Abstract
Commercially available health technologies such as smartphones and smartwatches, activity trackers and eHealth applications, commonly referred to as wearables, are increasingly available and used both in the leisure and healthcare sector for pulse and fitness/activity tracking. The aim of the Position Paper is to identify specific barriers and knowledge gaps for the use of wearables, in particular for heart rate (HR) and activity tracking, in clinical cardiovascular healthcare to support their implementation into clinical care. The widespread use of HR and fitness tracking technologies provides unparalleled opportunities for capturing physiological information from large populations in the community, which has previously only been available in patient populations in the setting of healthcare provision. The availability of low-cost and high-volume physiological data from the community also provides unique challenges. While the number of patients meeting healthcare providers with data from wearables is rapidly growing, there are at present no clinical guidelines on how and when to use data from wearables in primary and secondary prevention. Technical aspects of HR tracking especially during activity need to be further validated. How to analyse, translate, and interpret large datasets of information into clinically applicable recommendations needs further consideration. While the current users of wearable technologies tend to be young, healthy and in the higher sociodemographic strata, wearables could potentially have a greater utility in the elderly and higher-risk population. Wearables may also provide a benefit through increased health awareness, democratization of health data and patient engagement. Use of continuous monitoring may provide opportunities for detection of risk factors and disease development earlier in the causal pathway, which may provide novel applications in both prevention and clinical research. However, wearables may also have potential adverse consequences due to unintended modification of behaviour, uncertain use and interpretation of large physiological data, a possible increase in social inequality due to differential access and technological literacy, challenges with regulatory bodies and privacy issues. In the present position paper, current applications as well as specific barriers and gaps in knowledge are identified and discussed in order to support the implementation of wearable technologies from gadget-ology into clinical cardiology.
Keywords: Wearables, Digital health, Innovation, Prevention, Cardiovascular, Telemonitoring
Introduction
The last decade has seen a rapid increase in commercially available health technology such as smartphones and smartwatches, activity trackers and eHealth applications, commonly referred to as wearables. The worldwide wearable device sales is expected to reach 520 million units by 2025.1 Additionally, use of technologies capable of collecting physiological data may become even greater with widespread utilization of build-in smartphone sensors such as accelerometers, gyroscopes, video camera, microphones, skin conductance, as well as of other wearable technology.2 These sensors have the capability of providing readily accessible physiological information at a population level, which was previously available only in patient populations in the setting of provision of healthcare. At present, heart rate (HR) monitoring and activity tracking are the two most prevalent physiological measurements generally available. Both HR and measures of physical fitness are known to be robustly related to cardiovascular disease and longevity.3,4 There is a long-standing tradition for remote monitoring in cardiology spanning from ambulatory HR monitoring to implantable devices such as pacemakers and implantable loop recorders.5 Physicians are increasingly implementing wearables in their clinical practice.6 However, how to use and understand the data collected from commercially available wearables for primary and secondary cardiovascular prevention is currently unclear, with no guidelines or recommendations in this area.
The widespread availability of low-cost and high-volume physiological data from the community provides both unique opportunities and challenges. These issues need to be addressed in order to translate this data into meaningful clinical information on a user, provider, and healthcare system level.
Aims and scope
Aims
The aim of the present Position Paper is to identify specific barriers and knowledge gaps for the use of wearables, in particular for HR and activity tracking, in clinical cardiovascular healthcare to support their implementation into clinical care.
Scope
The scope of the present Position Paper, is focused on, but not limited to, use of wearables in primary and secondary prevention. In the current context, primary prevention is defined as prevention or delay of developing cardiovascular risk factors in healthy populations. Secondary prevention is defined as early cardiovascular disease detection and treatment in populations with known cardiovascular risk factors.7
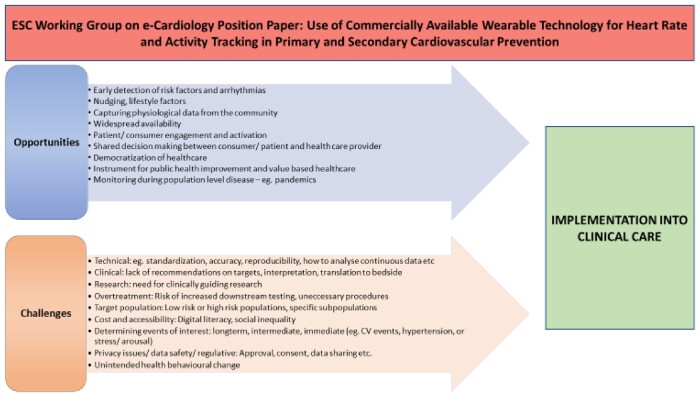
As the area of wearables is increasing exponentially in these years, the present Position Paper aims to provide a framework to constructively move the field forward from consumer products to clinical utility on an individual, healthcare provider, and healthcare system level (Figure 1).
Figure 1.
Overview of opportunities and challenges in the use of commercially available wearables for the implementation into clinical care.
Section 1: Technical aspects
Generally, current consumer devices provide HR estimates and heart rhythm information from one-lead electrocardiogram (ECG) or from the photoplethysmogram (PPG). An ECG can be obtained, for example, by chest straps wirelessly connected to a smartphone or smartwatch, or by a finger contact with a smartwatch crown. Using PPG, the sensor can be integrated into the smartphone, a wrist bracelet, an armband, or a smartwatch and the HR is estimated from the analysis of the pressure pulse detected by measuring changes in the LED light absorbed by the blood flowing into an artery.8 Other methods are currently under development to estimate HR from precordial vibrations measured with miniaturized accelerometers.9
In addition to single HR estimates, an increasing number of wearables enable continuous measurement of HR10 and thereby quantification of more advanced metrics such as HR variability (HRV) indices.11
It is challenging to assess the accuracy of HR measurement by consumer devices as published studies present data of different subsets of devices tested through different protocols, applied in different populations, where the accuracy varies based on the subjects’ activity and the prevalence of arrhythmias. Furthermore, the reported accuracy depends on which gold-standard was used: for example, in some studies benchmarking was performed using consumer-grade ECG chest straps rather than clinical-grade ECG equipment, producing discordant results.12,13 There is a need for standardized protocols and measures for a robust appraisal of the accuracy of these consumer systems as well as for the definition of their operational limitations.
The following general observations can be drawn:
accuracy decreases significantly with increasing activity level, and15,16
during exercise, PPG from smartwatches tends to be more sensitive to motion artefacts than ECG from chest straps.17
Only few consumer-grade systems have received FDA clearance or CE mark as personal ECG monitors and irregular rhythm detectors (both from ECG or PPG), but with specific operational constraints and their ability to reliably identify atrial fibrillation (AF) is under evaluation.18,19
In addition, smartphone applications (apps) are also commonly used for HR/rhythm assessment. These apps can measure HR by turning the smartphone into a PPG detector.20 Although some recent phones have a dedicated PPG sensor, in most cases the phone LED is exploited to illuminate the finger (to be positioned on the rear part of the phone), and the phone camera is used as PPG light-receptor.21 The performance of HR measurement from a conventional ECG, a finger pulse oximeter and four PPG based smartphone applications have been compared.22 It has been shown that HR estimates from ECG are well correlated with those from pulse oximetry, and from apps based on a PPG finger-contact measure. An additional smartphone-based method relies on a non-contact PPG assessment (a video is made of the subject’s face by the smartphone camera and PPG is derived from the changes of the red-colour band of the image over time).23 Performances of this technique are found to be significantly lower than those obtained by the contact PPG.21,24,25
Several consumer devices provide quantification of physical activity and posture obtained by the so-called IMUs (Inertial Measurement Unit), i.e. electronic chips including a 3D accelerometer, a 3D gyroscope, and sometimes a magnetometer. While the hardware technology embedded in such devices is mature, the algorithms used to analyse the data are still in their infancy (i.e. distance measurements accuracy depending on speed). Hence, the raw data obtained by the sensors are reliable, but how this information is processed for quantification of a subject's activity and clinical utility needs more research.26
Gaps in knowledge
Standardization of gold-standard to be used in validation protocols; for validation of HR-related measures, we recommend the use of clinical ECG equipment; for validation of activity measures, we recommend the use of video camera recordings.
Exact definition of range of measures and conditions in which the accuracy has been tested should be defined (i.e. posture-dependent, range of HR, range of walking speed, subject population, and for PPG skin colour, external light conditions, contact pressure).
The variability (i.e. test–retest reliability), bias and limits of agreement of the measurements should be reported.
Section 2: Heart rate and activity tracking for primary and secondary prevention
Resting heart rate
In individuals from the background population without known cardiovascular disease, elevated resting heart rate (RHR) has been shown to be associated with higher blood pressure, higher body mass index, impaired pulmonary function, lower levels of physical activity and with increased subclinical chronic inflammation.27–29 Although RHR is closely related to VO2max, its association with mortality is not explained by poor fitness alone.30 There is consistent epidemiological evidence of a significant independent relationship between elevated RHR and increased risk of cardiovascular events and mortality in general populations.29–34 While the majority of epidemiological research is based on single measurements of HR, few studies have investigated the association between temporal changes in HR and risk, which could be of greater relevance to wearable technologies.34,35 As a result, an increase in HR over time appears to be an indicator of deterioration of health.36 Increased HR at rest has also been found to be associated with adverse events in patient populations such as heart failure,37 chronic obstructive pulmonary disease,38 diabetes,39 and rheumatoid arthritis.40 Despite the well-established association between elevated HR, cardiovascular risk factors, and risk of cardiovascular disease, there are currently no general recommendations to guide the general public or healthcare providers in this area but also no trials in the general population to show that interventions directed at elevated HR has an effect on clinical outcomes.31
Heart rate variability
Beat-by-beat oscillations in RR interval (HRV) reflect the neural regulation on the cardiovascular system, providing a simple, non-invasive means to explore the complex and dynamic balance between sympathetic and parasympathetic cardiac neural influences in health and disease.11,41 Low HRV is associated with a number of cardiovascular risk factors, such as diabetes and hypertension, and has been shown to be associated with a 32–45% increase in the risk of development of a cardiovascular event in populations without known prevalent cardiovascular disease.42
The availability of wearable tools to measure HRV (and possibly also by coupling with blood pressure variability)41 opens new possibilities in risk prediction in secondary prevention. In particular, HRV and baroreflex sensitivity analysis may allow better characterization of cardiovascular neural modulation during sleep in normal and pathological conditions such as sleep apnoea or serve as a prognostic tool in patients with established CV diseases. For example, low HRV has been shown to be independently predictive of increased mortality in post-myocardial infarction patients and heart failure patients.43,44 However, HRV analysis in clinical practice has never reached a wide utilization due to its limitations in acquisition protocols detecting specific diseases (i.e. a lower HRV could be associated to different causes, as well as unbalanced neural influences).
Assessment of daily exercise behaviour
Improvement of physical activity behaviour is an important treatment target in cardiovascular prevention. Numerous physical activity devices are currently commercially available, but their accuracy, however, is differing considerably during walking at normal speed. Moreover, accurate assessment of physical activity at lower speeds than usual walking was shown to be even more challenging.12 A recent systematic review of consumer-wearable activity trackers indicated a lower validity for assessment of energy expenditure as compared to step counts.45 Focusing on the cardiac patient population, recent findings also demonstrated a low accuracy and sensitivity for estimating changes in energy expenditure of modern activity trackers.46,47 This illustrates the need for elaboration and definition of population-specific exercise measurement algorithms. In this regard, it has been shown that the combination of HR and accelerometric data enhances device performance on energy expenditure estimation.48
Gaps in knowledge
Clinical utility of HR and fitness tracking for monitoring or as a target for intervention need to be determined.
Recommendations on healthy levels of HR at rest and during continuous activity are needed, as well as recommendations for when and how to intervene or refer to specialist care.
Methods or algorithms for translating data from continuous fitness or HR tracking into clinically meaningful information that can be used for primary and secondary prevention are needed.
Research on how to interpret data from continuous HR and fitness tracking is needed.
Section 3: Who will benefit from wearable technology?
Wearables, properly selected and adopted, might be useful for both high- and low-risk individuals in allowing the identification of subjects needing further investigation.
The large and easy availability at population level make wearables the ideal technology for identification of early disease or monitoring of existing disease. For example, the use of wearables to objectively monitor physical activity can be of use in primary prevention, as it is well recognized that physical activity is inversely related to cardiovascular risk.49 In addition, physical activity plays a dual role for patients who have experienced a cardiovascular event, both as part of cardiac rehabilitation, but also as a tool to monitor treatment effects. Physical activity is a dynamic parameter, and the use of wearables in heart failure populations have shown a correlation between decline in physical activity and cognitive decline50 showing the potential of wearable technologies to monitor disease states and indicate the need for intensified medical attention.
The use of wearables as telemonitoring to reduce patient contacts may be beneficial for frail, immobile patients or in times of a pandemic.5 Dedicated patient populations can use wearable devices for monitoring of disease-specific parameters, e.g. activity in heart failure patients.43
Most currently available wearable tools are not ready to be considered medical devices,46,51 instead they offer a daily life approach to monitor well-being, such as physical activity in leisure-time or indicating the presence of irregular heartbeats. This can be done over relatively long time periods in a non-invasive manner, a possibility not easily allowed by conventional methods.
Previously undetected arrhythmias
Atrial fibrillation
In the large consumer-driven studies of wearables for detection of AF, younger individuals dominate the study population, reflecting current ownership and adoption of wearable technology.19,52 In contrast, AF prevalence and associated risks are mainly driven by increasing age.53 The performance of such wearable devices will depend on the prevalence of AF in the population that is studied. Younger participants (<40 years) also experience a larger number of false-positive alerts compared to the elderly,19 which may unnecessarily increase healthcare costs. In clinical studies focusing on high-risk individuals, much more AF has been detected,54 enabling stroke protective therapy and suggesting improved cost-effectiveness.55 For wearables to have an impact on health in the population, the wearers of the devices need to be at risk of an adverse outcome and likely to benefit from preventative therapy. The currently recruiting Heartline study (clinicaltrials.gov NCT04276441) aims to enroll 150 000 participants to evaluate if early AF diagnosis reduces the risk of thromboembolic events in a real-world setting.
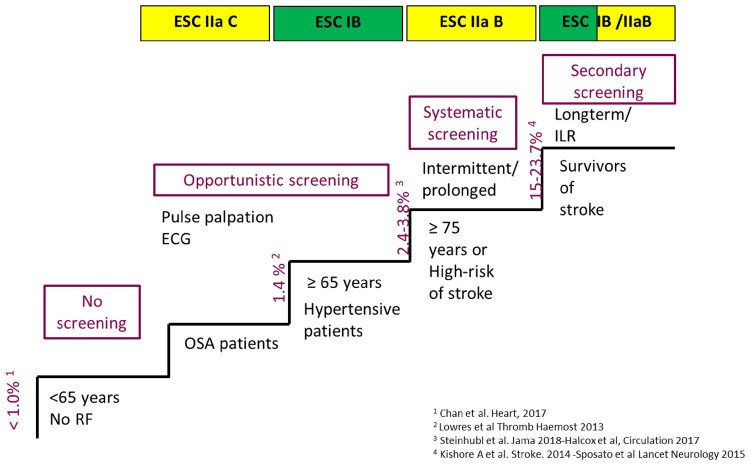
With regard to AF, risk factors for ischaemic stroke, such as age or cardiovascular co-morbidities included in the CHA2DS2-VASc score, are generally those that are also associated with increased incidence of AF.56 One would therefore expect that the use of wearables in the population, which is at an a priori greater risk for AF and its thromboembolic complications, would be associated with greater diagnostic yield and impact on risk management than indiscriminatory use of the technology in the population dominated by young and healthy (Figure 2).
Figure 2.
Recommendations for screening of atrial fibrillation.
Management of known arrhythmias
Wearable technologies have been proven useful, sometimes even beyond their indications for use as a medical device, for monitoring the effects of therapeutic interventions and documenting rhythm disorders underlying typical or atypical symptoms perceived to be caused by arrhythmias.57 A recent study showed that Apple Watch ECG tracings allowed adequate QT-interval measurements58 and thereby facilitated remote QT monitoring in quarantined outpatients receiving QT-prolonging treatments. However, it should be considered that in de novo classification request to FDA for the ECG app it is stated that ‘The clinical study did not quantitatively assess the quality of the ECG waveform produced by the ECG app. The ECG produced by the ECG app is not intended for clinical use or as the basis for diagnosis or treatment. The ECG waveform is only intended for informational use’. In the context of AF management, documentation of cardiac rhythm is pivotal for decision regarding the need for re-ablation procedures or self-administration of rhythm-control drugs in situations when pill-in-the-pocket strategy is employed. Nearly two thirds of patients with symptoms suggestive of AF do not have the arrhythmia, as shown in the studies using implantable loop recorders.59 Wearables can provide a comprehensive AF management enhancing teleconsultation during and after a pandemic, like recently shown in the Telecheck-AF project.60
Gaps in knowledge
Clinically relevant populations who would particularly benefit from use of wearables devices for HR and fitness monitoring should be defined.
Barriers, such as cost or technology literacy, should be identified and addressed in order to facilitate the use of wearables in at-risk populations.
While the wearable device ideally should be medically approved for clinical use, non-medically approved devices could contain clinically useful information. A therapeutic decision based on non-medical devices or off-label use of medical devices should therefore carefully weigh the source of data, validity of the information as well as clinical context before a clinical decision is made.
Section 4: Wearables—a means to patient empowerment?
Wearables are opening new avenues for patient engagement in self-management of cardiovascular health and in supporting shared decision making and goal setting. The European Society of Cardiology (ESC) defines patient engagement as a set of behaviours by which patients take more responsibility for their own health care, and healthcare professionals take more account of patients’ health needs.61 Wearable technologies may facilitate this process by enabling patients to self-monitor a range of aspects of health, including activity, body weight, HR and rhythm, blood pressure, blood glucose, and fatigue.62 This may also promote dynamic exchange of data with health professionals through visualization.
Visualization of health data has been mainly associated with electronic health records (EHR), gaining widespread adoption in the last two decades. A more recent approach aims to integrate data between EHR and medical devices, wearables and fitness tracking devices (a large number of existing wearables are EHR-compatible and this number is expected to increase exponentially). Mobile integration platforms, such as Google Fit and Apple HealthKit, pool data from multiple health apps and have the potential to integrate it with EHR, promoting visualization.63 However, there are concerns on data privacy and third-party utilization that would require further clarification.
One of the most advanced applications of health visualization is building an avatar using health information from a wide range of sources, including wearables. This enables a level of personalization of health that is key in facilitation of behaviour change. Personalization or tailoring is defined as any of a number of methods for creating communications individualized for their receivers.64 Personalization techniques, such as gamification, rewarding, goal setting, feedback and inter-human interaction maximize the opportunity for personal engagement.65 Personally controlled data alters power dynamics in health care, improving democratization of health.
Gaps in knowledge
Value-based initiatives to increase patient activation and engagement using wearables are needed.
Studies exploring the ability of a technology to maintain engagement over time (>3 or 6 months).
Tools and methods to characterize patient preference, increase personalization and improve engagement are needed.
Section 5: What are relevant clinical events of interest for prevention using wearables?
The use of continuous data using wearables is likely to challenge and expand our traditional way of thinking on clinical events of interest. Wearables have the potential to detect early markers of disease in ‘real-time’ or with a close temporal relationship to physiological changes and are therefore particularly suited for prevention. The conventional endpoints used for preventive measures and clinical epidemiology typically include all-cause mortality, cause-specific mortality, or single or aggregate comorbid endpoints based on administrative registers or other means of sampling information. Other cause-specific endpoints can be used, for instance incident AF or detection of other arrhythmias. Ideally, a marker of risk should be detected before a traditional endpoint/clinical event (e.g. manifest hypertension, AF, myocardial infarction, sudden death) occurs. More transient endpoints may be relevant—for instance, markers of physiological stress, and may potentially detect the very early markers of clinical events such as myocardial infarction or stroke. HR monitors would be able to detect increase in HR at rest, increase in HR during night-time, or other physiological markers. With the introduction of other wearable sensors (e.g. blood glucose), the potential for early detection of disease and risk would increase. Increased resting HR has been shown to predict future hypertension,66 which in turn is associated with increased risk of manifest cardiovascular disease. There is currently no recommendations, knowledge or consensus on how to advice individuals or the public in terms of very early markers of risk using wearable technology including HR or fitness trackers.
Information from wearables may be particularly useful in nudging or educating patients or caregivers about the effects of patient activities, underlying medical conditions and treatments. Ideally, these devices also help to support diagnosis and to tailor treatment strategy. The potential value of this technology is that the feedback loop can be shortened by offering automated input for immediate modification of therapy and behaviour. In this context, the data generated should be diagnostically meaningful, informative regarding the treatment effect and of prognostic value. Wearables may therefore allow a move towards ‘value-based pricing’ (programs/drugs/interventions paid for if they lead to results) as well as allowing a more holistic assessment of the value of any intervention to that individual.
Gaps in knowledge
Clinical endpoints and relevant events of interest need to be defined in the area of continuous monitoring in cardiovascular prevention.
Exploration of relevant immediate, intermediate, or clinical endpoints is needed.
Research in the area of continuous HR and fitness tracking needs to be explored particularly for non-classical clinical endpoints such as quality of life and psychosocial factors.
Early markers of disease should be explored in the area of continuous monitoring.
Section 6: Are there potential adverse consequences of wearables?
There are several areas where wearable technology may have adverse consequences, including unintended modification of behaviour, unintended creation of big datasets and its misuse, privacy and security issues, challenges facing regulatory bodies regarding safety and data interpretation, and lack of validation when used for health promotion.67
Wearables provide feedback on physiological and exercise parameters, giving users an opportunity to modify health behaviours. In a minority of individuals, this may lead to increasing anxiety about health, to device addiction, or to self-diagnosis or even to self-medication or self-management of clinical conditions.68,69 Patients could also suffer from negative consequences of excessive self-monitoring by finding it uncomfortable, intrusive, and unpleasant. Wearables may provide false assurances to the patient, with inaccuracy of activity trackers leading individuals to overestimate their level of physical activity, limiting the effectiveness for lifestyle interventions.70
Users who buy wearable devices today do not necessarily ‘own’ their data. Instead, the individual’s data is usually collected and stored on cloud severs by the manufacturer. This can create a paradox for the user in that they own the device, but not the captured data. The creation of such big datasets derived from an individual’s physiological data will have privacy and data storage/security implications, with the potential to expose patients to safety and cybersecurity risks, as has been the case in cardiac electronic implanted devices,71 having their technology infected with malware and vulnerability to unauthorized access through hacking.
Regulatory bodies do not regulate wearable sensors/devices designed purely for lifestyle purposes, such as smartwatches that generally promote health and fitness.72 In contrast, apps with medical purposes (diagnosis, prevention, monitoring, treatment or alleviation of disease) are currently classified as ‘medical devices’ by both the FDA73 and the European Union, where the new Medical Device Regulation (entering in force starting 22 May 2021) will strengthen the rules for obtaining certification.
Also, wearable devices are marketed as a means to improve general health and fitness, but manufacturers are not required to provide data to support the accuracy and effectiveness of their products. Furthermore, the use of wearables for cardiovascular health screening may medicalize healthy individuals, resulting in unnecessary medical investigations with possible patient harm and increased cost. False negatives can cause a potentially fatal condition to be missed while false positives can lead to overtreatment and/or anxiety.74
Furthermore, wearables may contribute to increasing the health inequalities and inequities in society, where those without access to these technologies (because of economic considerations or digital literacy issues) may become more disadvantaged. However, with decrease in cost of wearables devices and higher penetration of digital literacy this challenge may be attenuated in the near future.
Lastly, increased downstream testing and overtreatment with potential increase in cost and patient harm is a concern, especially when no clear definitions on indications for treatment or referral are established.
Gaps in knowledge
Data to show efficacy of wearable devices in improvement of meaningful clinical outcomes in asymptomatic patients without clinically manifest cardiovascular diseases.
The occurrence of unintended behavioural changes due to the use of devices and the resulting adverse clinical events in the population.
The health economic consequences of wearables should be determined, including benefits of early detection and risk of unnecessary downstream testing and overtreatment.
Section 7: Data security and privacy of heart rate and activity trackers in the light of new European legislation
When dealing with wearable technology in the context of cardiovascular health promotion, knowledge of the current legislation at EU level is needed.
The presence of a privacy policy is often lacking in most current commercially available HR and activity tracking technologies. In a review of the most downloaded health and fitness apps, the majority of apps did not have a privacy policy, while 74% of them gathered information classified as ‘sensitive’, sharing the collected data with a third party.75
The EU General Data Protection Regulation (GDPR) 2016/679, effective since 25 May 2018, has extended the concept of personal data to any information (a name, a photo, an email address, bank details, posts on social networking websites, medical information, or a computer IP, or also genetic, mental, cultural, economic and social data) related to a natural person or ‘Data Subject’ that can be used to directly or indirectly identify the person. Also, it has widened its jurisdiction, as it applies to all companies processing the personal data of data subjects residing in the EU, regardless of the company’s location. In addition, GDPR extends liability from data controller to all parties that get in touch with the personal data, together with the principle to hold and process only the data absolutely necessary for the completion of its duties (data minimization principle), as well as not to change the use of the data from the purpose for which it was originally collected. These changes should be reflected in the consent form that is provided with any tracker or activity app that require the subject to be enrolled in order to access the service.
The EU Medical Device Regulation (MDR) 2017/745, which will become effective starting 26 May 2021 extends the definition of medical device (any instrument intended by the manufacturer to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment, or alleviation of disease) to prediction and prognosis, thus including all digital health apps that have an intrinsic tendency to collect and evaluate physiological data, including wellness technologies, as well as predictive models, risk calculators, artificial intelligence. This could lead to the qualification as medical device for tools and software that are nowadays not under this category, as well as to the classification in higher classes of current class I medical devices, taking into account the intended purpose and the inherent risks.76
In particular, software intended to monitor vital physiological parameters (HR, blood pressure, respiration) could be classified as Class IIb, if the nature of variations of those parameters could result in immediate danger to the patient (depending on patient disease and associated risk).
Gaps in knowledge
Current legislation is not specific for novel technologies, such as wearables, that need different criteria to be tested, verified, and updated. It is important that professional medical associations such as the ESC follow the process of creation of new legislation in this field and to inform lawmakers on specific needs and risks related to healthcare in general and wearable technology in particular.
It should be established to what extent healthcare professionals should be informed about data security and privacy of a device/health and fitness app.
It should be established in what way patients are informed about data storage and transfer to third parties when using an HR and activity tracker.
Data safety and integration with other health platforms should be addressed.
The ability of patients to opt out should be verified.
Standards for accreditation processes should be established to avoid relying on developer self-certification to ensure adherence to data protection principles.
Section 8: Wearables and the COVID-19 pandemic
The pandemic of coronavirus disease (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has impacted clinical cardiology practice and the use of digital health. Patients with chronic cardiac diseases such as heart failure, arrhythmia, coronary artery disease, and congenital heart defects are traditionally followed including face-to-face contacts during outpatient visits.77–79 Due to the COVID-19 pandemic, outpatient visits of chronic patients have been replaced by virtual visits to limit disease transmission.80 Chronic cardiac patients need regular care and are at increased risk of infection with COVID-19 with worse outcome.81 Wearables should be considered in these vulnerable patients to continue regular care,82 to reduce risk of transmission, and to diagnose COVID-19 infection early.83 Wearables can also supplement conventional diagnostic testing for public health surveillance to track (asymptomatic) persons who can transmit SARS-CoV-2 to others.84 Wearables certified as medical devices have been shown able to track healthcare workers health status or to measure QT intervals.85 Zhuo et al.86 demonstrated that medical and nursing staff with insomnia showed clear signs of comorbid sleep apnoea attributable to stress. Wearables can be used to perform monitoring of vital signs such as oxygen saturation, respiratory rate, blood pressure, body temperature, but also pulmonary auscultation, ECGs, and cough monitoring.87 SpO2 measurement is available as both stand-alone finger oximeters and as smart phone systems, although the accuracy of the latter has been questioned.88 There are, however, also important challenges on wearables and COVID-19. Whereas only a few COVID-19 wearables studies are expected to generate high-quality evidence, the majority of recently initiated studies are expected to have a concerning low level of evidence.89 A joint decision with the patient (shared decision-making) to switch to remote care with wearables is recommended. The many political, economic, and time-consuming barriers could be considered discouraging for a quick introduction of wearables to monitor cardiac diseases in the COVID-19 era. However, the COVID era without a doubt has been of enormous importance for the general adoption and clinical implementation of digital health and wearable devices. The rapid initiation could possibly lead to the much needed will and decisiveness to create sustainable tools, to arrange for financial compensation, and to perform high-quality clinical outcome studies.
Gaps in knowledge
Large-scale evidence of the efficacy of wearables to diagnose and manage COVID-19 in cardiac patients is lacking.
The ideal physiological marker available for wearable technology to monitor, diagnose and manage COVID-19 with cardiac diseases need to be determined.
How to implement these findings from the individual user to a population level relevant for a pandemic needs further consideration.
Conclusion
The introduction of wearables represents an unprecedented situation in primary and secondary prevention of cardiovascular disease in relation to availability of low-cost physiological data, ‘democratization’ of health information, and possibility for early detection of disease or risk factors for disease. There are, however, significant issues and barriers that need to be addressed before wearables can be translated from nice-to-have consumer gadgets to clinical utility in the context of primary and secondary prevention. Healthcare providers are being presented with information from commercially available wearables with increasing frequency. However, there are presently no concrete guidelines for primary care physician or cardiologist on how to use, interpret or act on information from wearables. Even with the present absence of clinical evidence, the need for guidance is increasing to support the clinician faced with the daily challenges in the management of information from wearables. We encourage the professional associations of the ESC to develop clinical recommendations to guide the cardiologist in their respective fields. The present Position Paper represents a constructive framework for directing future research and policy issues in relation to use of wearables for cardiovascular prevention and the implementation into clinical care.
Conflict of interest: Dr. Jensen has nothing to disclose. Dr. Treskes reports personal fees from Boston Scientific, personal fees from Pfizer, personal fees from Sanofi, outside the submitted work; Dr. Caiani reports personal fees from Novartis, personal fees from Servier, personal fees from Medtronic, outside the submitted work; Dr. Casado-Arroyo has nothing to disclose. Dr. Cowie reports grants and personal fees from Boston Scientific, grants and personal fees from Medtronic, grants and personal fees from Abbott, personal fees from We-health, personal fees from AstraZeneca, grants and personal fees from Bayer, personal fees from Novartis, outside the submitted work; Dr. Dilaveris has nothing to disclose. Dr. Duncker reports grants and personal fees from Zoll CMS GmbH, personal fees from Abbott, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Boston Scientific, personal fees from Medtronic, personal fees from Pfizer, outside the submitted work; Dr. Frederix has nothing to disclose; Dr. Kemps has nothing to disclose; Dr. Mamas has nothing to disclose. Dr. Platonov has nothing to disclose. Dr. Di Rienzo has nothing to disclose. Dr. Schmidt-Trucksäss has nothing to disclose. Dr. Schuuring has nothing to disclose. Dr. Simova has nothing to disclose. Dr. Svennberg reports personal fees from Bayer, personal fees from Bristol-Myers-Squibb, personal fees from Pfizer, personal fees from Boehringer-Ingelheim, personal fees from Merck Sharp & Dr. Lumens has nothing to disclose. All other authors: none reported.
Contributor Information
Magnus T Jensen, Department of Cardiology, Copenhagen University Hospital Amager & Hvidovre, Kettegaard Alle 30, 2650 Hvidovre, Denmark.
Roderick W Treskes, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, the Netherlands.
Enrico G Caiani, Department of Electronics, Information and Biomedical Engineering, Politecnico di Milano, Via Ponzio 34/5, 20133 Milan, Italy; National Council of Research, Institute of Electronics, Information and Telecomunication Engineering, Milan, Italy.
Ruben Casado-Arroyo, Department of Cardiology, Erasme Hospital, Université Libre de Bruxelles, Route de Lennik 808, 1070 Brussels, Belgium.
Martin R Cowie, Department of Cardiology, Royal Bromptom Hospital, Sydney St, Chelsea, London SW3 6NP, UK.
Polychronis Dilaveris, Department of Cardiology, Hippokration Hospital, 114 Vas. Sofias avenue, 11527, Athens, Greece.
David Duncker, Department of Cardiology and Angiology, Hannover Heart Rhythm Center, Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany.
Marco Di Rienzo, Department of Biomedical Technology, IRCCS Fondazione Don Carlo Gnocchi, 20121 Milano, Italy.
Ines Frederix, Department of Cardiology, Jessa Hospital, Salvatorstraat 20, 3500 Hasselt, Belgium; Department of Cardiology, Antwerp University Hospital, Drie Eikenstraat 655, 2650 Edegm, Belgium; Faculty of Medicine & Life Sciences, Hasselt University, Martelarenlaan 42, 3500 Hasselt, Belgium; Faculty of Medicine & Health Sciences, Antwerp University, Campus Drie Eiken, Building S, Universiteitsplein 1, 2610 WILRIJK, Antwerp, Belgium.
Natasja De Groot, Department of Cardiology, Erasmus University Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
Philippe H Kolh, Department of Cardiovascular Surgery, University Hospital Liege, Quai Paul van Hoegaerden 2, 4000 Liege, Belgium.
Hareld Kemps, Department of Cardiology, Maxima Medical Centre, Dominee Theodor Fliednerstraat 1, 5631 BM Eindhoven, The Netherlands; Department of Industrial Design, Eindhoven University of Technology, 5612 AZ Eindhoven, The Netherlands.
Mamas Mamas, Academic Department of Cardiology, Royal Stoke Hospital, University Hospital North Midlands, Newcastle Rd, Stoke-on-Trent ST4 6QG, UK.
Paul McGreavy, ESC Patient’s Platform, European Society of Cardiology, Sophia Antipolis Cedex, France.
Lis Neubeck, School of Health and Social Care, Edinburgh Napier University, 9 Sighthill Ct, Edinburgh EH11 4BN, UK.
Gianfranco Parati, Department of Medicine and Surgery, University of Milano-Bicocca & Istituto Auxologico Italiano, IRCCS, Piazza dell'Ateneo Nuovo, 1, 20126 Milano MI, Italy; Department of Cardiovascular, Neural and Metabolic Sciences, San Luca Hospital, Piazzale Brescia 20, Milano, Italy.
Pyotr G Platonov, Department of Cardiology, Clinical Sciences, Lund University Hosptial, EA-blocket, 221 85 Lund, Sweden.
Arno Schmidt-Trucksäss, Department of Sport, Exercise and Health, University of Basel, Birsstrasse 320 B, 4052 Basel, Switzerland.
Mark J Schuuring, Department of Cardiology, Amsterdam University Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Iana Simova, Cardiology Clinic, Heart and Brain—University Hospital, One, G. M. Dimitrov Blvd. Sofia 1172, Pleven, Bulgaria.
Emma Svennberg, Department of Cardiology, Karolinska University Hospital, Anna Steckséns gata 41, 171 64 Solna, Stockholm, Sweden; Department of Clinical Sciences Danderyd University Hospital, 171 77 Stockholm, Sweden.
Axel Verstrael, ESC Patient’s Platform, European Society of Cardiology, Sophia Antipolis Cedex, France.
Joost Lumens, CARIM School for Cardiovascular Diseases, Maastricht University Medical Center, Duboisdomein 30, 6229 GT Maastricht, the Netherlands.
Data Availability
There are no new data associated with this article.
References
- 1.Global Wearable Computing Devices Market (2020 to 2025)—Growth, Trends & Forecasts. https://www.globenewswire.com/news-release/2020/06/24/2052588/0/en/Global-Wearable-Computing-Devices-Market-2020-to-2025-Growth-Trends-Forecasts.html.
- 2. Jang KI, Li K, Chung HU, Xu S, Jung HN, Yang Y, Kwak JW, Jung HH, Song J, Yang C, Wang A, Liu Z, Lee JY, Kim BH, Kim JH, Lee J, Yu Y, Kim BJ, Jang H, Yu KJ, Kim J, Lee JW, Jeong JW, Song YM, Huang Y, Zhang Y, Rogers JA. Self-assembled three dimensional network designs for soft electronics. Nat Commun 2017;8:15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen MT, Holtermann A, Bay H, Gyntelberg F. Cardiorespiratory fitness and death from cancer: a 42-year follow-up from the Copenhagen Male Study. Br J Sports Med 2017;51:1364–1369. [DOI] [PubMed] [Google Scholar]
- 4. Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife cardiorespiratory fitness and the long-term risk of mortality: 46 years of follow-up. J Am Coll Cardiol 2018;72:987–995. [DOI] [PubMed] [Google Scholar]
- 5. Varma N, Marrouche NF, Aguinaga L, Albert CM, Arbelo E,, Choi JI, Chung MK, Conte G, Dagher L, Epstein LM, Ghanbari H, Han JK, Heidbuchel H, Huang H, Lakkireddy DR, Ngarmukos T, Russo AM, Saad EB, Saenz Morales LC, Sandau KE, Sridhar ARM, Stecker EC, Varosy PD. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Europace 2020;22(3):450–495.31995197 [Google Scholar]
- 6. Manninger M, Kosiuk J, Zweiker D, Njeim M, Antolic B, Kircanski B, Larsen JM, Svennberg E, Vanduynhoven P, Duncker D. Role of wearable rhythm recordings in clinical decision making—the wEHRAbles project. Clin Cardiol 2020;43:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EPHO5: Disease prevention, including early detection of illness [Internet]. Last accessed: October 14th, 2020. http://www.euro.who.int/en/health-topics/Health-systems/public-health-services/policy/the-10-essential-public-health-operations/epho5-disease-prevention,-including-early-detection-of-illness2.
- 8. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 2007;28:R1–R39. [DOI] [PubMed] [Google Scholar]
- 9. Landreani F, Caiani EG. Smartphone accelerometers for the detection of heart rate. Expert Rev Med Devices 2017;14:935–948. [DOI] [PubMed] [Google Scholar]
- 10. Henriksen A, Haugen Mikalsen M, Woldaregay AZ, Muzny M, Hartvigsen G, Hopstock LA, Grimsgaard S. Using fitness trackers and smartwatches to measure physical activity in research: analysis of consumer wrist-worn wearables. J Med Internet Res 2018;20:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed] [Google Scholar]
- 12. Tedesco S, Sica M, Ancillao A, Timmons S, Barton J, O'Flynn B. Accuracy of consumer-level and research-grade activity trackers in ambulatory settings in older adults. PLoS One 2019;14:e0216891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sartor F, Gelissen J, van Dinther R, Roovers D, Papini GB, Coppola G. Wrist-worn optical and chest strap heart rate comparison in a heterogeneous sample of healthy individuals and in coronary artery disease patients. BMC Sports Sci Med Rehabil 2018;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang R, Blackburn G, Desai M, Phelan D, Gillinov L, Houghtaling P, Gillinov M. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol 2017;2:104–106. [DOI] [PubMed] [Google Scholar]
- 15. Cadmus-Bertram L, Gangnon R, Wirkus EJ, Thraen-Borowski KM, Gorzelitz-Liebhauser J. The accuracy of heart rate monitoring by some wrist-worn activity trackers. Ann Intern Med 2017;166:610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koshy AN, Sajeev JK, Nerlekar N, Brown AJ, Rajakariar K, Zureik M, Wong MC, Roberts L, Street M,, Cooke J, Teh AW. Smart watches for heart rate assessment in atrial arrhythmias. Int J Cardiol 2018;266:124–127. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Pi Z, Liu B. TROIKA: a general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans Biomed Eng 2015;62:522–531. [DOI] [PubMed] [Google Scholar]
- 18. Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol 2018;71:2381–2388. [DOI] [PubMed] [Google Scholar]
- 19. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M,, Turakhia MP. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruining N, Caiani E, Chronaki C, Guzik P, van d V. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur. J. Prev. Cardiol 2014;21:4–13. [DOI] [PubMed] [Google Scholar]
- 21. Coppetti T, Brauchlin A, Muggler S, Attinger-Toller A, Templin C, Schonrath F, Hellermann J, Luscher TF, Biaggi P, Wyss CA. Accuracy of smartphone apps for heart rate measurement. Eur J Prev Cardiol 2017;24:1287–1293. [DOI] [PubMed] [Google Scholar]
- 22. Stahl SE, An HS, Dinkel DM, Noble JM, Lee JM. How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Sport Exerc Med 2016;2:e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan BP, Lai WHS, Chan CKY, Au ACK, Freedman B,, Poh YC, Poh MZ. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020;5:105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan BP, Chan CK, Li CK, To OT, Lai WH, Tse G, Poh YC, Poh MZ. Resting and postexercise heart rate detection from fingertip and facial photoplethysmography using a smartphone camera: a validation study. JMIR Mhealth Uhealth 2017;5:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butler MJ, Crowe JA, Hayes-Gill BR, Rodmell PI. Motion limitations of non-contact photoplethysmography due to the optical and topological properties of skin. Physiol Meas 2016;37:N27–N37. [DOI] [PubMed] [Google Scholar]
- 26. O’Driscoll R, Turicchi J, Hopkins M, Gibbons C, Larsen SC, Palmeira AL, Heitmann BL, Horgan GW, Finlayson G, Stubbs RJ. The validity of two widely used commercial and research-grade activity monitors, during resting, household and activity behaviours. Health Technol 2020;10:637–648. [Google Scholar]
- 27. Inoue T, Iseki K, Iseki C, Kinjo K. Elevated resting heart rate is associated with white blood cell count in middle-aged and elderly individuals without apparent cardiovascular disease. Angiology 2012;63:541–546. [DOI] [PubMed] [Google Scholar]
- 28. Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 2004;25:363–370. [DOI] [PubMed] [Google Scholar]
- 29. Jensen MT, Marott JL, Allin KH, Nordestgaard BG, Jensen GB. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: the Copenhagen City Heart Study. Eur J Prev Cardiol 2012;19:102–108. [DOI] [PubMed] [Google Scholar]
- 30. Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart 2013;99:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen MT. Resting heart rate and relation to disease and longevity: past, present and future. Scand J Clin Lab Invest 2019;79:108–116. [DOI] [PubMed] [Google Scholar]
- 32. Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 1980;112:736–749. [DOI] [PubMed] [Google Scholar]
- 33. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 34. Palatini P, Rosei EA, Casiglia E, Chalmers J, Ferrari R, Grassi G, Inoue T, Jelakovic B, Jensen MT, Julius S, Kjeldsen SE, Mancia G, Parati G, Pauletto P, Stella A, Zanchetti A. Management of the hypertensive patient with elevated heart rate: statement of the Second Consensus Conference endorsed by the European Society of Hypertension. J Hypertens 2016;34:813–821. [DOI] [PubMed] [Google Scholar]
- 35. Palatini P, Parati G, Julius S. Office and out of office heart rate measurements: which clinical value? J Hypertens 2008;26:1540–1545. [DOI] [PubMed] [Google Scholar]
- 36. Hartaigh B, Allore HG, Trentalange M, McAvay G, Pilz S, Dodson JA, Gill TM. Elevations in time-varying resting heart rate predict subsequent all-cause mortality in older adults. Eur J Prev Cardiol 2015;22:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamill V, Ford I, Fox K, Böhm M, Borer JS, Ferrari R, Komajda M, Steg PG, Tavazzi L, Tendera M, Swedberg K. Repeated heart rate measurement and cardiovascular outcomes in left ventricular systolic dysfunction. Am J Med 2015;128:1102–1108.e6. [DOI] [PubMed] [Google Scholar]
- 38. Jensen MT, Marott JL, Lange P, Vestbo J,, Schnohr P, Nielsen OW, Jensen JS, Jensen GB. Resting heart rate is a predictor of mortality in COPD. Eur Respir J 2013;42:341–349. [DOI] [PubMed] [Google Scholar]
- 39. Hillis GS, Woodward M, Rodgers A, Chow CK, Li Q, Zoungas S, Patel A, Webster R, Batty GD, Ninomiya T, Mancia G, Poulter NR, Chalmers J. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia 2012;55:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koopman FA, Tang MW, Vermeij J, de Hair MJ, Choi IY, Vervoordeldonk MJ, Gerlag DM, Karemaker JM, Tak PP. Autonomic dysfunction precedes development of rheumatoid arthritis: a prospective cohort study. EBioMedicine 2016;6:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995;25:1276–1286. [DOI] [PubMed] [Google Scholar]
- 42. Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, Rosendaal FR, Dekkers OM. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace 2013;15:742–749. [DOI] [PubMed] [Google Scholar]
- 43. Werhahn SM, Dathe H, Rottmann T, Franke T, Vahdat D, Hasenfuß G, Seidler T. Designing meaningful outcome parameters using mobile technology: a new mobile application for telemonitoring of patients with heart failure. ESC Heart Fail 2019;6:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sessa F, Anna V, Messina G, Cibelli G, Monda V, Marsala G, Ruberto M, Biondi A, Cascio O, Bertozzi G, Pisanelli D, Maglietta F, Messina A, Mollica MP, Salerno M. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY) 2018;10:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herkert C, Kraal JJ, van Loon EMA, van Hooff M, Kemps HMC. Usefulness of modern activity trackers for monitoring exercise behavior in chronic cardiac patients: validation study. JMIR Mhealth Uhealth 2019;7:e15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Höchsmann C, Knaier R, Infanger D, Schmidt-Trucksäss A. Validity of smartphones and activity trackers to measure steps in a free-living setting over three consecutive days. Physiol Meas 2020;41:015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kraal JJ, Sartor F, Papini G, Stut W, Peek N, Kemps HM, Bonomi AG. Energy expenditure estimation in beta-blocker-medicated cardiac patients by combining heart rate and body movement data. Eur J Prev Cardiol 2016;23:1734–1742. [DOI] [PubMed] [Google Scholar]
- 49. Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: further evidence from the Whitehall study. Eur J Epidemiol 2001;17:863–869. [DOI] [PubMed] [Google Scholar]
- 50. Alosco ML, Spitznagel MB, Cohen R, Sweet LH, Hayes SM, Josephson R, Hughes J, Gunstad J. Decreases in daily physical activity predict acute decline in attention and executive function in heart failure. J Card Fail 2015;21:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parati G, Torlasco C, Omboni S, Pellegrini D. Smartphone applications for hypertension management: a potential game-changer that needs more control. Curr Hypertens Rep 2017;19:48. [DOI] [PubMed] [Google Scholar]
- 52. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol 2019;74(19):2365–2375. [DOI] [PubMed] [Google Scholar]
- 53. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 54. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, Topol EJ. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS Randomized Clinical Trial. JAMA 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, Frykman-Kull V, Levin LA. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17(7):1023–1029. [DOI] [PubMed] [Google Scholar]
- 56. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 57. Ajijola OA, Boyle NG, Shivkumar K. Detecting and monitoring arrhythmia recurrence following catheter ablation of atrial fibrillation. Front Physiol 2015;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strik M, Caillol T, Ramirez FD, Abu-Alrub S, Marchand H, Welte N, Ritter P, Haïssaguerre M, Ploux S, Bordachar P. Validating QT-interval measurement using the Apple Watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation 2020;142:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Turov A, Shirokova N, Karaskov A. Ablation of paroxysmal and persistent atrial fibrillation: 1-year follow-up through continuous subcutaneous monitoring. J Cardiovasc Electrophysiol 2011;22:369–375. [DOI] [PubMed] [Google Scholar]
- 60. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S, Vernooy K, Crijns H, Hendriks JM, Linz D. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: teleCheck-AF. Europace 2020. Sep 4:euaa201. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Graham I, Filippatos G, Atar D, Vardas PE, Pinto FJ, Fitzsimons D. Patient engagement. Eur Heart J 2017;38:3114–3115. [DOI] [PubMed] [Google Scholar]
- 62. Pevnick JM, Birkeland K, Zimmer R, Elad Y, Kedan I. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc Med 2018;28:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR mHealth uHealth 2019;7:e12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res 2008;23:454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monteiro-Guerra FM, Rivera-Romero O, Luque LF, Caulfield B. Personalization in real-time physical activity coaching using mobile applications: a scoping review. IEEE J Biomed Health Inform 2020;24:1738–1751. [DOI] [PubMed] [Google Scholar]
- 66. Palatini P, Dorigatti F, Zaetta V, Mormino P, Mazzer A, Bortolazzi A, D'Este D, Pegoraro F, Milani L, Mos L. Heart rate as a predictor of development of sustained hypertension in subjects screened for stage 1 hypertension: the HARVEST Study. J Hypertens 2006;24:1873–1880. [DOI] [PubMed] [Google Scholar]
- 67. Schukat M, McCaldin D, Wang K, Schreier G, Lovell NH, Marschollek M, Redmond SJ. Unintended consequences of wearable sensor use in healthcare. Contribution of the IMIA Wearable Sensors in Healthcare WG. Yearb Med Inform 2016:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ryan J, Edney S, Maher C. Anxious or empowered? A cross-sectional study exploring how wearable activity trackers make their owners feel. BMC Psychol 2019;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.A Fitbit fanatic's cry for help: I'm addicted to steps. Last accessed: October 14th, 2020. http://www.washingtonpost.com/news/to-your-health/wp/2015/05/11/a-fitbit-fanatics-cry-for-help/.
- 70. Wallen MP, Gomersall SR, Keating SE, Wisløff U, Coombes JS. Accuracy of heart rate watches: implications for weight management. PLoS One 2016;11:e0154420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nielsen JC, Kautzner J,, Casado-Arroyo R, Burri H, Callens S, Cowie MR, Dickstein K, Drossart I, Geneste G, Erkin Z, Hyafil F, Kraus A, Kutyifa V, Marin E, Schulze C, Slotwiner D, Stein K, Zanero S, Heidbuchel H, Fraser AG. Remote monitoring of cardiac implanted electronic devices: legal requirements and ethical principles—ESC Regulatory Affairs Committee/EHRA joint task force report. Europace 2020;22(11):1742–1758. [DOI] [PubMed] [Google Scholar]
- 72.FDA. General Wellness: Policy for Low Risk Devices. Last accessed: October 14th, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-wellness-policy-low-risk-devices.
- 73.FDA. Policy for Device Software Functions and Mobile Medical Applications. Last accessed: October 14th, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-device-software-functions-and-mobile-medical-applications.
- 74. Mandrola J, Foy A, Naccarelli G. Screening for atrial fibrillation comes with many snags. JAMA Intern Med 2018;178:1296–1298. [DOI] [PubMed] [Google Scholar]
- 75. Sunyaev A, Dehling T, Taylor PL, Mandl KD. Availability and quality of mobile health app privacy policies. J Am Med Inform Assoc 2015;22:e28–e 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.EU. Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745—MDR and Regulation (EU) 2017/746—IVDR. Last accessed: October 14th, 2020. https://ec.europa.eu/docsroom/documents/37581.
- 77. Ponikowski P, Voors AA,, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA,, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 78. Schuuring MJ, Backx AP, Zwart R, Veelenturf AH, Robbers-Visser D, Groenink M, Abu-Hanna A, Bruining N, Schijven MP, Mulder BJ, Bouma BJ. Mobile health in adults with congenital heart disease: current use and future needs. Neth Heart J 2016;24:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Treskes RW, van Winden LAM, van Keulen N, van der Velde ET, Beeres S, Atsma DE, Schalij MJ. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw Open 2020;3:e202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hermans ANL, van der Velden RMJ, Gawalko M, Verhaert DVM, Desteghe L, Duncker D, Manninger M, Heidbuchel H, Pisters R, Hemels M, Pison L, Sohaib A, Sultan A, Steven D, Wijtvliet P, Tieleman R, Gupta D, Dobrev D, Svennberg E, Crijns H, Pluymaekers N, Hendriks JM, Linz D. On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clin Cardiol 2020;43(11):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 2020;201:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016;375:154–161. [DOI] [PubMed] [Google Scholar]
- 83. Yang C, Yang J, Zhang J, Yang J. More clinical warning indicators should be explored for monitoring COVID-19 patients' condition. Int J Cardiol 2020;310:169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020;173:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chinitz JS, Goyal R, Morales DC, Harding M, Selim S, Epstein LM. Use of a smartwatch for assessment of the QT interval in outpatients with coronavirus disease 2019. J Innov Card Rhythm Manag 2020;11:4219–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhuo K, Gao C, Wang X, Zhang C, Wang Z. Stress and sleep: a survey based on wearable sleep trackers among medical and nursing staff in Wuhan during the COVID-19 pandemic. Gen Psychiatr 2020;33:e100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ding XR, Clifton D, Ji N, Lovell NH, Bonato P, Chen W, Yu X, Xue Z, Xiang T, Long X, Xu K, Jiang X, Wang Q, Yin B, Feng G, Zhang Y. Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic. IEEE Rev Biomed Eng 2021;14:48–70. [DOI] [PubMed] [Google Scholar]
- 88. Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc 2020;17:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pundi K, Perino AC, Harrington RA, Krumholz HM, Turakhia MP. Characteristics and strength of evidence of COVID-19 studies registered on ClinicalTrials.gov. JAMA Intern Med 2020;180:1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data associated with this article.