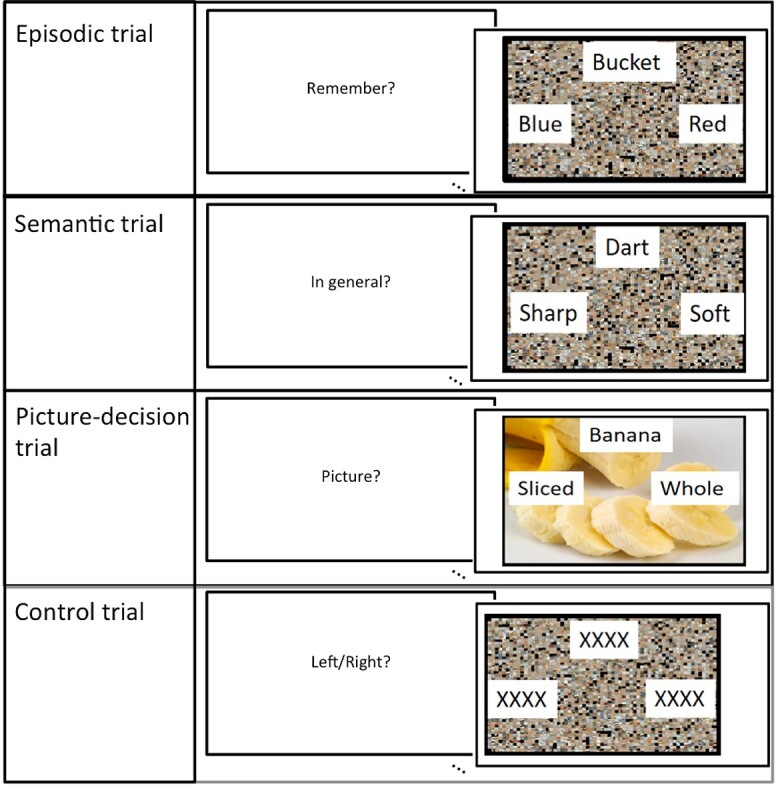
One example trial from each experimental condition.
The second goal was addressed in experiment 2: Here we first sought to determine the correspondence between structural and functional connectivity measures of LPC. We have previously shown varying functional connectivity patterns across LPC subregions in the dorsal–ventral and anterior–posterior directions. In the current study, we directly compared the same functional connectivity pattern with structural connectivity from the same regions of interest (ROIs) using DTI white-matter connectivity measures. Secondly, after establishing functional and structural input, we determined the extent to which varying functional and structural input directly maps onto variations in the pattern of task-based activation in the same ROIs across 2 independent fMRI datasets.
Materials and methods
Experiment 1: fMRI study
Participants
Twenty-two participants took part in the fMRI study (average age = 23.81, SD = 4.54; N female = 15). All participants were native English speakers with no history of neurological or psychiatric disorders and normal or corrected-to-normal vision.
Task design and procedures
There were 4 experimental tasks: episodic, semantic, picture-decision, and control. In each task, the participant was presented with word triads including a target word in the center of the screen and 2 words below, 1 on the left and 1 on the right. The participants had to select the correct option by button press. The words were presented on top of a scrambled or unscrambled picture depending on the condition. The trials lasted 4 s and were preceded by a 1.5-s instruction indicating the upcoming task. The trials were presented using an event-related design with the most efficient ordering of events determined using Optseq (http://www.freesurfer.net/optseq. Null time was intermixed between trials and varied between 0 and 26 s (average = 2.80 s, SD = 3.13) during which a fixation cross was presented. In total, 54 items were presented for each condition. The experiment was split into 3 runs (18 trials per condition), each run lasting 620 s, the order of which was counterbalanced across participants. An example trial from each task can be seen in Fig. 1.
Semantic task
Here the participants were presented with a target word (e.g. knife) and 2 alternative possible features of the object (e.g. sharp vs. bendy), such as its typical function, color, texture, shape, etc. The participants were instructed to determine which alternative was correct. The words were presented on top of a scrambled picture.
Picture-decision task
Here the word triads were presented on top of a color photograph of an object (e.g. a chair). The target word referred to a property of the picture (e.g. color) and 2 alternative choices (e.g. blue and red). The participants were instructed to select the option that best matched the target feature of the object.
Episodic task
Immediately prior to the scan, the participants were exposed to a selection of 54 color photographs of objects (e.g. a bucket) and told that they would be required to remember aspects of the pictures during the experiment. Each photograph was presented for 10 s and the participants were asked to describe the picture in as much detail to ensure that the pictures were sufficiently encoded. In each trial of the experiment, a target word would be presented (e.g. bucket) and the 2 alternative possible features of the remembered item (e.g. blue or red). The words were presented on top of a scrambled picture. The participants were instructed recall the feature that best described the target item. After a short jittered interval (varying from 0 to 1.5 s), the participants were given 3 s in which to rate the vividness of their memory of that particular item from 1 to 4 (1 = not vivid, 4 = very vivid). The episodic trial and the vividness rating were modeled separately in the general linear model.
Control task
In the control task, the word triads consisted of a string of Xs (e.g. xxxxxxxxx) on top of a scrambled picture. The picture was shifted slightly to the left or right. The participants had to indicate the direction of the shift. This acted as control for visual and motor activation.
fMRI acquisition parameters
Images were acquired using a 3T Philips Achieva scanner using a dual gradient-echo sequence, which has improved signal relative to conventional techniques, especially in areas associated with signal loss (Halai et al. 2014). Thirty-one axial slices were collected using a time repetition (TR) = 2.8 s, time echo (TE) = 12 and 35 ms, flip angle = 95°, 80 × 79 matrix, with resolution 3 × 3 mm, slice thickness 4 mm. B0 images were also acquired to correct for image distortion.
fMRI data analysis
Preprocessing
The dual-echo images were first B0 corrected and then averaged. Data were analyzed using SPM8. Images were motion-corrected and co-registered to the participants’ T1 structural image and then spatially normalized into Montreal Neurological Institute (MNI) space using DARTEL (Ashburner 2007). The functional images were then resampled to a 3 × 3 × 3 mm voxel size and smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
General linear modeling (GLM)
The data were filtered using a high-pass filter with a cut-off of 190 s and then analyzed using a general linear model. At the individual subject level, each condition was modeled with a separate regressor and events were convolved with the canonical hemodynamic response function. Time and dispersion derivatives were added and motion parameters were entered into the model as covariates of no interest. At the individual level, each task condition was contrasted separately against rest and entered at the second level into separate one-sample t-tests to test for a significant group effects for each condition versus rest, as well as one-way analysis of variance (ANOVA) to determine significant differences between tasks. A standard voxel height threshold P < 0.001, cluster-corrected using FWE P < 0.05, was used for all group analyses.
Regressor analyses
It has been previously shown that LPC activity is modulated by task difficulty, with dLPC showing a positive correlation with difficulty and ventral parietal cortex (VPC) showing a negative correlation with difficulty (Harrison et al. 2011; Gilbert et al. 2012; Humphreys and Lambon Ralph 2017); this might be true on the task level (harder tasks more strongly modulate activation) but also within each task at the item level. In order to determine whether there were significant correlations with trial-to-trial difficulty, we added trial-wise reaction time (RT) as a parametric modulator to the GLM, (i) across all tasks, as well as (ii) for each task independently. In addition to task difficulty, in terms of episodic memory retrieval, there is evidence that vLPC activation positively correlates with the self-reported vividness of the retrieved memory. In order to determine whether there were significant correlations with episodic vividness ratings, the item-level vividness ratings were added as a parametric modulator in a separate GLM.
ROI analyses
The specific hypothesis regarding the AG and IPS was tested using ROIs taken from a previous large-scale multidomain meta-analysis (AG and IPS) (Humphreys and Lambon Ralph 2015) and is therefore representative of the regions key regions highlighted in the literature. Note, the AG ROI corresponded to an 8 mm sphere centered on the coordinates showing maximum likelihood of activation for both semantic and episodic meta-analyses, and the region showing domain-general executive processing in the IPS (Humphreys and Lambon Ralph 2015). In addition, one primary aim of the current study is to test the theory that the AG functions as multimodal store of semantic information in a similar manner to the ATL. If true, the AG should show a similar pattern of task-related activation to that observed in the ATL. To test this, we compared task activation in the AG versus ATL ROIs. The ATL ROI was defined based on the results from a large-scale study that compared a variety of semantic tasks relative to nonsemantic control tasks (Humphreys et al. 2015).
Experiment 2: functional connectivity analysis
The second key aim of the current study was to determine whether the pattern of functional connectivity found across IPL regions in a previous study is mirrored in the structural connectivity. The full details of the previous study can be found in Humphreys et al. (2020a). Briefly, 24 participants completed a sequence processing task across 3 different domains: sentences, pictures, and numbers. On a given trial, a sequence of items (words, pictures, or numbers) was visually presented one item at a time and the participants indicated whether or not the sequence followed a coherent or incoherent structure. The preprocessed fMRI data were analyzed in a group spatial independent component analysis (ICA) using the GIFT toolbox (http://mialab.mrn.org/software/gift) (Calhoun et al. 2001) to decompose the data into its components and identify those that included the LPC. Four LPC components were identified, and functional labels were assigned to each based on the overlap with the built-in GIFT functional network template. The 4 components were labeled as follows: a DMN component, a frontoparietal executive control component, a language component, and a visual-parietal component (see Fig. 4 and description of components in results). The frontoparietal executive component had a peak in dLPC, within dorsal AG/IPS, whereas the other 3 components were located in vLPC. Specifically, the peak LPC coordinate for the DMN component was located in mid-AG (PGp), whereas the language component was slightly anterior (ventral PGa), and visual-parietal component was more posterior in posterior AG (posterior PGp). The same ICA networks were identified an independent resting-state ICA analysis, suggesting that the results are reliable.
Fig. 4.
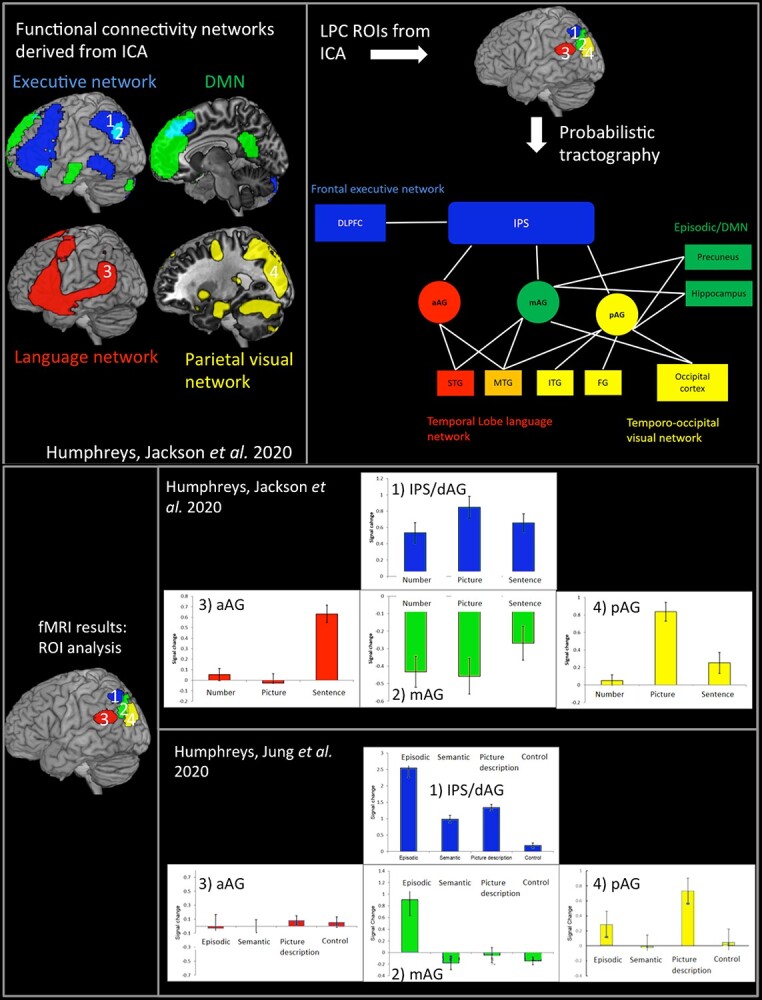
Top left: The 4 LPC functional connectivity networks derived from ICA. Top right: The results from the DTI analysis, using the ICA-derived LPC seed regions. Bottom panel: The results from 2 fMRI studies using the LPC ROIs.
Distortion-corrected diffusion-weighted imaging and probabilistic fiber tracking
Diffusion-weighted images were acquired in 24 healthy volunteers (11 females; mean age 25.9, range 19–47) without any record of neurological or psychiatric disorders. This dataset has described previously and utilized for various tractography-related explorations (Binney et al. 2012; Cloutman et al. 2012; Bajada et al. 2016, 2017; Jung et al. 2017, 2018). All participants were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield 1971). Participants gave written informed consent to the study protocol, which had been approved by the local ethics committee.
A 3T Philips Achieva scanner (Philips Medical System, Best Netherlands) was used for acquiring imaging data with an 8-channel SENSE head coil. Diffusion-weighted imaging was performed using a pulsed gradient spin echo-planar sequence, with TE = 59 ms, TR ≈ 11,884 ms, G = 62 mT m−1, half scan factor = 0.679, 112 × 112 image matrix reconstructed to 128 × 128 using zero padding, reconstructed resolution 1.875 × 1.875 mm, slice thickness 2.1 mm, 60 contiguous slices, 61 noncollinear diffusion sensitization directions at b = 1,200 smm−2 (∆ = 29.8 ms, δ = 13.1 ms), 1 at b = 0, SENSE acceleration factor = 2.5. Acquisitions were cardiac gated using a peripheral pulse unit positioned over the participants’ index finger or an electrocardiograph. For each gradient direction, 2 separate volumes were obtained with opposite polarity k-space traversal with phase encoding in the left–right/right–left direction to be used in the signal distortion correction procedure (Embleton et al. 2010). A co-localized T2-weighted turbo spin echo scan was acquired with in-plane resolution of 0.94 × 0.94 mm and slice thickness 2.1 mm, as a structural reference scan to provide a qualitative indication of distortion correction accuracy. A high-resolution T1-weighted 3D turbo field echo inversion recovery image (TR ≈ 2,000 ms, TE = 3.9 ms, TI = 1,150 ms, flip angle 8°, 256 × 205 matrix reconstructed to 256 × 256, reconstructed resolution 0.938 × 0.938 mm, slice thickness 0.9 mm, 160 slices, SENSE factor = 2.5) was obtained for the purpose of high-precision anatomical localization of seed regions for tracking.
In order to directly compare the results from the DTI to those from the functional connectivity analysis, 4 ROIs were identified for tractography based on the peak LPC coordinates from the 4 functional networks identified in the functional connectivity analysis: dLPC (dorsal PGa/IPS), anterior-vLPC (PGa), mid-vLPC (PGp), and posterior-vLPC (PGp) (Humphreys, Jackson, et al. 2020a). Note that the mid-LPC corresponds most closely to the DMN area, although the entire AG is often implicated. ROIs were defined as an 8-mm radius sphere in the left hemisphere and transformed into each individual’s native diffusion space using the diffeomorphic anatomical registration through an exponentiated lie algebra (DARTEL) toolbox (Ashburner 2007) based on each participant’s T1-weigthed images.
DTI analysis was performed using unconstrained probabilistic tractography using the PICo software package (Parker et al. 2003), sampling the orientation of probability density functions generated constrained spherical deconvolution (Tournier et al. 2008) and model-based residual bootstrapping (Haroon et al. 2009; Jeurissen et al. 2011). Twenty thousand Monte Carlo streamlines were initiated from each voxel within an ROI. Step size was set to 0.5 mm. Stopping criteria for the streamlines were set so that tracking terminated if pathway curvature over a voxel was greater than 180°, or the streamline reached a physical path limit of 500 mm.
The tracking results for each participant were spatially normalized into MNI space using the DARTEL toolbox. The brain regions associated with each fiber pathway were determined using brain masks from the AAL atlas or the Juelich histological atlas, which has a finer demarcation for the parietal cortex. The superior, middle, and inferior temporal lobes were further subdivided into anterior, middle, and posterior regions based on those used previously (Jung et al. 2017) since these regions have been shown to have large variations in connectivity profiles (Bajada et al. 2017; Jung et al. 2017). For each ROI, the atlas masks were overlaid over each participant’s tracking data and a maximum connectivity value (ranging from 0 to 20,000) between the ROI and each region of the brain was estimated. Thereby, we obtained a single probability estimate of a pathway between each pair of regions. These values were placed into an individual-specific matrix. Then, the connectivity matrices were subjected to a double threshold to ensure that only connections with high probability in the majority of participants were considered. For the first-level individual threshold, following the approach described by Cloutman et al (Cloutman et al. 2012), the λ-value of the Poisson distribution identified was used to determine a threshold value at P = 0.025. For the second-level group threshold, we used both a stringent (over 70% of participants, i.e. at least 17/24 participants) and a more relaxed (over 50% of participants, i.e. at least 12/24 participants) criteria for consistency.
fMRI ROI analysis
In order to determine the extent to which the results from the functional and structural connectivity analyses relate to functional differences in task-based activation, the same 4 LPC subregions (dLPC [dorsal PGa/IPS], anterior-vLPC [PGa], mid-vLPC [PGp], and posterior-vLPC [PGp]) were used as ROIs to examine the pattern of task-based activation from 2 fMRI studies: (i) the sentence, picture, and number sequence task described above and reported in detail by Humphreys et al. (2020a) and (ii) the current fMRI study reported in experiment 1.
Results
Experiment 1 behavioral results
On average, the participants produced relatively few incorrect responses (average proportion incorrect (SD): episodic task = 0.13 (0.05), semantic task = 0.06 (0.4), picture task = 0.09 (0.03), and control task = 0.01 (0.1); see Fig. 3). The average reaction time for each task was as follows (SD): episodic task = 2,149 ms (190), semantic task = 1,888 ms (182), picture task = 1,842 ms (231), and control task = 695 ms (122). A one-way within-subjects ANOVA revealed that the tasks varied significantly in difficulty both in terms of accuracy (F(21) = 111.88, P < 0.001) and reaction time (F(21) = 1202.20, P < 0.001). Pairwise comparisons showed that the episodic task was significantly more difficult than all other tasks in terms of accuracy (all ts > 3, all Ps < 0.006), the picture task was harder than the semantic task and control task (all ts > 3, all Ps < 0.005), and the control task was easier than all other tasks (ts > 3, all Ps < 0.001). The reaction time data mirrored the accuracy data (all ts > 6.58 and Ps < 0001), with the exception that the semantic task and picture task did not differ (t = 1.28, P = 0.2). Given the known effects of task difficulty on IPS activation and AG deactivation, then—all other things being equal—one might have expected the greatest IPS activation, and the greatest AG deactivation for the episodic task followed by the picture task, then semantic, and final the control task. Of theoretical relevance, while this was true in the IPS, this pattern was not the observed in AG.
Fig. 3.
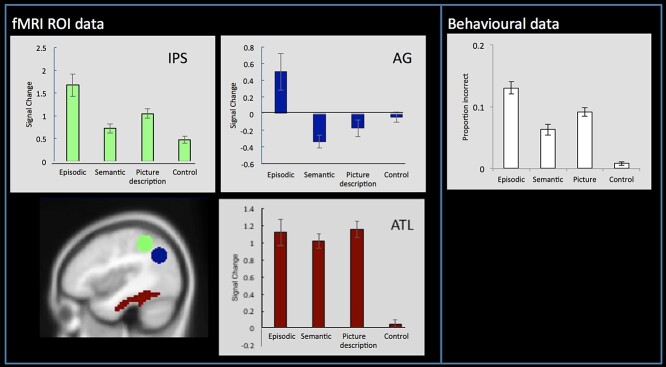
Left panel: ROI analyses showing mean activation relative to rest for each condition within the IPS, AG, and ATL. The AG and IPS ROIs were defined based on the results from a large-scale multidomain meta-analysis (Humphreys and Lambon Ralph 2015). The ATL ROI was defined based on the region identified by a study that contrasted the activation from a variety of semantic tasks relative to nonsemantic control tasks (Humphreys et al. 2015). Right panel: The behavioral data showing the average proportion of incorrect responses during the fMRI task. Note. The behavioral data closely mirror the fMRI activity in the IPS.
fMRI results
Whole-brain analyses were performed using a standard voxel height threshold P < 0.001 and cluster-corrected using FWE P < 0.05. The contrast of episodic task > control task was found to activate bilateral lateral and medial frontal areas, dorsal (IPS) and ventral LPC (AG), medial parietal (precuneus), and posterior and medial occipitotemporal areas (see Fig. 2). As with many other tasks with written word stimuli (Rice, Hoffman, et al. 2015a; Rice, Lambon Ralph, et al. 2015b), the contrast of semantic task > control task activation was mainly left-sided but overlapped with the episodic task in the lateral and medial frontal cortex, and posterior occipitotemporal areas although the activation for the semantic task spread more anteriorly along fusiform gyrus into the ATL, in congruence with its established role in semantic processing (Lambon Ralph et al. 2017). In contrast to the episodic task, no parietal activation was found for the semantic task (in fact the vLPC was more strongly activated by the control task relative to semantics) (Fig. 2). Indeed, direct task comparisons showed these network differences to be statistically significant (Fig. 2). Importantly, given the importance of the direction of activation relative to rest, the same AG region that is “positively” activated in the episodic task (episodic task > rest) is “deactivated” in the semantic task (rest > semantic task) (Fig. 2).
Fig. 2.
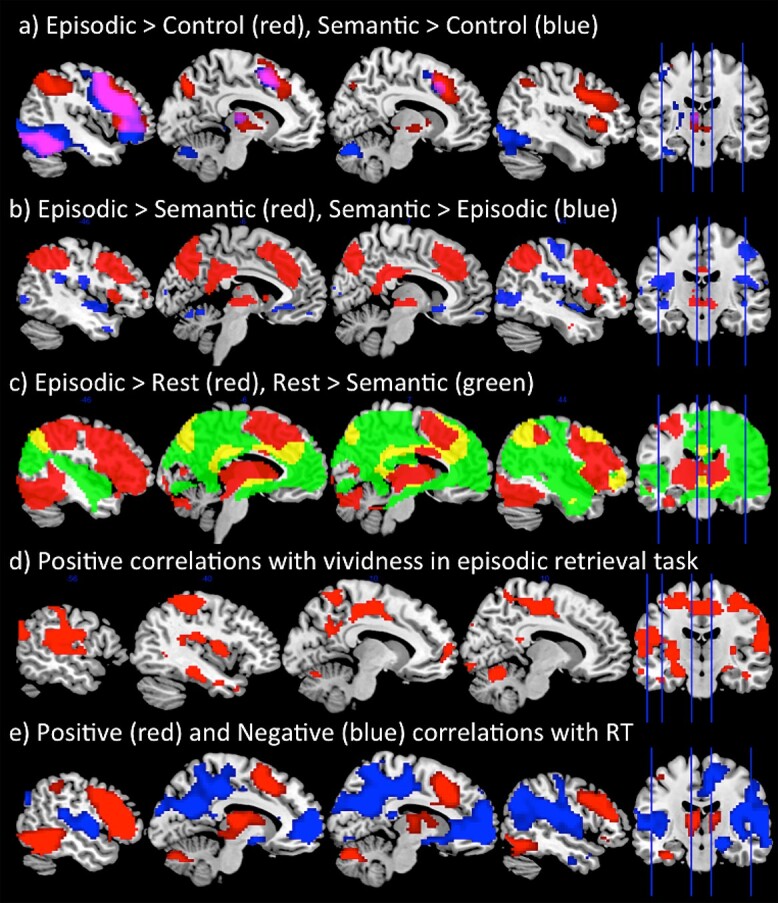
A) Whole-brain responses to the contrast of Episodic > Control (red) and Semantic > Control (blue). B) Whole-brain responses to the contrast of Episodic > Semantic (red) and Semantic > Episodic (blue). C) Whole-brain responses showing positive activation relative to rest for the episodic task (Episodic > Rest (red)) and deactivation relative to rest for the semantic task (Rest > Semantic (green)). This shows positive LTC activation for the episodic task but deactivation for the semantic task. D) The areas that positively correlated with vividness ratings during the episodic retrieval task, using a trial-wise parametric modulator. E) The network showing a positive correlation (red) and a negative correlation (blue) with task difficulty using a trial-wise parametric modulator. Analyses were performed using a standard voxel height threshold P < 0.001 and cluster-corrected using FWE P < 0.05.
Within AG, the results from the ROI analysis provided strong support that this region is primarily involved in episodic retrieval rather than any other task. Despite being the hardest task, AG was found to be significantly “positively” activated relative to rest during episodic processing, as demonstrated by a one-sample t-test (t(21) = 2.30, P = 0.03). In contrast, AG showed significant deactivation relative to rest during semantic retrieval (t(21) = −4.39, P < 0.00), thus strongly contradicting the semantic hypothesis. Indeed, semantic activation in the AG was found to also be lower than even the control task which required no semantic processing (t(21) = −2.84, P = 0.01) (Fig. 3). This provides clear evidence that the AG is engaged by episodic retrieval but is disengaged during semantic retrieval. Unlike the AG, however, the ATL (Fig. 3), a known semantic region, showed a very different pattern of activation: strong positive activation relative to rest across the 3 experimental tasks (since all tasks required semantic processing) (all ts > 7.34, Ps < 0.001), but no modulation for the control task (t = 0.86, P = 0.4). In further support of the role of AG in episodic retrieval, the network was found to be highly correlated with the vividness ratings. A whole-brain analysis showed a strong correlation with vividness within lateral and medial parietal cortices, as well as medial frontal areas (Fig. 2). This network shows close correspondence to the DMN. Therefore, AG activation appears closely related to vivid episodic retrieval rather than semantic retrieval.
The IPS was found to be positively activated relative to rest for all tasks (all ts > 5.44, P < 0.000) (Fig. 3). Indeed, activation closely mirrored task difficulty (Fig. 3), with significantly greater activation for the episodic task relative to all others (all ts > 2.70, P < 0.01), the picture task showing greater activation compared to the semantic task and control task (all ts > 4.20, all Ps < 0.001), and the control task showing the weakest activation compared to all tasks (ts > 2.19, all Ps < 0.001).
In order to investigate the relationship between task difficulty and activation across experimental items, we added RT as a parametric modulator to GLM analysis to examine the positive and negative correlations with trial difficulty in a whole-brain analysis. This revealed that a large network of dorsal parietal cortex, as well as lateral frontal, and posterior temporal areas were strongly positively correlated with task difficulty. In contrast, VPC, as well as the wider DMN in medial frontal and parietal cortices, was strongly negatively related to task difficulty, thus supporting the notion that this network is disengaged when a task becomes harder to perform (Fig. 2). Note that the exception to this rule is during episodic retrieval, which was the hardest task behaviorally but nevertheless most strongly engaged VPC. Indeed, when the same whole-brain correlation analysis was performed but only including the episodic retrieval trials, no correlation was found with task difficulty within the DMN (even at the very lenient threshold P < 0.05 uncorrected).
To summarize, the AG was actively engaged by the episodic retrieval task, and this positively correlated with the vividness of the memory. In contrast, the semantic task, picture-decision task, and control task all resulted in AG deactivation, and the level of deactivation was proportionate to task difficulty. The IPS showed the reverse response, with increasing activation for increased task difficulty.
Experiment 2: functional connectivity results
Four LPC functional networks were identified from the functional ICA analysis (Fig. 4). These included 1 dLPC component, which encompassed a frontoparietal executive control network with peaks in dorsal AG/IPS, left lateral frontal, pMTG, and posterior superior frontal gyrus (referred to as the executive network from here on). The remaining 3 components implicated vLPC, with an anterior–posterior organization. These included (i) a DMN component with a peak in mid-AG (PGp), precuneus (PCC), medial frontal, middle temporal gyrus (MTG); (ii) a component that clearly resembled the language network including the anterior AG (PGa), left IFG, and large section of the temporal lobe (STG and MTG) (Vigneau et al. 2006); and (iii) in posterior AG (posterior PGp), a visual-parietal network that involved visual cortex, SPL, and PGp.
DTI results
In order to examine the extent to which the functional connectivity networks reflect direct structural connections, the coordinates for the 4 peak LPC subregions (dorsal AG/IPS, mid-AG, anterior-AG, and posterior-AG) from the functional ICA were used as ROIs for the DTI analysis. Note that the mid-AG and dorsal AG/IPS ROIs overlap with the AG and IPS ROIs used in experiment 1, respectively.
Dorsal connectivity
Consistent with the role of dLPC in executive processing, the IPS/AG ROI showed long-range connectivity with lateral frontal lobe, specifically, dorsolateral frontal cortex (DLPFC) and IFG (BA44), which are known to be involved in top-down executive control (Fig. 4, Table 1).
Table 1.
Group-level connectivity matrix.
| Dorsal IPS |
Ventral | ||||
|---|---|---|---|---|---|
| Anterior | Middle | Posterior | |||
| Temporal lobe | mMTG | 92 | |||
| pSTG | 96 | ||||
| pMTG | 96 | ||||
| pITG | 58 | 54 | |||
| Hippocampus | 83 | 92 | |||
| Frontal lobe | DLPFC | 71 | 71 | ||
| BA44 | 50 | ||||
| Parietal lobe | S1 | 100 | 75 | 88 | 58 |
| 5Ci | |||||
| 5M | |||||
| 5L | 63 | ||||
| 7PC | 100 | 63 | 92 | 67 | |
| 7A | 96 | 96 | 79 | ||
| 7P | 83 | 71 | |||
| 7M | 54 | 75 | |||
| IPS1 | 100 | 75 | 92 | ||
| IPS2 | 100 | 67 | |||
| IPS3 | 100 | 96 | 63 | ||
| PFo | 63 | ||||
| PFt | 83 | ||||
| PF | 96 | 88 | |||
| PFm | 75 | 88 | |||
| PFcm | 96 | 88 | |||
| PGa | 67 | 92 | 88 | ||
| PGp | 63 | 100 | 83 | ||
| PCC | 88 | 100 | |||
| Occipital lobe | Superior OCC | 92 | 100 | ||
| Middle OCC | 79 | 67 | 100 | 100 | |
| Inferior OCC | 50 | 83 | |||
Bold font indicates that the connection probability was over 70% for the group analysis. The individual threshold was set at 2.5%.
mMTG: middle temporal gyrus (from the middle-slice); pSTG: posterior-superior temporal gyrus; pMTG: posterior-middle temporal gyrus; pITG: posterior-inferior temporal gyrus; DLPFC: dorsolateral prefrontal cortex; BA: Brodmann's area 44; S1: primary sensory cortex; 5C1, 5M, 5L, 7PC, 7A, 7P, 7M: superior parietal cortex; IPS: intraparietal sulcus; PFo, PFt, PF, PFcm, PFm: supramarginal gyrus; PGa, PGp: angular gyrus; PCC: precuneus; OCC: occipital cortex.
Anterior–posterior gradient within the ventral parietal lobes
Within the vLPC, there was a graded variation in connectivity between anterior and posterior parietal cortex control (Fig. 4, Table 1). The anterior vLPC showed connectivity with the temporal lobe areas implicated in language processing (Vigneau et al. 2006; Binder et al. 2009; Lambon Ralph et al. 2017). In contrast, moving in a posterior direction, in mid vLPC, the connectivity tended to become stronger with areas associated with the DMN and episodic memory, including the inferior temporal cortex and the hippocampus, as well as large portions of the PCC (Sestieri et al. 2011; Rugg and Vilberg 2013). Finally, the posterior vLPC connected with areas, including the medial parietal cortex and occipital lobe, associated with visual processing and spatial attention (Corbetta and Shulman 2002; Zacks 2008). Together, these results appear highly consistent with the functional networks identified in the functional ICA.
fMRI ROI analyses
A core assumption of the PUCC model is that differences in the underlying structural connectivity inputs across LPC give rise to variations in the emergent functional activation profile across the region. To test this assumption, we examined the functional activation within each of the 4 LPC ROIs in 2 independent fMRI datasets: (i) the sentence, picture, and number sequence task, reported previously (Humphreys et al. 2020a), and (ii) the fMRI data from experiment 1 involving the episodic, semantic, picture-description, and control task (see Fig. 4). For the first fMRI dataset, dorsal AG/IPS and mid-AG ROIs exhibited opposing directions of activation relative to fixation; activation for the dorsal AG/IPS, which is part of the executive network, was significantly greater than rest for all conditions (one-sample t-test, all ts > 3.49, Ps < 0.002). In contrast, the ventral mid-AG, which is part of the DMN/episodic memory network, showed significant negative activation for all conditions (one-sampled t-test, all ts > −3.68, Ps < 0.002). The anterior and posterior ventral AG showed a different pattern: the anterior ventral AG, which formed part of the language network, was only activated for the sentence task, showing significantly positive activation for the sentence conditions only (ts > 4.72, Ps < 0.001, d = 1.01), with the picture and number conditions showing no difference from zero (ts < 0.92, Ps > 0.37). In contrast, the posterior ventral AG, which formed part of the visual-parietal network was specifically engaged by the picture task only (ts > 6.35, Ps < 0.001, d = 1.42), with no modulation of the sentence and number tasks (ts < 2, Ps > 0.05).
For the second fMRI dataset from the current study (Fig. 4), like in the first, the dorsal AG/IPS was found to be positively activated by all tasks relative to rest (all ts > 8.50, P < 0.001). In contrast, whereas the sentence, picture, and number sequences deactivated the mid-AG in the first dataset, here mid-AG was positively activated for the episodic task (t(21) = 3.28, P = 0.005) (all other tasks showed no difference from zero (all ts < −1.55, Ps > 0.14). The fact that the mid-AG is positively engaged by the episodic retrieval task in isolation is consistent with what one would expect if it functions as part of the DMN/episodic retrieval network, since no other task required the retrieval of information from episodic memory. The posterior AG, which was positively engaged by picture sequences in the first fMRI dataset, similarly showed positive activation relative to rest for the picture-description task (t(21) = 4.70, P < 0.001) but no other (all ts < 1.38, Ps > 0.18), thereby supporting the role of this region as part of the visual-parietal network. Finally, whereas the anterior AG was engaged by the sentence sequence task in the first fMRI dataset, no modulation was found for any task in the second dataset which only included single-word items (all ts < 1.40, Ps > 0.18); this might thereby imply a greater role of this region in sentence/multi-item rather than single-word processing.
Discussion
Summary of main results
The results from experiment 1 showed that the dLPC and vLPC were positively engaged during episodic retrieval (but not in any other task), and this activation was found to correlate positively with the vividness of the episodic memory. The vLPC (AG) showed strong deactivation compared to rest during semantic memory retrieval (indeed the LPC was less engaged by the semantic task than the control task that involved little/no semantic processing). This provides compelling evidence against theories that posit a role in semantic processing, or in all forms of internally directed thought. This pattern contrasts sharply with the results from the ATL, which showed strong positive activation for the semantic, episodic, and the picture-description tasks, since all 3 tasks necessitate the retrieval of information from semantic, but no modulation for the control task (which had no semantic memory requirements). Indeed, if the AG served a primarily semantic function, one would predict a similar pattern of task-related activation to the ATL. In terms of task difficulty, with the exception of the episodic task, activation within the vLPC (AG), as part of the wider DMN, showed a strong negative correlation with reaction time thereby suggesting that this region is “turned-off” when a task becomes increasingly difficult. This is in contrast to the dLPC, as part of a wider multidemand frontoparietal network, which increased activation in relation to task difficulty. Critically, the episodic retrieval task was the only task to activate both the dLPC and vLPC regions concurrently. These data were complemented by the results from experiment 2, which showed that in terms of functional and structural connectivity, dLPC forms part of a frontoparietal network and was positively engaged by all fMRI tasks. The vLPC showed an anterior–posterior variation in connectivity. Specifically, the mid vLPC region (corresponding to the mid-PGp subregion of AG associated with the DMN) connected with areas associated with episodic memory retrieval (hippocampus and PCC) and was only actively engaged during the episodic retrieval task consistent with its role as part of the episodic retrieval network (Sestieri et al. 2011; Rugg and Vilberg 2013). In contrast, the anterior vLPC (PGa) showed functional and structural connectivity with temporal lobe language processing areas (Vigneau et al. 2006; Binder et al. 2009; Lambon Ralph et al. 2017) and was found to respond only to a sentence processing task, consistent with existing evidence from sentence processing studies (Humphreys and Lambon Ralph 2015; Branzi et al. 2020; Humphreys, Jackson, et al. 2020a). Whereas, the posterior vLPC (posterior PGp) connected with the occipital lobe and medial parietal areas associated with visual attention (Corbetta and Shulman 2002; Zacks 2008) and was only actively engaged by the picture-sequence and picture-description tasks. Together these results fit with the PUCC model that suggests a shift in the functional engagement of vLPC based on variations in the underlying structural connectivity of the network (see Fig. 4 for a schematic model).
Implications for semantic theories of LPC function
The current data provide clear evidence that the AG is not engaged during semantic retrieval. How can this result be aligned with evidence showing AG apparent sensitivity to semantic contrasts (Binder et al. 2009)? One key explanation is based on the type of contrast being performed: typical contrasts being words > nonword, or concrete > abstract. A meta-analysis of semantic versus nonsemantic studies that used other forms of contrast found no evidence of AG engagement despite engagement of the wider frontotemporal semantic network (Visser et al. 2010), and another study found stronger AG engagement during nonverbal tongue movements compared to meaningful speech (Geranmayeh et al. 2012). As highlighted in Section 1, when interpreting these data, it is important to take into account 2 variables: (i) the polarity of activation relative to rest and (ii) difficulty-related differences.
With regard the first point, the semantic retrieval task showed deactivation relative to rest. It is of course difficult to interpret “rest,” as it could involve spontaneous semantic and linguistic processing (Binder et al. 1999) (although one could also make the argument that the same is also true for episodic retrieval (Buckner et al. 2008; Andrews-Hanna 2012) yet episodic memory tasks positively engage the AG, as demonstrated here and elsewhere (Humphreys and Lambon Ralph 2015)). Importantly, while AG deactivates for semantic and nonsemantic tasks, other key semantic areas do not show the same pattern as AG; for instance, the ATL is positively engaged during semantic tasks but deactivated by nonsemantic tasks (Humphreys et al. 2015; Humphreys and Lambon Ralph 2017).
With regard to the second point, AG deactivation is known to relate to task difficulty (Hahn et al. 2007; Mason et al. 2007; Humphreys and Lambon Ralph 2017), with increasing deactivation for harder tasks. Indeed, the contrast of word > nonword and concrete > abstract typically involves comparing an easier task versus a harder task. Compellingly, the same word > nonword and concrete > abstract contrasts can be inverted by reversing the difficulty of the task/stimuli (Pexman et al. 2007; Graves et al. 2017). Indeed, when task difficulty is directly manipulated in a semantic and visuospatial tasks, one observes a main effect of task difficulty (easy vs. hard) in the AG but no semantic versus nonsemantic difference, whereas the IPS shows the reverse pattern of difficulty-related activation (hard vs. easy) (Humphreys and Lambon Ralph 2017).
Implications for episodic memory retrieval
The episodic retrieval task showed positive activation in both the AG and IPS despite being the most difficult task, making it the exception to the difficulty-related activation pattern. The influence of connectivity into various IPC areas, embraced in the PUCC framework (see below), can explain these episodic findings in the ventral and dorsal parietal cortices. Specifically, the mid-PGp region of the AG connects with the hippocampus and PCC, operating as part of a wider episodic retrieval network. Based on evidence that the patients with AG damage are not profoundly amnesic, unlike those with damage to the medial temporal lobe (Berryhill et al. 2007; Simons et al. 2008; Humphreys, Lambon Ralph, et al. 2020b), we propose that episodic information stored elsewhere in the system is temporally buffered online during episodic retrieval. Indeed the notion of an AG episodic buffer has been proposed elsewhere (Wagner et al. 2005; Vilberg and Rugg 2008). This notion is consistent with observation that patients with parietal damage lack clarity or vividness of episodic memories, as one might predict from a deficit in buffering multimodal contextual information (Davidson et al. 2008; Shimamura 2011; Yazar et al. 2014; Moscovitch et al. 2016; Bonnici et al. 2018; St. Jacques 2019), as well as fMRI studies showing that the level of AG activation varies depending on the extent to which information is retrieved from episodic/autobiographical memory compared to semantic memory (Brown et al. 2018). In addition, due to its connectivity with DLPFC executive systems, the IPS takes on a domain-general ability for selection/manipulation of internally buffered information—which is required in many episodic and semantic tasks. Indeed, previous studies of episodic retrieval have also suggested that the IPS plays an executive role in decision-making during episodic tasks (Gonzalez et al. 2015; Sestieri et al. 2017). Overall, we propose that episodic retrieval is an active construction process where the memory is reconstructed online thus needs both buffering of information from episodic-related areas and executively demanding shaping, thereby recruiting both dLPC and vLPC systems.
A unifying account of LPC functions
By exploring both the variation in functional response and connectivity profiles across the LPC, the current study found evidence in favor of the neurocomputational principles embraced by the PUCC model (Humphreys and Lambon Ralph 2015; Humphreys, Jackson, et al. 2020a; Humphreys, Lambon Ralph, et al. 2020b). According to the PUCC model, the LPC does not support long-term stored information per se but rather is an online temporary buffer of multimodal spatiotemporal input. Indeed, this hypothesis appears consistent with other proposals that AG acts a temporary buffer (Wagner et al. 2005; Vilberg and Rugg 2008), or a “schematic-convergence zone” which binds information, if we assume that this binding is temporary (Shimamura 2011; Wagner et al. 2015). An online buffer would seem to be a necessary neurocomputation for the construction of internal models of the world, reconstruction of autobiographical memories, or the envisioning of possible future events, and, perhaps, for the ongoing buffering of combinatorial meaning generated over a time-extended period (Hasson et al. 2008; Lerner et al. 2011; Ramanan and Bellana 2019). We would predict that if the LPC was operating as a “buffering-system” then the content of the system would align with what participants buffer at each temporal interval. Indeed, consistent results have been found from episodic memory literature using MVPA (Wagner et al. 2015; Lee and Kuhl 2016), whereby the episodic content of a person’s current recall (in this case the visual features of a face) directly align with decoding in the AG (Lee and Kuhl 2016).
A key notion of PUCC is that the expressed functions of a cortical region will reflect the combination of the local computation and its pattern of connectivity. This tenet has been formally demonstrated by computational models, whereby the resultant “behavior” of a processing unit depends not only on its local computations but also on its long-range connectivity (more recently referred to as “connectivity-constrained cognition – C3”: Lambon Ralph et al. 2001; Plaut 2002; Chen et al. 2017). How this might apply to the LPC is sketched out in Fig. 5. In its most simple form (left panel), the LPC might have a single local computation—an online multimodal buffer of internal and external sequentially experienced information. Computational models with recurrent connections (shown as a generic “Elman” network in Fig. 4A) show this general property and the same type of model can be applied to any time-extended series of inputs and outputs (Elman 1990; Botvinick and Plaut 2006). Accordingly, across a larger region such as the LPC, even if the local buffering computation itself was the same, the emergent observed cognitive function will depend on what sources of information and influences arrive at each subregion, i.e. the differential connectivity pattern. Figure 5 (right panel) shows a schematic of the varying pattern of connectivity to LPC subregions. We can consider the influence of this connectivity profile in 2 steps. First, the long-range connectivity from executively related DLPFC primarily terminates in the IPS/dLPC region (Crowe et al. 2013) and not the VPC. This should generate a fundamental, counterpointed difference in the emergent observed functions: In receiving top-down signals from frontal executive processing areas, dLPC (IPS) will operate as a domain-general executive system engaged in the selection/manipulation processes on internally buffered information (Fedorenko et al. 2013; Humphreys and Lambon Ralph 2015, 2017). The IPS region itself connects to all subregions of the VPC and thus can become involved in any particular activity irrespective of information type (c.f., the DLPFC and IPS are 2 key components of the “multidemand” system: Fedorenko et al. 2013; Assem et al. 2020). In contrast to the IPS, most of the VPC does not seem to receive this same level of DLPFC connectivity and thus its underlying buffering will not be so executively penetrated, i.e. its buffering will be more “automatic” (e.g. a distinction envisaged in the differentiation between the central executive and “slave” subsystems in classical models of short-term working memory: (Broadbent 1982; Baddeley 2000, 2003). More generally, the differential connectivity of DLPFC to IPS and not VPC might also help to explain why in many situations dLPC and vLPC show anticorrelated functional activity (Fox et al. 2009; Chai et al. 2012; Keller et al. 2013; Humphreys and Lambon Ralph 2017), whereby the dLPC is increasingly activated and the vLPC increasingly deactivated based on task difficulty (Humphreys and Lambon Ralph 2017). When engaged in a goal-oriented task, on-going automatic information accumulation in VPC subregions would presumably be unhelpful/disruptive unless this input is necessary for task performance. In this case, activation in the irrelevant VPC subregions would be suppressed/deactivated (Humphreys, Jackson, et al. 2020a). This would be especially needed with increasing demands on task performance, thereby explaining the common observed pattern of anticorrelated activation modulated by task difficulty (Humphreys and Lambon Ralph 2017).
Fig. 5.
A schematic illustration of the assumptions of the PUCC model: (i) The LPC acts as a multimodal buffer in terms of its basic underlying neurocomputation (left panel) and then (ii) the varying long-range connectivity to LPC influences the emergent function of each subregion.
Different functions across the VPC itself should emerge, given that there are differences in connectivity of VPC subregions to distinct neural networks (Figs 4 and 5) (Nelson et al. 2012; Daselaar et al. 2013; Humphreys and Lambon Ralph 2015; Humphreys, Jackson, et al. 2020a). Specifically, the more anterior VPC through its primary connections to pSTG and pMTG becomes involved in sound, phonological, and language processing (Griffiths and Warren 2002; Humphreys and Lambon Ralph 2015). The most posterior VPC subregion is most heavily influenced by the dorsal connectivity from visual regions, consistent with its involvement in visuospatial processing (Corbetta and Shulman 2002; Zacks 2008; Humphreys and Lambon Ralph 2015, 2017; Humphreys, Jackson, et al. 2020a), while the mid-AG region’s involvement in episodic tasks seems entirely consistent with its connectivity through to key nodes of the extended episodic network (Sestieri et al. 2011; Rugg and Vilberg 2013).
To conclude, we propose a unified model of LPC function in LPC acts as multimodal buffer of information. Despite a common core mechanism, graded subdivisions in how this function is expressed can arise based on varying long-range connectivity inputs to dorsal–ventral and anterior–posterior areas.
Funding
This research was supported by an MRC Programme grant to MALR (MR/R023883/1) and an intramural award (MC_UU_00005/18).
Conflict of interest statement. The authors declare no conflict of interest.
Contributor Information
Gina F Humphreys, MRC Cognition & Brain Sciences Unit, University of Cambridge, 15 Chaucer Road, Cambridge CB2 7EF, United Kingdom.
JeYoung Jung, School of Psychology, University of Nottingham, Nottingham NG9 2RD, United Kingdom.
Matthew A Lambon Ralph, MRC Cognition & Brain Sciences Unit, University of Cambridge, 15 Chaucer Road, Cambridge CB2 7EF, United Kingdom.
References
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012:18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007:38:95–113. [DOI] [PubMed] [Google Scholar]
- Assem M, Glasser MF, Van Essen DC, Duncan J. A domain-general cognitive core defined in multimodally parcellated human cortex. Cereb Cortex. 2020:30:4361–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000:4:417–423. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003:4:829–839. [DOI] [PubMed] [Google Scholar]
- Bajada CJ, Haroon HA, Azadbakht H, Parker GJ, Lambon Ralph MA, Cloutman LL. The tract terminations in the temporal lobe: their location and associated functions. Cortex. 2016:155:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada CJ, Jackson RL, Haroon HA, Azadbakht H, Parker GJM, Lambon Ralph MA, Cloutman LL. A graded tractographic parcellation of the temporal lobe. NeuroImage. 2017:155:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007:27:14415–14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999:11:80–93. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009:19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Parker GJ, Lambon Ralph MA. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion-weighted imaging probabilistic tractography. J Cogn Neurosci. 2012:24:1998–2014. [DOI] [PubMed] [Google Scholar]
- Bonnici HM, Cheke LG, Green DAE, FitzGerald THMB, Simons JS. Specifying a causal role for angular gyrus in autobiographical memory. J Neurosci. 2018:38:10438–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Plaut DC. Doing without schema hierarchies: a recurrent connectionist approach to normal and impaired routine sequential action. Psychol Rev. 2004:111:395–429. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Plaut DC. Short-term memory for serial order: a recurrent neural network model. Psychol Rev. 2006:113:201–233. [DOI] [PubMed] [Google Scholar]
- Branzi FM, Humphreys GF, Hoffman P, Lambon Ralph MA. Revealing the neural networks that extract conceptual gestalts from continuously evolving or changing semantic contexts. NeuroImage. 2020:220:116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE. Task combination and selective intake of information. Acta Psychol. 1982:50:253–290. [DOI] [PubMed] [Google Scholar]
- Brown TI, Rissman J, Chow TE, Uncapher MR, Wagner AD. Differential medial temporal lobe and parietal cortical contributions to real-world autobiographical episodic and autobiographical semantic memory. Sci Rep. 2018:8:6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network - anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008:1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012:16:338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001:14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008:212:481–495. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, Kapri A, Kuhlen T, Huang R, Shah NJ, Zilles K. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. NeuroImage. 2011:58:362–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XQJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012:59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lambon Ralph MA, Rogers TT. A unified model of human semantic knowledge and its disorders. Nat Hum Behav. 2017:1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. NeuroImage. 2012:59:3514–3521. [DOI] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Morris DM, Parker GJM, Lambon Ralph MA. Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain. Brain Lang. 2013:127:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002:3:201–215. [DOI] [PubMed] [Google Scholar]
- Crowe DA, Goodwin SJ, Blackman RK, Sakellaridi S, Sponheim SR, MacDonald AW, Chafee MV. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013:16:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989:33:25–62. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Huijbers W, Eklund K, Moscovitch M, Cabeza R. Resting-state functional connectivity of ventral parietal regions associated with attention reorienting and episodic recollection. Front Hum Neurosci. 2013. 10.3389/fnhum.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008:46:1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003:20:487–506. [DOI] [PubMed] [Google Scholar]
- Elman JL. Finding structure in time. Cogn Sci. 1990:14:179–211. [Google Scholar]
- Embleton KV, Haroon HA, Morris DM, Ralph MA, Parker GJ. Distortion correction for diffusion-weighted MRI tractography and fMRI in the temporal lobes. Hum Brain Mapp. 2010:31:1570–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013:110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang DY, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009:101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SLE, Leech R, Beckmann CF, Woodhead Z, Wise RJS. The contribution of the inferior parietal cortex to spoken language production. Brain Lang. 2012:121:47–57. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965:88:237–294. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Language and brain. Sci Am. 1972:226:76. [DOI] [PubMed] [Google Scholar]
- Gilbert S, Bird G, Frith C, Burgess P. Does “task difficulty” explain “task-induced deactivation?”. Front Psychol. 2012. 10.3389/fpsyg.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AJ, Hutchinson JB, Uncapher MR, Chen J, LaRocque KF, Foster BL, Rangarajan V, Parvizi J, Wagner AD. Parietal dynamics during recognition memory. Proc Natl Acad Sci U S A. 2015:112:11066–11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Boukrina O, Mattheiss SR, Alexander EJ, Baillet S. Reversing the standard neural signature of the word-nonword distinction. J Cogn Neurosci. 2017:29:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002:25:348–353. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007:17:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Welbourne SR, Embleton K, Parkes LM. A comparison of dual gradient-echo and spin-echo fMRI of the inferior temporal lobe. Hum Brain Mapp. 2014:35:4118–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon HA, Morris DM, Embleton KV, Alexander DC, Parker GJ. Using the model-based residual bootstrap to quantify uncertainty in fiber orientations from Q-ball analysis. IEEE Trans Med Imaging. 2009:28:535–550. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Contreras-Rodríguez O, Soriano-Mas C, López-Solà M, Deus J, Ortiz H, Blanco-Hinojo L, Alonso P, Hernández-Ribas R, et al. Task-induced deactivation from rest extends beyond the default mode brain network. PLoS One. 2011:6:e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008:28:2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex. 2015:25:3547–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cereb Cortex. 2017:27:4199–4212. [DOI] [PubMed] [Google Scholar]
- Humphreys GF, Hoffman P, Visser M, Binney RJ, Ralph MAL. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc Natl Acad Sci U S A. 2015:112:7857–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Jackson RL, Lambon Ralph MA. Overarching principles and dimensions of the functional organization in the inferior parietal cortex. Cereb Cortex. 2020a:30:5639–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA, Simons JS. A unifying account of angular gyrus contributions to episodic and semantic cognition. PsyArXiv. 2020b:26. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Jones DK, Tournier JD, Sijbers J. Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum Brain Mapp. 2011:32:461–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Cloutman LL, Binney RJ, Lambon Ralph MA. The structural connectivity of higher order association cortices reflects human functional brain networks. Cortex. 2017:97:221–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Visser M, Binney RJ, Lambon Ralph MA. Establishing the cognitive signature of human brain networks derived from structural and functional connectivity. Brain Struct Funct. 2018:223:4023–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci. 2013:33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuro-psychological evidence and a computational model. J Cogn Neurosci. 2001:13:341–356. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017:18:42–55. [DOI] [PubMed] [Google Scholar]
- Lee H, Kuhl BA. Reconstructing perceived and retrieved faces from activity patterns in lateral parietal cortex. J Neurosci. 2016:36:6069–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Hacker CD, Snyder AZ, Corbetta M, Zhang D, Leuthardt EC, Shimony JS. Clustering of resting state networks. PLoS One. 2012:7:e40370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci. 2011:31:2906–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O'Reilly JX, Croxson PL, Olivier E, Noonan MP, Bergmann C, Mitchell AS, Baxter MG, et al. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011:31:4087–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007:315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, St. John M, Taraban R.. Sentence comprehension: a parallel distributed processing approach. Lang Cogn Process. 1989:4:287–335. [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu Rev Psychol. 2016:67:105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Petersen SE. In favor of a 'fractionation' view of ventral parietal cortex: comment on Cabeza et al. Trends Cogn Sci. 2012:16:399–400author reply 400-391. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:9:97–113. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Haroon HA, Wheeler-Kingshott CA. A framework for a streamline-based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. J Magn Reson Imaging. 2003:18:242–254. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini PA, Ungerleider LG. Neural correlates of visual working memory. Neuron. 2002:35:975–987. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG. Neural correlates of concreteness in semantic categorization. J Cogn Neurosci. 2007:19:1407–1419. [DOI] [PubMed] [Google Scholar]
- Plaut DC. Graded modality-specific specialization in semantics: a computational account of optic aphasia. Cogn Neuropsychol. 2002:19:603–639. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013:23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011:72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001:98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Bellana B. A domain-general role for the angular gyrus in retrieving internal representations of the external world. J Neurosci. 2019:39:2978–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L, Irish M, Moscovitch M, Rugg MD. From knowing to remembering: the semantic–episodic distinction. Trends Cogn Sci. 2019:23:1041–1057. [DOI] [PubMed] [Google Scholar]
- Rice GE, Hoffman P, Lambon Ralph MA. Graded specialization within and between the anterior temporal lobes. Ann N Y Acad Sci. 2015a:1359:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Lambon Ralph MA, Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: an ALE meta-analysis of 97 functional neuroimaging studies. Cereb Cortex. 2015b:25:4374–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, King DR. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. 2018:107:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013:23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011:31:4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. The contribution of the human posterior parietal cortex to episodic memory. Nat Rev Neurosci. 2017:18:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011:11:277–291. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008:46:1185–1191. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010:53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL. A new perspective on visual perspective in memory. Curr Dir Psychol Sci. 2019: 0963721419850158. [Google Scholar]
- Tournier JD, Yeh CH, Calamante F, Cho KH, Connelly A, Lin CP. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. NeuroImage. 2008:42:617–625. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010:20:2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011:72:385–396. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006:30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008:46:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008:100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010:22:1083–1094. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005:9:445–453. [DOI] [PubMed] [Google Scholar]
- Wagner IC, Buuren M, Kroes MCW, Gutteling TP, Linden M, Morris RG, Fernandes G. Schematic memory components converge within angular gyrus during retrieval. elife. 2015:4:e09668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn Sci. 2003:7:483–488. [DOI] [PubMed] [Google Scholar]
- Yazar Y, Bergstrom ZM, Simons JS. Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One. 2014:9:e110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. NeuroImage. 2013:88C:212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. J Cogn Neurosci. 2008:20:1–19. [DOI] [PubMed] [Google Scholar]