Abstract

The development of inexpensive and well-activated water-splitting catalysts is required to reduce the use of conventional fossil fuels. In this study, a trimetallic Fe–Co–Ni catalyst was fabricated using a simple ion electrodeposition method. The metal deposition was performed using cyclic voltammetry, which was more efficient than constant-voltage deposition and significantly increased the stability of the catalyst. The synthesized material presented the morphology of a nanoflower in which the nanosheets were agglomerated. The Fe–Co–Ni catalyst exhibited excellent oxygen evolution reaction (OER) properties because the charge-transfer rate was improved owing to the synergistic effect of the metals. The OER was performed in a 1 M KOH solution using a three-electrode system, and the overpotential was 302 mV at 100 mA/cm2. In addition, the Fe–Co–Ni catalyst exhibited excellent stability in alkaline solution for more than 48 h at 200 mA/cm2. The results show that the method for preparing Fe–Co–Ni significantly improves its catalytic activity, and the resulting material could be used as an economical and efficient catalyst in future.
1. Introduction
Renewable hydrogen energy and electrochemical water splitting are actively being studied as alternatives to conventional fossil fuels.1,2 Water splitting involves the hydrogen evolution reaction and oxygen evolution reaction (OER), and the half-reactions of these two electrolysis reactions are limited owing to their slow kinetics. Precious metal catalysts, which are expensive and scarce, are generally used to accelerate the slow OER.3−5 Therefore, the development of inexpensive alternative catalyst materials with OER properties similar to those of noble metal catalysts is being actively pursued. Layered double hydroxides (LDHs) have been widely used as substitutes for OER catalyst materials.6−10 However, LDH has limitations owing to insufficient electron-transport capacity and the shortage of exposed active sites.11 In addition, the electrodes developed in many previous studies showed deterioration in electrode performance when a polymer binder with low electrical conductivity was used to form the catalyst layer. Therefore, research on catalyst materials that utilize the synergistic effect of the ternary metal structure to circumvent this limitation is being actively conducted.12−15 The metal-to-metal interaction reduces the electron migration resistance, accelerates electron migration, and improves catalytic properties.16,17
In this study, an Fe–Co–Ni catalyst was fabricated using a simple one-step electrolytic plating method on a nickel foam substrate to improve the overall water decomposition properties. This deposition method uses a small amount of precursor and is very efficient. Moreover, economical catalyst production is possible using this strategy because precious metals are not used.18−20 An electrode deposited using constant voltage and an electrode deposited using cyclic voltammetry (CV) under the same conditions were compared. The catalyst manufactured using the CV method had a more uniform surface than that manufactured using constant voltage, and the influence of oxygen was significantly reduced in the former. Consequently, the difference in stability between the two catalysts was large, and the catalyst manufactured using the CV method showed better characteristics. The prepared catalyst exhibited good catalytic activity, with a low overpotential (302 mV at 100 mA/cm2) and good durability. In addition, it could be a promising economical alternative for the existing water splitting catalysts.
2. Experimental Section
2.1. Preparation of the Fe–Ni–Co on Nickel Foam Catalyst
Briefly, 0.035 mol of FeSO4·7H2O, 0.035 mol of CoSO4·7H2O, and 0.038 mol of NiSO4·6H2O were added to 200 mL of deionized water and stirred for 2 h. Ni foam (1 cm × 1 cm) was cleaned using water and ethanol and used as the substrate. A three-electrode system was used in the electrochemical station (CH Instruments, CHI 660E) for the deposition of the catalytic material; Ag/AgCl was used as the reference electrode, and a Pt plate (1 cm × 1 cm) was used as the counter electrode. The catalyst deposition via the constant-voltage method was performed by applying −1 V (vs reversible hydrogen electrode (RHE)) for 5 min. In the CV method, the catalyst was manufactured by performing repeated sweeping from 0 to −1 V (vs RHE). After the deposition of the catalyst material, each sample was cleaned several times with deionized water and dried at 60 °C for 2 h.
2.2. Characterization
The morphology and nanostructure of the samples were obtained using scanning electron microscopy (SEM, Philips, FEI XL 30 FEG) and transmission electron microscopy (TEM, JEOL, JEM-2100F). X-ray photoelectron spectroscopy (XPS; Vg Scienta, ESCA 2000) was performed to analyze the surfaces of the as-prepared catalysts. An inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer, NexION 2000) was used to compare the metal elements of the prepared catalyst.
2.3. Electrochemical Measurements
The electrochemical activity of the prepared electrode catalyst was evaluated using a three-electrode system with an electrochemical station (CH instrument, CHI 660E) with 1 M KOH as the electrolyte. A Hg/HgO electrode (in 1 M NaOH solution) and platinum wire were used as the reference and counter electrodes, respectively. The linear sweep voltammetry (LSV) scanning range was 0–1 V (vs Hg/HgO) at a scan rate of 5 mV/s. The kinetic properties of the catalysts were evaluated using overpotential and Tafel plots. The electrical characteristics of the sample were confirmed via electrochemical impedance spectroscopy (EIS) in the range of 0.1–100 kHz at a potential of 302 mV with an amplitude of 5 mV. All measured potentials were calibrated to the reversible hydrogen electrode (RHE) using eq 1. The pH used in the calculation was measured with a pH meter, and the value was 13.96.
| 1 |
3. Results and Discussion
3.1. Morphological and Structural Analyses
The electrode deposited using the constant voltammetry method was referred to as Fe–Co–Ni(co) and that deposited via the CV method was referred to as Fe–Co–Ni(cv). The Fe–Co–Ni electrodes deposited using both methods showed a two-dimensional nanosheet structure similar to those of LDHs. The SEM images in Figure 1 show a comparison of the morphologies of the prepared catalysts. Many cracks were generated on the surface of Fe–Ni–Co(co) compared to that of Fe–Ni–Co(cv), showing an unstable morphology. The surface of the Fe–Ni–Co(co) microstructure was nonuniform and rough owing to the influence of gases generated during electrochemical deposition.21 The TEM image in Figure 2 shows that the surface of the sample exhibits entangled nanosheets, and each element is evenly distributed. TEM analysis confirms the layer spacing of the fabricated Fe–Ni–Co(cv) catalysts (Figure 3). The nanostructures of the fabricated samples exhibit different interplanar distances. The crystal structure with an interplanar distance of 0.206 nm corresponds to the (111) plane of FeNi3, whereas the interplanar distances of 0.27 and 0.25 nm correspond to the (012) plane of NiFe layered double hydroxide (LDH) and FeNiCo LDH, respectively. Furthermore, the catalyst analyzed via fast Fourier-transform (FFT) analysis exhibits polycrystalline characteristics. The molar ratio of CV and CO was determined by the ICP-MS (Table S1). The molecular ratio analysis result of Fe, Ni, and Co of Fe–Ni–Co(cv) was 1.03:1.56:1, and in order to see the effect of the counter electrode on the plating process, molecular detection of Pt was attempted, but it was not detected. The binding interactions and surface oxidation states of the synthesized catalysts were analyzed using XPS. The XPS survey spectrum showed the presence of Ni, Fe, Co, and O in the sample. In Figure 4a,b, the peaks at 530.2 and 531.5 eV correspond to the metal oxide bonds and oxygen in the OH group.22−24 For the Fe–Ni–Co(cv) sample, the peak at 533.4 eV corresponds to the oxygen-based species of surface-adsorbed H2O.24 The peaks at 710.6 and 725.1 eV in the Fe 2p spectrum are ascribed to the Fe2+ 2p3/2 and Fe2+ 2p1/2 states, respectively.25 In addition, the peaks at 712.6 and 726.8 eV correspond to Fe3+ 2p3/2 and Fe3+ 2p1/2 states, respectively.25−27 In Figure 4e,f, the peaks at 780.1 and 795.2 eV are attributed to Co2+ 2p3/2 and Co2+ 2p1/2.27 In the case of Fe–Ni–Co(co), the Co3+ 2p3/2 and Co3+ 2p1/2 peaks appeared at 782.5 and 797.1 eV; however, in Fe–Ni–Co(cv), they were slightly shifted owing to surface oxidation, and peaks appeared at 785.1 and 800.5 eV.27 The peaks at 854.4 and 872.5 eV in the Ni 2p spectrum are attributed to Ni2+ 2p3/2 and Ni2+ 2p1/2. Furthermore, the peaks at 856.8 and 874.2 eV are associated with the Ni3+ 2p3/2 and Ni3+ 2p1/2 states.27−29 The peaks at approximately 860.0 and 877.1 eV are ascribed to the satellite peaks.
Figure 1.
SEM images of Fe–Ni–Co(co) (a, b) and Fe–Ni–Co(cv) (c, d).
Figure 2.

(a, b) TEM images of Fe–Ni–Co(cv). (c–f) Energy-dispersive X-ray spectroscopy (EDS) elemental mapping of Fe–Ni–Co(cv) sample.
Figure 3.
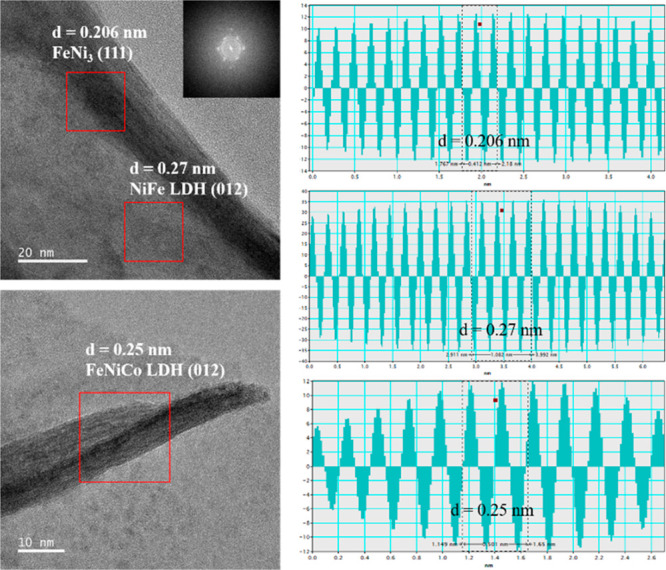
High-resolution transmission electron microscopy (HRTEM) images and d-spacing analyses of the Fe–Ni–Co(cv) catalyst.
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of Fe–Ni–Co(co) and Fe–Ni–Co(cv): (a, b) O 1s peak, (c, d) Fe 2p peak, (e, f) Co 2p peak, and (g, h) Ni 2p peak, (i) survey spectrum.
3.2. OER
The Fe–Ni–Co(cv) catalyst exhibited excellent catalytic performance in reactions with alkali solutions. Figure 5a shows the LSV curve of the prepared Fe–Ni–Co(cv) catalyst; it exhibits properties superior to those of the RuO2 catalyst. The overpotentials of the Fe–Ni–Co(cv), Fe–Ni–Co(cv), and RuO2 catalysts measured at 100 mA/cm2 were 302, 406, and 441 mV, respectively. Figure 5b shows a Tafel plot used to evaluate the reaction kinetics of each catalyst. The first step of the OER is the discharge step, in which the electrons adsorbed on the active site, and protons are combined to form adsorbed oxygen ions.30−32 Thereafter, the recombination step (Tafel reaction) or desorption step (Heyrovsky reaction) occurs.33,34 In the OER, the reaction rate is governed by the Tafel slope. Among the analyzed catalysts, the measured Tafel slope of Fe–Ni–Co(cv) was the lowest at 63 mV/dec, indicating that the catalytic reaction kinetics were the fastest. EIS analysis was performed to measure and substantiate the double-layer capacitance (Cdl) and charge-transfer resistance (Rct) in the CV analysis, disproving the effective surface area of the catalyst.35−38 The Nyquist plots of all samples are shown in Figure 5c, and the results show the lowest Rct (1.42 Ω) value for Fe–Ni–Co(cv). In addition, the stability evaluation of the Fe–Ni–Co(cv) catalyst shown in Figure 5d indicates that it has excellent durability for more than 48 h at high current density (200 mA/cm2). Figure 6a shows CV measurements for all samples at different scan rates (4, 8, 12, 16, and 20 mV/s) in the 0.1–0.2 V vs RHE scale region. The electrochemical surface area (ECSA) can be calculated using eq 2, and it is directly proportional to Cdl because Cs represents a constant theoretical value of the material.39−42
| 2 |
Figure 5.

(a) LSV curves of Fe–Ni–Co(cv), Fe–Ni–Co(co), and commercial RuO2 catalyst. (b) Corresponding Tafel plots. (c) Corresponding Nyquist plots. (d) Stability test for Fe–Ni–Co(cv) catalyst.
Figure 6.

(a) Scan-rate dependence of the current densities of Fe–Ni–Co(cv), Fe–Ni–Co(co), and RuO2 catalyst. (b) Comparison of OER properties before and after stability test of Fe–Ni–Co(cv) and Fe–Ni–Co(co) catalysts.
Fe–Ni–Co(cv) has a Cdl value of 33.78 mF/cm2, which is higher than those of Fe–Ni–Co(co) (22.87 mF/cm2) and RuO2 (4.78 mF/cm2), indicating the highest catalytic activity (Figure S4). As a result of ECSA analysis, it was confirmed that the surface area and active site exposure of the Fe–Ni–Co(cv) sample were higher than that of the RuO2 and Fe–Ni–Co(co) samples. Figure 6b shows a comparison of the LSV curves before and after the stability test. In the case of Fe–Ni–Co(cv), the overpotential before and after the test showed a minimal difference of less than 0.5%, whereas in the case of Fe–Ni–Co(co), a loss of more than 6% occurred. The results show that the amount of gas generated on the surface during the deposition process of the Fe–Ni–Co(cv) sample was reduced, preventing oxygen from entering the metal and improving the adhesion. The OER characteristics of the prepared catalyst were compared with other reported transition-metal-based catalysts, and the results was listed in Table 1.
Table 1. Summary of Research on Ni-, Fe-, and Co-Based Catalysts in Recent Years.
| Material | Overpotential at 10 mA/cm2 (mV) | Overpotential at 100 mA/cm2 (mV) | Tafel slope (mV/dec) | Ref |
|---|---|---|---|---|
| Co Ni | 280 | 331 | 79 | (1) |
| Fe–Co–Ni | 280 | 409 | 86 | (3) |
| Fe–Co–B | 228 | 65 | (7) | |
| NiCoFe–LDH | 276 | 332 | 56 | (8) |
| FeCoNi alloy | 285 | 42 | (10) | |
| NiFeW | 249 | 79 | (12) | |
| NiFeOOH | 320 | 60 | (18) | |
| NiFeCo@CNS | 213 | 350 | 62 | (19) |
| NiCoFe nanosheet | 333 | 56 | (20) | |
| Fe–Ni–Co(cv) | ∼207 | 302 | 6 | this work |
4. Conclusions
In this study, Fe–Co–Ni catalyst was fabricated using a simple electrochemical deposition method. The catalysts prepared via constant-voltage deposition and CV deposition were compared. The CV deposition method was advantageous for achieving a uniform deposition because it reduced the amount of gas generated on the surface compared to constant-voltage deposition. In addition, because the use of the CV method prevented oxygen from penetrating the metal, the two catalysts showed a large difference in stability. The structure of the prepared sample provided a large amount of hydroxide, which acted as an intermediate for the OER and significantly reduced the overpotential. In addition, compared to single metal or bimetallic catalysts, the trimetallic catalyst has a discontinuous lattice and has an advantageous structure for OER due to increased exposure of surface defects due to the cation-exchange process. The measured overpotential of the catalyst was 302 mV at 100 mA/cm2, which indicated better catalytic properties than those of RuO2. The excellent OER performance in this paper is attributed to the structure with a large surface area of Fe–Ni–Co and the exposure of many active sites. In addition, it exhibits easy electron transport kinetics due to the synergistic effect between metal elements. This study demonstrates a method for preparing a catalyst with a high catalytic activity using a low-cost metal, which could be used in oxygen-based energy-conversion technologies.
Acknowledgments
This work was supported by the Gyeonggi Regional Research Center (GRRC) program of Gyeonggi Province (GRRC Sungkyunkwan 2017-B01) and the Korea Basic Science Institute (KBSI) National Research Facilities & Equipment Center (NFEC) grant funded by the Korean government (Ministry of Education) (2019R1A6C1010031).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06461.
SEM image according to applied voltage in manufacturing Fe–Ni–Co(co) catalyst, SEM image according to the number of repetitions in manufacturing Fe–Ni–Co(cv) catalyst, LSV curves for each condition, cyclic voltammograms for each catalyst, HER characterization of Fe–Ni–Co(cv) samples, ICP-MS analysis results (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Charles V.; Zhang X.; Yuan M.; Zhang K.; Cui K.; Zhang J.; Zhao T.; Li Y.; Liu Z.; Li B.; Zhang G. CoNi nano-alloy anchored on biomass-derived N-doped carbon frameworks for enhanced oxygen reduction and evolution reactions. Electrochim. Acta 2022, 402, 402. 10.1016/j.electacta.2021.139555. [DOI] [Google Scholar]
- Chae M.; Jung H. y.; Suh S. J. Fabrication of platinum-doped WS2 hollow spheres catalyst for high-efficient hydrogen evolution reaction. J. Appl. Electrochem. 2022, 52 (3), 499–507. 10.1007/s10800-021-01658-7. [DOI] [Google Scholar]
- Zhu S.; Lei J.; Wu S.; Liu L.; Chen T.; Yuan Y.; Ding C. Construction of Fe-Co-Ni-Sx/NF nanomaterial as bifunctional electrocatalysts for water splitting. Mater. Lett. 2022, 311, 131549. 10.1016/j.matlet.2021.131549. [DOI] [Google Scholar]
- Gu X.; Zheng S.; Huang X.; Yuan H.; Li J.; Kundu M.; Wang X. Hybrid Ni3S2-MoS2 nanowire arrays as a pH-universal catalyst for accelerating the hydrogen evolution reaction. Chem. Commun. 2020, 56 (16), 2471–2474. 10.1039/C9CC10090C. [DOI] [PubMed] [Google Scholar]
- Tian X.; Li X.; Duan S.; Du Y.; Liu T.; Fang Y.; Yang X. Room Temperature Benzofused Lactam Synthesis Enabled by Cobalt (III)-Catalyzed C (sp2)–H Amidation. Advanced Synthesis and Catalysis 2021, 363 (4), 1050–1058. 10.1002/adsc.202001254. [DOI] [Google Scholar]
- Liu J.; Choi H. J.; Meng L. Y.. A review of approaches for the design of high-performance metal/graphene electrocatalysts for fuel cell applications. Journal of Industrial and Engineering Chemistry; Korean Society of Industrial Engineering Chemistry, 2018; Vol. 64, pp 1–15. [Google Scholar]
- Yang P.; Li E.; Xiao F.; Zhou P.; Wang Y.; Tang W.; He P.; Jia B. Nanostructure Fe–Co–B/bacterial cellulose based carbon nanofibers: An extremely efficient electrocatalyst toward oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47 (26), 12953–12963. 10.1016/j.ijhydene.2022.02.053. [DOI] [Google Scholar]
- Wang Y.; Tao S.; Lin H.; Wang G.; Zhao K.; Cai R.; Yang S. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy 2021, 81, 105606. 10.1016/j.nanoen.2020.105606. [DOI] [Google Scholar]
- Chen J.; Zheng F.; Zhang S. J.; Fisher A.; Zhou Y.; Wang Z.; Sun S. G. Interfacial Interaction between FeOOH and Ni-Fe LDH to Modulate the Local Electronic Structure for Enhanced OER Electrocatalysis. ACS Catal. 2018, 8 (12), 11342–11351. 10.1021/acscatal.8b03489. [DOI] [Google Scholar]
- Zhang X.; Zhao Y.; Zhao Y.; Shi R.; Waterhouse G. I. N.; Zhang T. A Simple Synthetic Strategy toward Defect-Rich Porous Monolayer NiFe-Layered Double Hydroxide Nanosheets for Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2019, 9 (24), 1900881. 10.1002/aenm.201900881. [DOI] [Google Scholar]
- Guo R.; Wen H.; Zhang S.; Yu T.; He Y.; Ni Z.; You J. Anionic sulfur-modified FeNi-LDH at various Fe/Ni molar ratios for high-performance OER electrocatalysis. Mater. Lett. 2021, 285, 285. 10.1016/j.matlet.2020.129132. [DOI] [Google Scholar]
- Qin Y.; Wang F.; Shang J.; Iqbal M.; Han A.; Sun X.; Xu H.; Liu J. Ternary NiCoFe-layered double hydroxide hollow polyhedrons as highly efficient electrocatalysts for oxygen evolution reaction. Journal of Energy Chemistry 2020, 43, 104–107. 10.1016/j.jechem.2019.08.014. [DOI] [Google Scholar]
- Zhang L.; Lu P.; Luo Y.; Zheng J. Y.; Ma W.; Ding L. X.; Wang H. Graphene-quantum-dot-composited platinum nanotube arrays as a dual efficient electrocatalyst for the oxygen reduction reaction and methanol electro-oxidation. Journal of Materials Chemistry A 2021, 9 (15), 9609–9615. 10.1039/D0TA12418D. [DOI] [Google Scholar]
- Stevens M. B.; Enman L. J.; Korkus E. H.; Zaffran J.; Trang C. D. M.; Asbury J.; Kast M. G.; Toroker M. C.; Boettcher S. W. Ternary Ni-Co-Fe oxyhydroxide oxygen evolution catalysts: Intrinsic activity trends, electrical conductivity, and electronic band structure. Nano. Research 2019, 12 (9), 2288–2295. 10.1007/s12274-019-2391-y. [DOI] [Google Scholar]
- Xia J.; Huang K.; Yao Z.; Zhang B.; Li S.; Chen Z.; Wu F.; Wu J.; Huang Y. Ternary duplex FeCoNi alloy prepared by cathode plasma electrolytic deposition as a high-efficient electrocatalyst for oxygen evolution reaction. J. Alloys Compd. 2022, 891, 161934. 10.1016/j.jallcom.2021.161934. [DOI] [Google Scholar]
- Peng L.; Yang N.; Yang Y.; Wang Q.; Xie X.; Sun-Waterhouse D.; Waterhouse G. I. N. Atomic Cation-Vacancy Engineering of NiFe-Layered Double Hydroxides for Improved Activity and Stability towards the Oxygen Evolution Reaction. Angewandte Chemie - International Edition 2021, 60 (46), 24612–24619. 10.1002/anie.202109938. [DOI] [PubMed] [Google Scholar]
- Park H. K.; Ahn H.; Lee T. H.; Lee J. Y.; Lee M. G.; Lee S. A.; Jang H. W. Grain Boundaries Boost Oxygen Evolution Reaction in NiFe Electrocatalysts. Small Methods 2021, 5 (2), 2170003. 10.1002/smtd.202170003. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Song Z.; Li Z.; Han M.; Cheng Y.; Zheng Z. Standing NiFe LDH nanosheets on stainless steel fibers felt: A synergistic impact on the oxygen evolution reaction (OER) for the water splitting. Catal. Commun. 2022, 164, 164. 10.1016/j.catcom.2022.106425. [DOI] [Google Scholar]
- Guo P. F.; Yang Y.; Wang W. J.; Zhu B.; Wang W. T.; Wang Z. Y.; Wang J. L.; Wang K.; He Z. H.; Liu Z. T. Stable and active NiFeW layered double hydroxide for enhanced electrocatalytic oxygen evolution reaction. Chemical Engineering Journal 2021, 426, 130768. 10.1016/j.cej.2021.130768. [DOI] [Google Scholar]
- Lyu F.; Wang Q.; Choi S. M.; Yin Y.. Noble-Metal-Free Electrocatalysts for Oxygen Evolution. Small;Wiley-VCH Verlag, 2019; Vol. 15, Issue (1), . [DOI] [PubMed] [Google Scholar]
- Qi J.; Zhang W.; Cao R.. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Advanced Energy Materials; Wiley-VCH Verlag, 2018; Vol. 8, Issue (5), . [Google Scholar]
- Wang X.; Liu H.; Li M.; Li J.; Lu Y.; Wang L.; Wang Z.; Zhang X.; Ding X. Modulation of electronic structure and oxygen vacancies of perovskites SrCoO3-δ by sulfur doping enables highly active and stable oxygen evolution reaction. Electrochim. Acta 2021, 390, 138872. 10.1016/j.electacta.2021.138872. [DOI] [Google Scholar]
- Zhang Y.; Kizilkaya O.; Bilan H. K.; Kurtz R.; Podlaha E. J. Activity and Regeneration of Electrodeposited Fe-Ni-Co-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction. ACS Applied Energy Materials 2020, 3 (8), 7239–7245. 10.1021/acsaem.0c00985. [DOI] [Google Scholar]
- Chen C.; Tuo Y.; Lu Q.; Lu H.; Zhang S.; Zhou Y.; Zhang J.; Liu Z.; Kang Z.; Feng X.; Chen D. Hierarchical trimetallic Co-Ni-Fe oxides derived from core-shell structured metal-organic frameworks for highly efficient oxygen evolution reaction. Applied Catalysis B: Environmental 2021, 287, 119953. 10.1016/j.apcatb.2021.119953. [DOI] [Google Scholar]
- Kathale B. M.; Xiao H.; Yang S.; Yin H.; Yu T.; Zhou X.; Qian L.; Xiao J.; Lei P.; Li X. Fluoride mediated conversion of FeOOH into NiFeOOH for outstanding oxygen evolution reaction. Electrochim. Acta 2022, 406, 406. 10.1016/j.electacta.2022.139831. [DOI] [Google Scholar]
- Yaseen W.; Ullah N.; Xie M.; Yusuf B. A.; Xu Y.; Tong C.; Xie J. Ni-Fe-Co based mixed metal/metal-oxides nanoparticles encapsulated in ultrathin carbon nanosheets: A bifunctional electrocatalyst for overall water splitting. Surfaces and Interfaces 2021, 26, 26. 10.1016/j.surfin.2021.101361. [DOI] [Google Scholar]
- Hu H.-S.; Li Y.; Deng G.; Shao Y.-R.; Li K.-X.; Wang C.-B.; Feng Y.-Y. The importance of the iron valence state in NiCoFe nanosheet array catalysts for the oxygen evolution reaction. Inorganic Chemistry Frontiers 2021, 8 (3), 766–776. 10.1039/D0QI01179G. [DOI] [Google Scholar]
- Chandrasekaran P.; Nesakumar Jebakumar Immanuel Edison T.; Gopalakrishnan Sethuraman M. Electrocatalytic study of carbon dots/Nickel iron layered double hydroxide composite for oxygen evolution reaction in alkaline medium. Fuel 2022, 320, 123947. 10.1016/j.fuel.2022.123947. [DOI] [Google Scholar]
- Wang Y.; Zhu R.; Wang Z.; Huang Y.; Li Z. Cu induced formation of dendritic CoFeCu ternary alloys on Ni foam for efficient oxygen evolution reaction. J. Alloys Compd. 2021, 880, 160523. 10.1016/j.jallcom.2021.160523. [DOI] [Google Scholar]
- Zhou H.; Zhang H.; Lai C.; Wang H.; Hu J.; Ji S.; Lei L. Rapidly electrodeposited NiFe(OH)x as the catalyst for oxygen evolution reaction. Inorg. Chem. Commun. 2022, 139, 109350. 10.1016/j.inoche.2022.109350. [DOI] [Google Scholar]
- Huang F.; Yao B.; Huang Y.; Dong Z. L. NiFe layered double hydroxide nanosheet arrays for efficient oxygen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2022, 47 (51), 21725–21735. 10.1016/j.ijhydene.2022.04.296. [DOI] [Google Scholar]
- Kim C.; Kim S. H.; Lee S.; Kwon I.; Kim S.; Seok C.; Park Y. S.; Kim Y. Boosting overall water splitting by incorporating sulfur into NiFe (oxy) hydroxide. Journal of Energy Chemistry 2022, 64, 364–371. 10.1016/j.jechem.2021.04.067. [DOI] [Google Scholar]
- Wang S.; Xu B.; Huo W.; Feng H.; Zhou X.; Fang F.; Xie Z.; Shang J. K.; Jiang J. Efficient FeCoNiCuPd thin-film electrocatalyst for alkaline oxygen and hydrogen evolution reactions. Applied Catalysis B: Environmental 2022, 313, 121472. 10.1016/j.apcatb.2022.121472. [DOI] [Google Scholar]
- Wei Y.; Lv Y.; Guo B.; Gong J. Hierarchical molybdenum disulfide nanosheet arrays stemmed from nickel-cobalt layered double hydroxide/carbon cloth for highly-efficient hydrogen evolution reaction. Journal of Energy Chemistry 2021, 57, 587–592. 10.1016/j.jechem.2020.09.024. [DOI] [Google Scholar]
- Jung S. Y.; Kang S.; Kim K. M.; Mhin S.; Kim J. C.; Kim S. J.; Enkhtuvshin E.; Choi S.; Han H. S. Sulfur-incorporated nickel-iron layered double hydroxides for effective oxygen evolution reaction in seawater. Appl. Surf. Sci. 2021, 568, 150965. 10.1016/j.apsusc.2021.150965. [DOI] [Google Scholar]
- Zhang W.; Li D.; Zhang L.; She X.; Yang D.. NiFe-based nanostructures on nickel foam as highly efficiently electrocatalysts for oxygen and hydrogen evolution reactions. Journal of Energy Chemistry; Elsevier B.V, 2019; Vol. 39, pp 39–53. [Google Scholar]
- Rong M.; Mo Y.; Cao Z.; Ma X.; Wang S.; Zhong H. MoSe2 regulates Ce-doped NiFe layered double hydroxide for efficient oxygen evolution reaction: The increase of active sites. Int. J. Hydrogen Energy 2022, 47 (43), 18688–18699. 10.1016/j.ijhydene.2022.04.046. [DOI] [Google Scholar]
- Gu X.; Liu Z.; Li M.; Tian J.; Feng L. Surface structure regulation and evaluation of FeNi-based nanoparticles for oxygen evolution reaction. Applied Catalysis B: Environmental 2021, 297, 120462. 10.1016/j.apcatb.2021.120462. [DOI] [Google Scholar]
- Gebreslase G. A.; Martínez-Huerta M. V.; Lázaro M. J.. Recent progress on bimetallic NiCo and CoFe based electrocatalysts for alkaline oxygen evolution reaction: A review. Journal of Energy Chemistry; Elsevier B.V, 2022; Vol. 67, pp 101–137. [Google Scholar]
- Cao X.; Wang T.; Jiao L. Transition-Metal (Fe, Co, and Ni)-Based Nanofiber Electrocatalysts for Water Splitting. Advanced Fiber. Materials 2021, 3 (4), 210–228. 10.1007/s42765-021-00065-z. [DOI] [Google Scholar]
- Yang J.; Xuan H.; Yang J.; Meng L.; Wang J.; Liang X.; Li Y.; Han P. Metal-organic framework-derived FeS2/CoNiSe2 heterostructure nanosheets for highly-efficient oxygen evolution reaction. Appl. Surf. Sci. 2022, 578, 152016. 10.1016/j.apsusc.2021.152016. [DOI] [Google Scholar]
- Wang L.; Zhang L.; Ma W.; Wan H.; Zhang X.; Zhang X.; Zhou Z. In Situ Anchoring Massive Isolated Pt Atoms at Cationic Vacancies of α-NixFe1-x(OH)2 to Regulate the Electronic Structure for Overall Water Splitting. Adv. Funct. Mater. 2022, 32, 2203342. 10.1002/adfm.202203342. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




