Abstract
Aims
Finerenone, a selective, non-steroidal mineralocorticoid receptor antagonist, improves cardiovascular (CV) and kidney outcomes in patients with type 2 diabetes (T2D) and chronic kidney disease (CKD). This subgroup analysis of FIDELITY, a pre-specified, pooled, individual patient-data analysis of FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049), compared finerenone vs. placebo in patients with and without baseline history of atherosclerotic CV disease (ASCVD).
Methods and results
Outcomes included a composite CV outcome [CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure (HHF)]; CV death or HHF; a composite kidney outcome (kidney failure, sustained estimated glomerular filtration rate decrease ≥57%, or kidney-related death); all-cause mortality; and safety by baseline history of ASCVD.
Of 13 026 patients, 5935 (45.6%) had a history of ASCVD. The incidence of the composite CV outcome, CV death or HHF, and all-cause mortality was higher in patients with ASCVD vs. those without, with no difference between groups in the composite kidney outcome. Finerenone consistently reduced outcomes vs. placebo in patients with and without ASCVD (P-interaction for the composite CV outcome, CV death or HHF, the composite kidney outcome, and all-cause mortality 0.38, 0.68, 0.33, and 0.38, respectively). Investigator-reported treatment-emergent adverse events were consistent between treatment arms across ASCVD subgroups.
Conclusion
Finerenone reduced the risk of CV and kidney outcomes consistently across the spectrum of CKD in patients with T2D, irrespective of prevalent ASCVD.
Keywords: Atherosclerotic cardiovascular disease, Chronic kidney disease, Finerenone, Mineralocorticoid receptor antagonist, Type 2 diabetes
Graphical Abstract
Graphical Abstract.
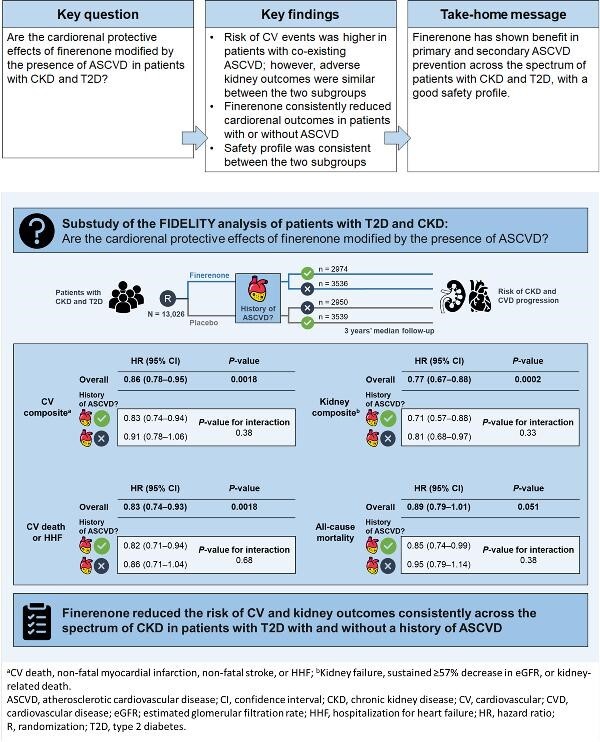
Finerenone reduced the risk of clinically important cardiorenal outcomes vs. placebo, irrespective of history of atherosclerotic cardiovascular disease, across the spectrum of chronic kidney disease in patients with type 2 diabetes.
Introduction
Type 2 diabetes (T2D) and chronic kidney disease (CKD) commonly co-exist and are associated with major adverse health outcomes, such as heart failure (HF) and atherosclerotic events, as well as premature mortality from cardiovascular (CV) causes.1–3 Patients with T2D have up to a four-fold higher risk of atherosclerotic CV disease (ASCVD) compared with populations without T2D,4,5 and the severity of kidney impairment correlates with a higher incidence of CV events.3,6,7 Furthermore, the risk of all-cause mortality is incremental, with the highest risk reported in patients with ASCVD on top of CKD and T2D.8 Given that CV complications are among the most frequent causes of death among patients with T2D and CKD, prevention of CV complications in this patient population is a key therapeutic focus.9 However, there are currently limited data on how CV events could be effectively prevented or reduced in patients with CKD and T2D.
Finerenone, a selective, non-steroidal mineralocorticoid receptor antagonist, demonstrated CV and kidney benefits in patients with T2D and CKD in the FIDELIO-DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease; NCT02540993) and FIGARO-DKD (FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease; NCT02545049) phase III trials.10–12 The two trials were complementary in design, with FIDELIO-DKD including patients with T2D and more advanced CKD compared with FIGARO-DKD, which included patients with T2D with earlier stages of CKD but at high CV risk.10,12 As prospectively planned, the individual patient-level data from these two trials were pooled in the FIDELITY (The FInerenone in chronic kiDney diseasE and T2D: Combined FIDELIO-DKD and FIGARO-DKD Trial programme analysis) database, providing the opportunity to evaluate outcomes with finerenone in a population of patients with T2D across a broader spectrum of CKD than either trial enrolled.13 The results of the primary analysis of FIDELITY on the overall dataset for the primary trial outcome provided confirmation of the benefits of finerenone in reducing the risks of CV and kidney events.13
Given that almost 50% of patients with CKD and T2D in the FIDELITY population had a history of ASCVD,13 and that patients with ASCVD are at greater risk for HF,14 we sought to investigate whether the cardiorenal benefits of finerenone were consistent, irrespective of comorbid ASCVD. Here, the results of the analyses of the FIDELITY dataset stratified by presence or absence of ASCVD at baseline are presented.
Methods
Study design and participants
FIDELITY is a pre-specified pooled database of individual patient data from the FIDELIO-DKD and FIGARO-DKD trials, two phase III, randomized, double-blind, placebo-controlled, multi-centre clinical trials of finerenone in patients with T2D and CKD.10,12,13 The present subgroup analyses of FIDELITY were pre-specified. These trials were performed in accordance with the principles of the Declaration of Helsinki and were approved by the competent authorities and ethic committees at each site. All participants provided written informed consent.
In brief, eligible patients were ≥18 years of age, clinically diagnosed with T2D and CKD defined as either (i) persistent, moderately increased urine albumin-to-creatinine ratio (UACR) ≥30—<300 mg/g and estimated glomerular filtration rate (eGFR) 25—≤90 mL/min/1.73 m2, or (ii) persistent, severely increased UACR ≥300—≤5000 mg/g and eGFR ≥25 mL/min/1.73 m2. Potential participants were required to be treated with a maximum tolerated dose of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker ≥4 weeks prior to the screening visit, preferably without adjustment to dose or choice of agent or to any other antihypertensive or antiglycaemic treatment, and to have a serum potassium level of ≤4.8 mEq/L at the run-in and screening visits. Key exclusion criteria included: HF with reduced ejection fraction and persistent symptoms of New York Heart Association class II–IV; prior stroke, transient ischaemic cerebral attack, acute coronary syndrome, or hospitalization for worsening HF in the 30 days prior to the screening visit; uncontrolled hypertension [i.e. mean sitting systolic blood pressure (SBP) ≥170 mmHg, mean sitting diastolic blood pressure ≥110 mmHg at the run-in visit, mean sitting SBP ≥160 mmHg, or mean sitting diastolic blood pressure ≥100 mmHg at the screening visit]; or known significant non-diabetic kidney disease. Concomitant therapy with eplerenone, spironolactone, any renin inhibitor, potassium-sparing diuretic or potent cytochrome P450 isoenzyme 3A4 inhibitors, or inducers was prohibited in both trials.
Procedures and outcomes
Patients were randomized in a 1:1 ratio to receive once-daily oral treatment with finerenone (10 or 20 mg at titrated doses) or matching placebo. Patients with an eGFR at screening <60 mL/min/1.73 m2 received 10 mg, and those with an eGFR ≥60 mL/min/1.73 m2 received 20 mg. Blinded up-titration of the study drug was encouraged from visit 2 onward (i.e. >1 month of treatment), provided that serum potassium concentrations were ≤4.8 mEq/L and kidney function was stable. Down-titration was allowed any time after treatment initiation for safety reasons.
Pre-specified subgroups were categorized by the presence or absence of ASCVD at baseline, as reported by the investigators. ASCVD was defined as investigator-reported medical history of coronary artery disease [i.e. previous myocardial infarction (MI), coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or angiographically proven stenosis ≥50% in at least one major coronary artery], previous ischaemic stroke, peripheral artery disease, or carotid endarterectomy. A history of HF alone was not included in this definition (Supplemental Methods).
Efficacy outcomes included: a primary composite CV outcome [time to first event of CV death, non-fatal MI, non-fatal stroke, or hospitalization for HF (HHF)]; a composite of time to CV death or HHF; a composite kidney outcome [time to first event of kidney failure (defined as the occurrence of end-stage kidney disease or an eGFR <15 mL/min/1.73 m2), a sustained ≥57% decrease in eGFR ≥4 weeks from baseline, or kidney-related death]; and all-cause mortality. Other outcomes assessed were: the composites of time to first event of non-fatal or fatal HHF, non-fatal or fatal stroke, and non-fatal or fatal MI. All outcomes were adjudicated by an independent clinical event committee (including cardiologists, neurologists, and nephrologists) blinded to treatment assignment.10,12
Treatment-emergent adverse events (AEs) were defined as AEs that first occurred or were exacerbated during the study drug treatment period or ≤3 days after temporary or permanent interruption of study drug, with evaluation stratified by a history of ASCVD. Hyperkalaemia AEs included investigator-reported events with the use of the Medical Dictionary for Regulatory Activities terms ‘hyperkalaemia’ or ‘blood potassium increased.’
Statistical analyses
Efficacy analyses were performed in the full analysis set, consisting of all randomized participants without any critical Good Clinical Practice violations. Baseline characteristics of patients were stratified by history of ASCVD. History of ASCVD was a stratification factor in the FIGARO-DKD trial but not in the FIDELIO-DKD trial.10,12 Efficacy outcomes were captured from randomization up to the end-of-trial visit in both trials. Data on patients without an event were censored at the date of their last contact, and complete information on all components of their respective outcomes was recorded. Incidences and their associated 95% confidence intervals (CIs) were expressed per 100 patient-years (PY). Time-to-event analysis of clinical outcomes was performed using stratified Cox proportional hazards models with stratification factors: geographic region (North America, Latin America, Europe, Asia, or others), eGFR category at screening (25—<45, 45—<60, or ≥60 mL/min/1.73 m2), albuminuria category at screening (moderately or severely elevated), ASCVD status, and study (FIDELIO-DKD or FIGARO-DKD). Estimates of treatment effects for time-to-event outcomes are expressed as hazard ratios (HRs) with corresponding 95% CIs.
Stratified Cox proportional hazards models were used to estimate the treatment effects by ASCVD status and the interaction between treatment and ASCVD status. The models included treatment, ASCVD subgroup, and ASCVD subgroup by treatment interaction terms as fixed effects. χ2 tests were used to report the P-interaction terms. The two-slope linear spline mixed model repeated measure method was used to estimate the rate of change in eGFR.15 Safety analyses were performed in the safety analysis set, consisting of data from all randomized patients without critical Good Clinical Practice violations who received at least one dose of finerenone or placebo. In all cases, the threshold for assessing statistical significance was set at level 0.05. Statistical analyses were performed with SAS statistical software, version 9.4.15
Results
Patients
FIDELITY comprised 13 026 patients in the full analysis set followed for a median of 3 years. Among these patients, 5935 (45.6%) had a history of ASCVD at baseline; of whom, 2979 (50.2%) were treated with finerenone and 2956 (49.8%) with matching placebo. At baseline, patients with a history of ASCVD were older and more frequently male and White, had a longer duration of T2D, were more likely to have a history of atrial fibrillation or coronary heart disease, and were less likely to have hypertension (Table 1 and supplementary material online, Table S1). Patients with ASCVD also had lower baseline mean eGFR and median UACR, while SBP, glycated haemoglobin, and body mass index did not differ by ASCVD status. Patients with ASCVD were more likely to be treated with beta-blockers, loop diuretics, statins, and insulin and were less likely to be receiving metformin and glucagon-like peptide 1 receptor agonists. Sodium-glucose co-transporter-2 inhibitor use did not differ between the two subgroups and no patients were treated with angiotensin receptor—neprilysin inhibitors at baseline. The mean daily dose exposure (mean ± standard deviation) achieved for finerenone was 16.0 ± 4.4 mg for patients with ASCVD and 16.9 ± 4.1 mg for patients without ASCVD, corresponding to a standardized mean difference of 0.21 between the two patient subgroups. The effect of finerenone on SBP did not differ between patients with and without ASCVD (Supplementary material online, Figure S1).
Table 1.
Patient baseline characteristics by history of atherosclerotic cardiovascular disease
| Characteristic | With history of ASCVD (n = 5935) | Without history of ASCVD (n = 7091) |
|---|---|---|
| Age, years, mean ± SD | 66.8 ± 8.5 | 63.1 ± 10.0 |
| Sex, male, n (%) | 4374 (73.7) | 4714 (66.5) |
| Race, n (%)WhiteBlack/African AmericanAsian | 4407 (74.3)233 (3.9)1002 (16.9) | 4462 (62.9)289 (4.1)1892 (26.7) |
| SBP, mmHg, mean ± SD | 136.7 ± 14.4 | 136.8 ± 14.0 |
| DBP, mmHg, mean ± SD | 75.3 ± 9.8 | 77.22 ± 9.4 |
| BMI, kg/m2, mean ± SD | 31.2 ± 5.7 | 31.3 ± 6.2 |
| Duration of diabetes, years, mean ± SD | 16.5 ± 8.9 | 14.5 ± 8.4 |
| HbA1c, %, mean ± SD | 7.7 ± 1.4 | 7.7 ± 1.4 |
| Serum potassium, mEq/L, mean ± SD | 4.4 ± 0.5 | 4.3 ± 0.4 |
| eGFR, mL/min/1.73 m2, mean ± SD | 53.8 ± 19.6 | 60.7 ± 22.8 |
| eGFR, mL/min/1.73 m2, n (%) <25 25—<45 45—<60 ≥60 | 79 (1.3)2225 (37.5)1746 (29.4)1883 (31.7) | 83 (1.2)2007 (28.3)1688 (23.8)3312 (46.7) |
| UACR, mg/g, median (IQR) | 456 (152–1094) | 564 (250–1195) |
| UACR, mg/g, n (%) <30 30—<300 ≥300 | 136 (2.3)2143 (36.1)3655 (61.6) | 94 (1.3)1956 (27.6)5037 (71.0) |
| Mean waist—hip ratio, mean ± SD | 1.01 ± 0.11 | 0.99 ± 0.11 |
| Waist circumference, cm, mean ± SD | 107.8 ± 14.7 | 106.4 ± 15.4 |
| hs-CRP, mg/L, mean ± SD | 4.9 ± 9.5 | 4.6 ± 10.1 |
| Heart rate, bpm, mean ± SD | 71.2 ± 11.2 | 74.7 ± 11.4 |
| History of HF, n (%) | 1555 (26.2) | 287 (4.0) |
| History of AF, n (%) | 685 (11.5) | 421 (5.9) |
| History of MI, n (%) | 2017 (34.0) | 5 (<0.1)a |
| History of hypertension, n (%) | 5730 (96.5) | 6836 (96.4) |
| Current smoker, n (%) | 876 (14.8) | 1217 (17.2) |
| Medication use at baseline, n (%)RAAS inhibitorsBeta-blockersDiureticsLoop diureticsThiazide diureticsAngiotensin receptor—neprilysin inhibitorsAspirinPlatelet aggregation inhibitorsbStatinsPotassium supplementsPotassium-lowering agentsAnti-hyperglycemic therapiesInsulin and analoguesMetforminSulfonylureasDPP-4 inhibitorsGLP-1RAsSGLT-2 inhibitorsAlpha glucosidase inhibitors | 5923 (99.8)3854 (64.9)3299 (55.6)1583 (26.7)1377 (23.3)03823 (64.4)4539 (76.5)4809 (81.0)215 (3.6)86 (1.4)5802 (97.8)3736 (62.9)3193 (53.8)1425 (24.0)1381 (23.3)405 (6.8)405 (6.8)284 (4.8) | 7080 (99.8)2650 (37.4)3411 (48.1)1220 (17.2)1775 (25.0)02532 (35.7)2762 (39.0)4590 (64.7)170 (2.4)96 (1.4)6918 (97.6)3894 (54.9)4364 (61.5)1964 (27.7)1897 (26.8)539 (7.6)472 (6.7)372 (5.2) |
ASCVD history (yes/no) was determined by pre-specified medical loglines of carotid endarterectomy, coronary artery disease, MI, ischaemic stroke, and peripheral arterial occlusive disease. History of MI was determined by medical history that may not have had a conclusive diagnosis for ASCVD conditions.
bExcluding heparin.
AF, atrial fibrillation; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; bpm, beats per minute; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin; HF, heart failure; hs-CRP, high-sensitivity C-reactive protein; IQR, interquartile range; MI, myocardial infarction; RAAS, renin—angiotensin—aldosterone system; SBP, systolic blood pressure; SD, standard deviation; SGLT-2, sodium-glucose co-transporter-2; UACR, urine albumin-to-creatinine ratio.
Cardiovascular and kidney outcomes and all-cause mortality by history of atherosclerotic cardiovascular disease
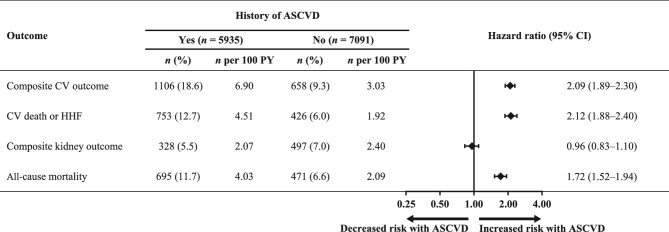
The incidence of the composite CV outcome (CV death, non-fatal MI, non-fatal stroke, or HHF) was higher in patients with a history of ASCVD vs. those without (incidence/100 PY 6.9 vs. 3.0; HR 2.09; 95% CI 1.89–2.30; Figure 1). The incidence of the composite of CV death or HHF was also higher in patients with ASCVD vs. those without (incidence/100 PY 4.5 vs. 1.9; HR 2.12; 95% CI 1.88–2.40). In contrast, the risk of the composite kidney outcome [time to first event of kidney failure [defined as the occurrence of end-stage kidney disease or an eGFR <15 mL/min/1.73 m2], a sustained ≥57% decrease in eGFR ≥4 weeks from baseline, or kidney-related death) did not differ between patients with and without a history of ASCVD (incidence/100 PY 2.1 vs. 2.4; HR 0.96; 95% CI 0.83–1.10). Patients with a history of ASCVD had a higher risk of all-cause mortality vs. those without (incidence/100 PY 4.0 vs. 2.1; HR 1.72; 95% CI 1.52–1.94).
Figure 1.
Overall incidence and relative risk of patient outcomes by history of atherosclerotic cardiovascular disease. ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CV, cardiovascular; HHF, hospitalization for heart failure; PY, patient-years.
Effects of finerenone on composite cardiovascular and kidney outcomes by history of atherosclerotic cardiovascular disease
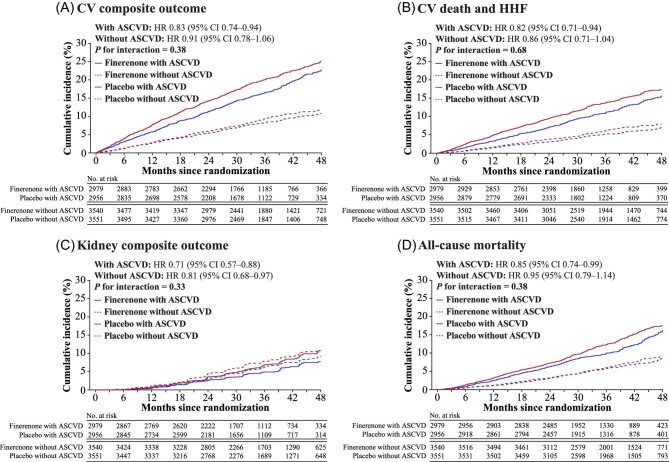
Overall, finerenone significantly reduced the risk of the primary composite CV outcome compared with placebo (HR 0.86; 95% CI 0.78–0.95; P = 0.0018). Finerenone consistently lowered the risk of the composite CV outcome compared with placebo in patients with ASCVD (HR 0.83; 95% CI 0.74–0.94) and without ASCVD (HR 0.91; 95% CI 0.78–1.06; P-interaction = 0.38; Figure 2A and supplemental material online, Figure 2). Overall, the risk of CV death or HHF was also reduced with finerenone compared with placebo (HR 0.83; 95% CI 0.74–0.93; P = 0.0018), with no treatment effect modification by ASCVD (with ASCVD, HR 0.82; 95% CI 0.71–0.94; and without ASCVD, HR 0.86; 95% CI 0.71–1.04; P-interaction = 0.68; Figure 2B and supplementary material online, Figure S3).
Figure 2.
Composite cardiovascular and kidney outcomes and all-cause mortality by history of atherosclerotic cardiovascular disease. (A) composite cardiovascular outcome of time to first onset of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure. (B), outcome of time to cardiovascular death and hospitalization for heart failure. (C), composite kidney outcome of time to first onset of kidney failure, a sustained ≥57% decrease in eGFR from baseline ≥4 weeks, or kidney-related death. (D) outcome on all-cause mortality. ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; HR, hazard ratio.
The risk of the composite kidney outcome was significantly lower with finerenone compared with placebo in the overall population (HR 0.77; 95% CI 0.67–0.88; P = 0.0002; Figure 2C and supplementary material online, Figure S4). There was no treatment effect modification of finerenone on the composite kidney outcome by ASCVD status (with ASCVD, HR 0.71; 95% CI 0.57–0.88; without ASCVD, HR 0.81; 95% CI 0.68–0.97; P-interaction = 0.33). The effects of finerenone vs. placebo on the least-squares mean change in eGFR and UACR from baseline did not differ by ASCVD baseline status (Supplementary material online, Figure S5).
Effect of finerenone on all-cause mortality and other outcomes by history of atherosclerotic cardiovascular disease
Overall, the incidence of all-cause mortality was numerically lower in the finerenone group than with placebo (incidence/100 PY 2.8 vs. 3.1; HR 0.89; 95% CI 0.79–1.01), but this difference was not significant (P = 0.051). The effect of finerenone on all-cause mortality was not modified by history of ASCVD (with ASCVD, HR 0.85; 95% CI 0.74–0.99; and without ASCVD, HR 0.95; 95% CI 0.79–1.14; P-interaction = 0.38; Figure 2D and supplementary material online, Figure S6).
Overall, a lower incidence of CV death due to HF or HHF was observed in finerenone-treated patients compared with placebo (incidence/100 PY 1.3 vs. 1.8; HR 0.75; 95% CI 0.64–0.89). This effect was consistent in patients with ASCVD (HR 0.72; 95% CI 0.59–0.88) and without ASCVD (HR 0.82; 95% CI 0.63–1.08; P-interaction = 0.43).
The overall incidence of fatal or non-fatal stroke did not differ between finerenone and placebo (incidence/100 PY 1.1 vs. 1.2; HR 0.96; 95% CI 0.80–1.16); similarly, no overall difference was observed for fatal or non-fatal MI (incidence/100 PY 1.0 vs. 1.1). There was no interaction for either of these composite outcomes by ASCVD status (Supplementary material online, Figure S7).
Safety outcomes and vital signs by history of atherosclerotic cardiovascular disease
The number of AEs in the ASCVD subgroups were consistent with the overall trial results (Table 2). Serious AEs were more common in patients with a history of ASCVD, independent of randomized treatment assignment. Similar to the overall results, the increased frequency of hyperkalaemia with finerenone vs. placebo was evident in those with ASCVD (16.7% vs. 8.9% of patients) and those without ASCVD (14.3% vs. 8.1% of patients). A low number of patients were hospitalized because of finerenone-related hyperkalaemia in both groups (1.2% of patients with a history of ASCVD and 0.7% of patients without a history of ASCVD; incidence/100 PY 0.49 and 0.27, respectively), and discontinuation of finerenone was infrequent during the trial (0.2% of patients with a history of ASCVD and 0.1% of patients without a history of ASCVD).
Table 2.
Safety outcomes by history of atherosclerotic cardiovascular disease at baseline
| With history of ASCVD | Without history of ASCVD | |||
|---|---|---|---|---|
| Treatment-emergent AEs, n (%) | Finerenone (n = 2974) | Placebo (n = 2950) | Finerenone (n = 3536) | Placebo (n = 3539) |
| Any AE | 2547 (85.6) | 2543 (86.2) | 3055 (86.4) | 3064 (86.6) |
| Maximum intensity for any AEMildModerateSevere | 775 (26.1)1202 (40.4)570 (19.2) | 742 (25.2)1118 (37.9)683 (23.2) | 1119 (31.6)1366 (38.6)570 (16.1) | 1089 (30.8)1397 (39.5)578 (16.3) |
| Any study drug—related AE | 561 (18.9) | 413 (14.0) | 645 (18.2) | 449 (12.7) |
| Any AE leading to discontinuation of study drug | 207 (7.0) | 155 (5.3) | 207 (5.9) | 196 (5.5) |
| Any SAEaStudy drug—relatedLeading to discontinuation of study drug | 1022 (34.4)46 (1.5)70 (2.4) | 1086 (36.8)32 (1.1)67 (2.3) | 1038 (29.4)37 (1.0)75 (2.1) | 1100 (31.1)29 (0.8)87 (2.5) |
| HyperkalaemiaAny hyperkalaemiaDrug-relatedLeading to permanent discontinuation of study drugSAEaDrug-relatedLeading to hospitalizationLeading to permanent discontinuation of study drug | 496 (16.7)284 (9.5)54 (1.8)41 (1.4)29 (1.0)35 (1.2)6 (0.2) | 264 (8.9)108 (3.7)20 (0.7)8 (0.3)3 (0.1)3 (0.1)2 (<0.1) | 504 (14.3)289 (8.2)56 (1.6)28 (0.8)14 (0.4)26 (0.7)4 (0.1) | 287 (8.1)141 (4.0)18 (0.5)8 (0.2)5 (0.1)7 (0.2)0 |
History of atherosclerotic cardiovascular disease was not formally assessed but defined by medical records of patients; therefore, patients with undetected atherosclerotic cardiovascular disease may be categorized as patients without atherosclerotic cardiovascular disease.
SAEs were defined as treatment-emergent events that: (i) resulted in death; (ii) were life-threatening; (iii) required inpatient hospitalization (or prolongation of existing hospitalization); (iv) caused persistent or significant disability/incapacity; (v) were congenital abnormalities or birth defects; or (vi) were judged by the investigator to be serious or important medical events.
AE, adverse event; ASCVD, atherosclerotic cardiovascular disease; SAE, serious adverse event.
Discussion
These analyses were pre-specified in the FIDELITY pooled dataset of individual patient data from the FIDELIO-DKD and FIGARO-DKD trials that offer a higher level of analytic precision than the two trials alone. Across the FIDELITY population, finerenone significantly lowered the risks of CV events and progression of CKD compared with placebo in patients with T2D and a broad spectrum of CKD.13 The secondary analyses of FIDELITY, having included nearly 6000 patients with ASCVD and 7000 without, demonstrate that the benefits of finerenone are not modified by the co-existence of ASCVD at baseline. These findings were consistent for the composite CV outcome (time to CV death, non-fatal MI, non-fatal stroke, or HHF), a composite of time to CV death or HHF, and the composite kidney outcome (time to kidney failure, sustained eGFR decrease, or kidney-related death), and the results presented here are in line with a previous analysis of data from FIDELIO-DKD that showed that the effects of finerenone on CV outcomes were consistent in patients with or without a history of ASCVD.11 In addition to confirming the results of FIDELIO-DKD, the present study extends the effect of finerenone to a broader spectrum of patients with CKD and to kidney outcomes, including patients with less advanced CKD who are at higher CV risk.
The CV benefits of finerenone in patients with T2D and CKD were previously reported to be associated with a reduced risk of HHF, CV death, and non-fatal MI.11 In FIDELITY, the CV benefit of finerenone was primarily driven by a reduction in HHF.13 Importantly, results from the analyses suggest that the protection conferred by finerenone against HF is independent of a history of ASCVD, of which coronary artery disease constitutes the main cause of HF,16 indicating that finerenone addresses some of the underlying pathogenetic mechanisms that lead to HF in patients with T2D and CKD, even in the absence of overt ASCVD.
In this study and in accordance with a previous report, the prevalent ASCVD at baseline (defined in the FIDELITY trial as a history of documented coronary artery disease, ischaemic stroke, peripheral artery disease, or carotid endarterectomy) was associated with a higher risk of CV events, including the primary composite CV outcome and the composite of time to CV death or HHF, irrespective of treatment arm.17 Interestingly, a higher risk of the composite kidney outcome in patients with ASCVD at baseline compared with those without was not observed, irrespective of treatment arm. Overall, patients with a history of ASCVD also had a higher risk of serious AEs. Thus, the frequent combination of T2D, CKD, and ASCVD defines a particularly high-risk population that should be targeted early by clinicians with effective therapies. In this regard, recent advances in the diagnosis and treatment of kidney function impairment are particularly promising and represent an important focus of research offering new therapeutic strategies in a more comprehensive cardiorenal approach.10,12,18–21 Indeed, finerenone contributes to the better outcome in these patients, improving CV and kidney outcomes as documented in this study.
The safety profile of finerenone did not differ between patients with and without ASCVD, and finerenone was generally well tolerated in both groups. There was a 6–8% absolute increase in treatment-associated hyperkalaemia with finerenone vs. placebo; approximately one in 100 patients required hospitalization for hyperkalaemia, but the risk did not appear to be modified by ASCVD history.
Limitations of this analysis include that the history of ASCVD was not formally assessed but was defined according to medical records; therefore, some patients with undetected ASCVD may theoretically have been categorized as patients without ASCVD. In addition, ASCVD was not a stratification factor in FIDELIO-DKD, which may have impacted the quality of assessment.
In conclusion, finerenone reduced the risk of CV and kidney outcomes consistently in patients with and without a history of ASCVD at baseline and was generally well tolerated in both patient subgroups. These results indicate that finerenone may be used for the prevention of ASCVD and for protection from worsening of kidney disease in patients with T2D and a broad spectrum of CKD.
Supplementary Material
Acknowledgments
The authors and study sponsor are indebted to the patients and their families, as well as the investigators and sites participating in the studies. Medical writing assistance was provided by Connie Lam, PhD, of Chameleon Communications International and was funded by Bayer AG.
Contributor Information
Gerasimos Filippatos, Department of Cardiology, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Athens, Chaidari 12462, Greece.
Stefan D Anker, Department of Cardiology (CVK), and Berlin Institute of Health Center for Regenerative Therapies, German Centre for Cardiovascular Research Partner Site Berlin, Charité Universitätsmedizin, 10117 Berlin, Germany; Institute of Heart Diseases, Wrocław Medical University, Borowska 213, 50-556 Wrocław , Poland.
Bertram Pitt, Department of Medicine, University of Michigan School of Medicine, Ann Arbor, MI 48109, USA.
Darren K McGuire, The Division of Cardiology, University of Texas Southwestern Medical Center, and Parkland Health and Hospital System, Dallas, TX 75390, USA.
Peter Rossing, Steno Diabetes Center Copenhagen, 2730 Herlev, Denmark; Department of Clinical Medicine, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Luis M Ruilope, Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, s/n, 28041, Madrid, Spain; CIBER-CV, Hospital Universitario 12 de Octubre, s/n, 28041, Madrid, Spain; Faculty of Sport Sciences, European University of Madrid, s/n, 28670, Villaviciosa de Odón, Madrid, Spain.
Javed Butler, Baylor Scott and White Research Institute, Dallas, TX 75204, USA; The Department of Medicine, University of Mississippi School of Medicine, Jackson, MS 39216, USA.
Ewa A Jankowska, Institute of Heart Diseases, Wrocław Medical University, Borowska 213, 50-556 Wrocław , Poland.
Erin D Michos, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Dimitrios Farmakis, Statistics and Data Insights, University of Cyprus Medical School, Nicosia 2029, Cyprus.
Alfredo E Farjat, Research and Development, Statistics and Data Insights, Bayer PLC, Reading, RG2 6AD, UK.
Peter Kolkhof, Research and Development, Cardiovascular Precision Medicines, Bayer AG, 42117, Wuppertal, Germany.
Andrea Scalise, Pharmaceutical Development, Bayer Hispania, S.L., 08970 Barcelona, Spain.
Amer Joseph, Cardiology and Nephrology Clinical Development, Bayer AG, Berlin 13353, Germany.
George L Bakris, Department of Medicine, University of Chicago Medicine, Chicago, IL 60637, USA.
Rajiv Agarwal, Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN 46202, USA.
Funding
Bayer AG, Berlin, Germany. The data underlying this article will be shared on reasonable request to the corresponding author.
Conflict of interest: G.F. reports lecture fees and/or that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor Pharma. He is a senior consulting editor for JACC Heart Failure and has received research support from the European Union.
S.D.A. has received research support from Abbott Vascular and Vifor Pharma, and personal fees from Abbott Vascular, Bayer, BRAHMS, Boehringer Ingelheim, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor Pharma.
B.P. reports consultant fees for Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, BioSciences KBP, G3 Pharmaceuticals, PhaseBio, Sanofi/Lexicon, Sarfez Pharmaceuticals, scPharmaceuticals, SQ Innovation, Tricida, and Vifor Pharma/Relypsa. He has stock options for Ardelyx, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP BioSciences, Sarfez Pharmaceuticals, scPharmaceuticals, SQ Innovation, Tricida, and Vifor Pharma/Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9 931 412) and a provisional patent for histone acetylation—modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045 784).
P.R. reports personal fees from Bayer during the conduct of the study. He has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas Pharma, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor Pharma; all fees are given to Steno Diabetes Center Copenhagen.
L.M.R. reports receipt of consultancy fees from Bayer.
D.K.M. has received trial leadership committee fees and consulting fees from AstraZeneca, Boehringer Ingelheim, and Sanofi US, data monitoring committee fees from CSL Behring, executive committee fees, consulting fees, and advisory board fees from Lilly USA and Novo Nordisk, executive committee fees from Eisai and Lexicon, steering committee fees from Esperion, consulting fees from Afimmune, Applied Therapeutics, and Metavante, and advisory board fees from Pfizer and Merck Sharp & Dohme.
J.B. receives consulting fees from American Regent, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceuticals, Janssen, Medtronic, Novartis, Novo Nordisk, and Vifor Pharma.
E.A.J. reports grants and personal fees from Vifor Pharma and personal fees from Abbott, AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Cardiac Dimensions, Gedeon Richter, Novartis, Pfizer, Radcliffe Group, Respicardia, Servier, Swixx BioPharma, Takeda, and Translational Medicine Academy, outside the submitted work.
E.D.M. reports advisory board fees from Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, and Novo Nordisk.
D.F. reports advisory board and/or lecture fees from Abbott Laboratories, Bayer, Boehringer Ingelheim, Leo Pharma, Novartis, and Orion.
A.E.F. is a full-time employee of Bayer PLC, Division Pharmaceuticals, Reading, UK.
A.S. is a full-time employee of Bayer AG, Division Pharmaceuticals, Spain.
P.K. is a full-time employee of Bayer AG, Division Pharmaceuticals, Germany. He is the co-inventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1).
A.J. was a full-time employee of Bayer AG, Division Pharmaceuticals, Germany at the time of the studies and analysis; he is now a full-time employee of Chiesi Farmaceutici S.p.A, Parma, Italy.
G.L.B. reports research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study, as well as research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics. He acted as a consultant and received personal fees from for Alnylam, Merck, and Relypsa. He is an editor of the American Journal of Nephrology, Nephrology, and Hypertension; section editor of UpToDate; and an associate editor of Diabetes Care and Hypertension Research.
R.A. reported personal fees and non-financial support from Bayer HealthCare Pharmaceuticals Inc. during the conduct of the study. He also reported personal fees and non-financial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius, Janssen, Relypsa, Sanofi, and Vifor Pharma. He has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co, and Reata Pharmaceuticals and non-financial support from E. R. Squibb & Sons, OPKO Health, and Otsuka America Pharmaceutical. He is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa; and a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen. He has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis Transplantation and has been an author for UpToDate. He has received research grants from the U.S. Veterans Administration and the National Institutes of Health.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goderis G, Vaes B, Mamouris P, van Craeyveld E, Mathieu C. Prevalence of atherosclerotic cardiovascular disease, heart failure, and chronic kidney disease in patients with type 2 diabetes mellitus: a primary care research network-based study. Exp Clin Endocrinol Diabetes 2022;130:447–453. [DOI] [PubMed] [Google Scholar]
- 3. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossing P, Persson F, Frimodt-Møller M, Hansen TW. Linking kidney and cardiovascular complications in diabetes-impact on prognostication and treatment: the 2019 Edwin Bierman award lecture. Diabetes 2021;70:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jialal I, Chaudhuri A. Targeting inflammation to reduce ASCVD in type 2 diabetes. J Diabetes Complications 2019;33:1–3. [DOI] [PubMed] [Google Scholar]
- 6. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium . Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 7. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG, Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Branch M, German C, Bertoni A, Yeboah J. Incremental risk of cardiovascular disease and/or chronic kidney disease for future ASCVD and mortality in patients with type 2 diabetes mellitus: ACCORD trial. J Diabetes Complications 2019;33:468–472. [DOI] [PubMed] [Google Scholar]
- 9. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G, FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 11. Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Schloemer P, Tornus I, Joseph A, Bakris GL, FIDELIO-DKD Investigators . Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021;143:540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–2263. [DOI] [PubMed] [Google Scholar]
- 13. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - Mechanisms, management, and clinical considerations. Circulation 2016;133:2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vonesh E, Tighiouart H, Ying J, Heerspink HL, Lewis J, Staplin N, Inker L, Greene T. Mixed-effects models for slope-based endpoints in clinical trials of chronic kidney disease. Stat Med 2019;38:4218–4239. [DOI] [PubMed] [Google Scholar]
- 16. Bragazzi NL, Zhong W, Shu J, Abu-Much A, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol 2021;28:1682–1690. [DOI] [PubMed] [Google Scholar]
- 17. Wan EYF, Chin WY, Yu EYT, Wong ICK, Chan EWY, Li SX, Cheung NKL, Wang Y, Lam CLK. The impact of cardiovascular disease and chronic kidney disease on life expectancy and direct medical cost in a 10-year diabetes cohort study. Diabetes Care 2020;43:1750–1758. [DOI] [PubMed] [Google Scholar]
- 18. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 19. Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabet Endocrinol 2021;9:22–31. [DOI] [PubMed] [Google Scholar]
- 20. Górriz JL, Soler MJ, Navarro-González JF, García-Carro C, Puchades MJ, D'Marco L, Martínez Castelao A, Fernández-Fernández B, Ortiz A, Górriz-Zambrano C, Navarro-Pérez J, Gorgojo-Martinez JJ. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med 2020;9:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B, Investigators E-RO . Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137:119–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.