Abstract
We previously reported that DNA vaccination was able to elicit cellular immune responses and partial protection against Chlamydia trachomatis infection. However, DNA immunization alone did not generate immune responses or protection as great as that induced by using live organisms. In this study, we evaluated the immunologic effects of a combinational vaccination approach using C. trachomatis mouse pneumonitis (MoPn) major outer membrane protein (MOMP) DNA priming followed by boosting with immune-stimulating complexes (ISCOM) of MOMP protein (MOMP ISCOM) for protection of BALB/c mice against MoPn lung infection. Substantially better protection to challenge infection was observed in mice given combinational vaccination compared with mice given MOMP ISCOM immunization alone, and the protection approximated that induced by live organisms. Enhanced protection was correlated with stronger delayed-type hypersensitivity, higher levels of gamma interferon production, and increased immunoglobulin A antibody responses in lung homogenates. The results indicate that DNA priming followed by ISCOM protein boosting may be useful in designing a fully protective chlamydial vaccine.
DNA vaccination has provided a new approach for prevention of a wide range of infectious diseases. Chlamydia trachomatis is a common cause of several sexually transmitted diseases, such as urethritis, cervicitis, and salpingitis, and is the causative agent of trachoma, the leading cause of preventable blindness worldwide (14, 19). Chlamydial genital infection is also an important risk factor for human immunodeficiency virus (HIV) transmission (5, 9). Clearly, a vaccine to prevent C. trachomatis infection would be highly desirable but has been difficult to develop. In general, an effective vaccine to prevent C. trachomatis infection should elicit strong T-cell responses and neutralizing mucosal antibody (1, 2, 7, 12, 15, 16, 20–22). We previously reported that C. trachomatis major outer membrane protein (MOMP) DNA immunization induced partial protection against C. trachomatis mouse pneumonitis (MoPn) lung infection, which was associated with Th1 cellular immune responses, variable and low serum antibody responses, and absent immunoglobulin A (IgA) antibody responses (23, 24).
DNA priming and protein boosting has been used for vaccination against HIV-1. Immunization with HIV-1 envelope (env) DNA followed by env DNA plus HIV Env protein induced high titers of neutralizing antibody and completely protected monkeys from infection after intravenous challenge with a chimeric HIV strain (10). Similar results were observed when mice of different genetic backgrounds (CBA, BALB/c, and C57BL/6) were primed with plasmid DNA encoding a sequence derived from the Plasmodium falciparum antigen Pf155/RESA and boosted with the recombinant malarial protein (4). In the current study, we immunized BALB/c mice with MOMP DNA followed by boosting with MOMP immune-stimulating complex (ISCOM) protein and characterized the resulting immune responses and protective efficacy against C. trachomatis MoPn lung challenge infection.
MATERIALS AND METHODS
Animals and organisms.
Female BALB/c mice (4 to 5 weeks old) were purchased from Charles River Canada (Saint Constant, Canada). All animals were maintained and used in strict accordance with the guidelines issued by the Canadian Council on Animal Care.
C. trachomatis MoPn biovar was grown in HeLa cells, and elementary bodies (EBs) were purified by step gradient density centrifugation and kept at −70°C as previously described (23).
Vaccination vectors and proteins.
The MOMP gene was amplified from C. trachomatis MoPn genomic DNA by PCR and cloned into eukaryotic expression plasmid pcDNA3 (Invitrogen, San Diego, Calif.) as described previously (23, 24). The MOMP gene-encoding plasmid was transferred by electroporation into Escherichia coli DH5α, which was grown in Luria-Bertani broth containing 100 μg of ampicillin per ml. The plasmid was extracted by a DNA purification system (Wizard Plus Maxiprep; Promega, Madison, Wis.), and the sequence of recombinant MOMP DNA was verified by PCR direct sequence analysis as described before (23). Purified plasmid was dissolved in saline at a concentration of 1 mg/ml. The DNA concentration was determined by spectrophotometry (DU-62; Beckman, Fullerton, Calif.) at 260 nm, and the size of the plasmid was compared with a DNA standard in an ethidium bromide-stained agarose gel.
To prepare MoPn MOMP, purified chlamydial EBs were extracted with 10 mM phosphate buffer (pH 7.4) in 1% Sarkosyl and 10 mM dithiothreitol (DTT). EBs were incubated at 37°C for 30 min, with occasional 20-s pulses in a sonicating waterbath. Following incubation, soluble and insoluble fractions were separated by centrifugation at 15,000 × g for 1 h at 20°C. The pellet (which comprises the outer membrane complexes) was extracted with 10 mM phosphate buffer (pH 7.4) containing 10 mM DTT and decanoyl-N-methyglucamide (Mega 10) and/or n-octylglucopyranoside at a total combined concentration of 1%. The suspended pellet was incubated at 37°C for 30 min with occasional 20-s pulses in a sonicating waterbath. Following incubation, soluble and insoluble fractions were separated by centrifugation at 150,000 × g for 1 h at 20°C. MOMP is the predominant protein component of the soluble fraction (>90%).
ISCOMs were prepared by diluting the MOMP solution to 0.2 mg/ml with 10 mM phosphate buffer (pH 6.8). Phosphatidylcholine and cholesterol were dissolved at 5 mg/ml each. Quil A was added to a concentration of 1 mg/ml. A 20% concentration of Mega 10 was added to bring the final concentration in the mixture to 1%. The mixture was rocked at 20 to 25°C overnight and then dialyzed at 20 to 25°C against three changes of 10 mM phosphate buffer (pH 6.8) for about 8 to 16 h per change. When prepared by this method, ISCOMs are uniform particles about 40 to 50 nm in diameter. Circular dichroism studies showed that the MOMP in the ISCOM exists in a predominantly β-sheet conformation (A. Murdin and K. Sokoll, unpublished data). For intramuscular immunization, ISCOMs containing 0.5 μg of MOMP were diluted to 100 μl with saline and injected into the quadriceps muscle group.
Immunization.
Female BALB/c mice (4 to 5 weeks old) were intramuscularly immunized with plasmid DNA on three occasions, 0, 2, and 4 weeks. For each immunization, a total of 200 μl of plasmid DNA (200 μg) was injected into the two quadriceps muscles (100 μg of DNA per injection site) with a 27-gauge needle. Negative control animals were injected intramuscularly with saline or with the blank plasmid vector lacking an inserted chlamydial gene. The mice primed with the plasmid DNA were boosted at week 6 with MOMP ISCOM. For protein boosting, 0.5 μg of MOMP ISCOM per mouse was injected into the quadriceps muscles. Where indicated, groups of mice were immunized intranasally with 1,000 inclusion-forming units (IFU) of viable MoPn EBs twice on two occasions at 2-week intervals. A total of 1,000 IFU of MoPn EBs was suspended in 40 μl of sucrose-phosphate-glutamate (SPG) buffer (160 g of sucrose, 0.415 g of KH2PO4, 0.976 g of NaHPO4, 0.576 g of glutamic acid diluted in 800 ml of water [pH 7.4 to 7.6]) and delivered onto the nostrils of mice following anesthesia with isoflurane (Aerrane; Fort Dodge Animal Health, Fort Dodge, Iowa).
Challenge infection and quantification of MoPn.
Mice were challenged intranasally with 103 IFU of MoPn in 40 μl 14 days after the last immunization as described before (22–24). The body weight of the mice was measured daily for 10 days following challenge infection. Mice were sacrificed, and their lungs were aseptically isolated and homogenized with a cell grinder in 4 ml of SPG, and the tissue homogenates were centrifuged at 500 × g for 10 min at 4°C to remove coarse tissue and debris. The supernatant was frozen at −70°C until tested. For assessment of MoPn growth, lung homogenates were inoculated onto HeLa-229 cells grown to confluence in flat-bottomed 96-well microtiter plates and pretreated with 100 μl of Hank's balanced salt solution containing 30 μg of EDTA per ml for 15 min. The monolayers were inoculated in triplicate with 50 μl of serially diluted supernatants. After 2 h of incubation at 37°C on a rocker platform, each well was supplemented with 200 μl of Eagle's minimal essential medium containing 10% fetal calf serum, 1.5 mg of cycloheximide per ml, and 12 μg of gentamicin per ml. Plates were incubated for 48 h at 37°C in CO2. The cell monolayers were fixed with absolute methanol and incubated with a Chlamydia genus-specific murine monoclonal antibody (MAb) followed by staining with goat anti-mouse IgG antibody conjugated to horseradish peroxidase to detect inclusion formation. The stained inclusions were developed with 4-chloro-1-naphthol (Sigma, St. Louis, Mo.) and H2O2. Inclusion number was counted under a light microscope, and chlamydial growth in each lung was calculated from the dilution titer of the original inoculum.
ELISA for antibody.
Chlamydia-specific IgG2a and IgG1 antibodies in the serum were determined with an alkaline phosphatase-based enzyme-linked immunosorbent assay (ELISA) as described previously (22, 24). Briefly, mice were bled from the tail 2 weeks following boosting. ELISA plates (Corning 25805; Corning Science Products, Corning, N.Y.) were coated with 50 μl of 105 IFU of MoPn EBs in bicarbonate buffer (0.05 M, pH 9.6) overnight at 4°C. Plates were blocked with 2% bovine serum albumin dissolved in phosphate-buffered saline (PBS) for 2 h at room temperature, and the plates were incubated with serially diluted mouse sera for 4 h at room temperature. After being washed four times with PBS-Tween 20, biotinylated goat anti-mouse IgG2a or goat anti-mouse IgG1 (Southern Biotechnology Associates Inc., Birmingham, Ala.) antibody was added to the wells and incubated overnight at 4°C. Alkaline phosphatase-conjugated streptavidin was added and incubated for 45 min at 37°C. After extensive washing, p-nitrophenyl phosphate was added to the plates, which were then read with a microplate reader at 405 nm.
Chlamydia-specific IgA antibody in extracts of lung homogenates was also tested by ELISA at day 10 after challenge infection. The lungs of mice were ground with a tissue homogenizer and centrifuged at 10,000 rpm. Anti-MoPn EB IgA antibody in the supernatant of the lung extracts was tested in an ELISA with biotin-labeled goat anti-mouse IgA (PharMingen, San Diego, Calif.).
MoPn-specific DTH.
Delayed-type hypersensitivity (DTH) was evaluated 6 weeks after the last immunization as previously described (23, 24). Briefly, 25 μl of UV-killed MoPn EBs (2 × 105 IFU) in SPG buffer were injected into one hind footpad of the mice, and the same volume of SPG buffer was injected into the opposite hind footpad as a control. Footpad swelling was measured at 48 and 72 h following injection using a dial-gauge calliper (Walter Stern 601; Fisher Scientific, Ottawa, Canada). The difference between the thickness of the two foot pads was used as a measure of the DTH response.
Cytokine ELISA.
Gamma interferon (IFN-γ) and interleukin-10 (IL-10) production by splenic mononuclear cells following specific antigen stimulation in vitro was determined as previously described (21, 22, 24). Briefly, a single-cell suspension of spleen cells was cultured at 5 × 106 cells/ml (2 ml/well) alone or with UV-killed MoPn (2 × 105 IFU/ml) in 24-well plates at 37°C in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum, 1% l-glutamine, and 5 × 10−5 M 2-mercaptoethanol. Culture supernatants were harvested at 72 and 96 h. IFN-γ and IL-10 in culture supernatants were detected by a sandwich ELISA with MAbs purchased from PharMingen as previously described (22).
Statistics.
All data were expressed as means ± standard errors. Student's t test was used for analysis of statistical significance (P value).
RESULTS
DTH response.
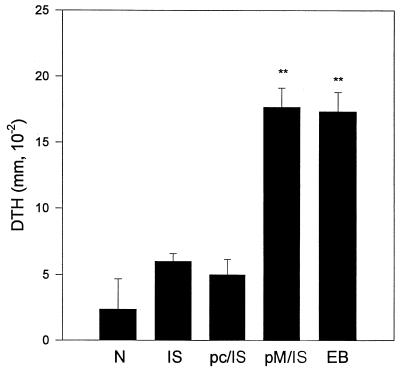
Previous work has shown that DTH reactions correlate with protective immunity to C. trachomatis MoPn infection in this model system (21–24). DTH responses are demonstrated by footpad swelling following UV-inactivated EB challenge among immunized mice. A strong DTH response was elicited in mice immunized with MOMP DNA followed by MOMP ISCOM boosting and was comparable in magnitude to that measured in mice immunized with viable EBs (Fig. 1). MOMP ISCOM alone or with pcDNA3 priming failed to induce significant DTH responses. Thus, mice immunized with protein alone did not show strong T-cell responses, and empty vector DNA priming (without the specific antigen gene) was unable to enhance the T-cell responses in mice immunized with MOMP ISCOM.
FIG. 1.
DTH response. BALB/c mice (three per group) were immunized three times with DNA (pc, vector pcDNA3, pM, MOMP gene in vector pcDNA3) intramuscularly at 2-week intervals or immunized once with live MoPn EBs (EB; 1,000 IFU) intranasally. Fourteen days after the third immunization, the DNA-immunized groups were boosted with MOMP ISCOM protein (pc/IS, pcDNA primed and MOMP ISCOM boosted; pM/IS, MOMP DNA primed and MOMP ISCOM boosted). N, naive mice as a control. All mice were injected with 25 μl of 2 × 104 IFU of UV-killed MoPn EBs in SPG into one hind footpad 14 days after boosting; 25 μl of SPG buffer was injected into the other hind footpad as a control. Footpad swelling was measured at 48 h following the injection. The difference in thickness between the two footpads was used as a measure of DTH. The data are means ± SEM. ∗∗, P < 0.01 compared with N, IS, and pc/IS.
Antibody response.
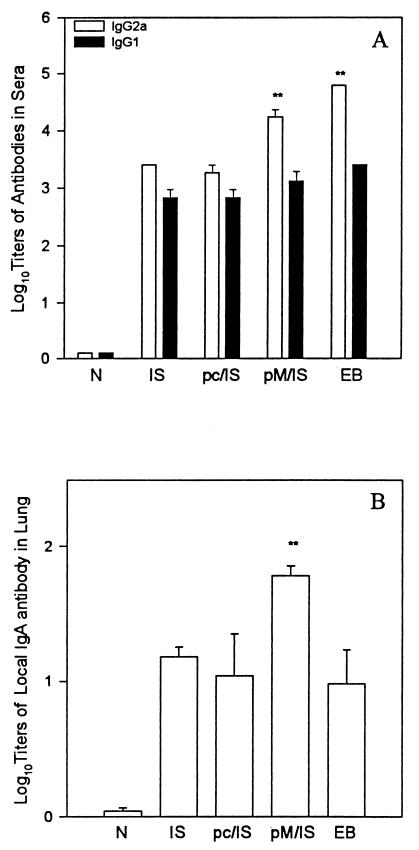
Serum antibody responses to MoPn EBs were tested by ELISA (Fig. 2A). MOMP ISCOM alone induced significant antibody responses, including IgG2a and IgG1 antibody isotypes. Antibody production was enhanced when MOMP ISCOM immunization was preceded by MOMP DNA priming. In particular, IgG2a levels in the MOMP DNA-primed/MOMP ISCOM-boosted group were significantly higher than those in the groups given MOMP ISCOM immunization alone or pcDNA3 priming and MOMP ISCOM boosting. The isotype-specific antibody data also suggest that Th1-biased immune responses to chlamydial antigens are enhanced when MOMP ISCOM immunization is preceded by MOMP DNA priming.
FIG. 2.
Antibody response against MoPn EBs. (A) Serum antibody. BALB/c mice (five per group) were immunized as described in the legend to Fig. 1. Serum samples were collected 2 weeks after boosting. MoPn-specific IgG2a and IgG1 in serum were tested by ELISA. An optical density of 0.5 was the cutoff point. The data are means ± SEM. ∗∗, P < 0.01 compared with N, IS, and pc/IS. (B) IgA antibody against MoPn EBs in extracts of mouse lung homogenates. BALB/c mice (five per group) were immunized as above. The mice were challenged with 1,000 IFU of MoPn EBs 14 days after the last immunization and sacrificed on day 10 postinfection. The lungs of the mice were ground and centrifuged at 10,000 rpm. IgA antibody in the supernatant of the lung extracts was tested by ELISA against MoPn EBs. ∗∗, P < 0.001, compared with ISCOM alone and P < 0.05 compared with pc/IS or EB.
We tested IgA levels to MoPn EBs in extracts of lung homogenates at day 10 after challenge infection in order to evaluate the effects of immunization on IgA antibody responses. IgA levels in the lungs (Fig. 2B) of the mice vaccinated with the MOMP DNA priming/MOMP ISCOM boosting protocol were significantly higher than those in the mice immunized with MOMP ISCOM alone, given pcDNA priming and MOMP ISCOM boosting, or immunized with MoPn EBs. The increased IgA levels in the lungs of MOMP DNA-primed/MOMP ISCOM-boosted mice indicate that MOMP DNA priming was able not only to enhance systemic Th1 cellular immune responses but also to increase IgA B-cell responses.
Cytokine production by spleen cells.
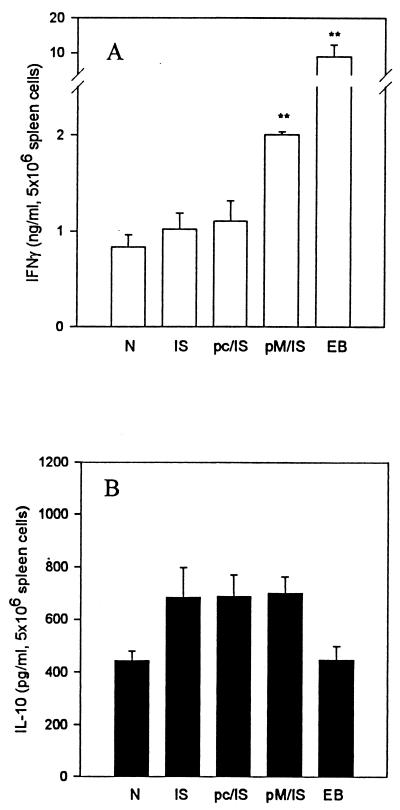
IFN-γ production plays an important role in DTH responses and in protection against C. trachomatis MoPn infection (20, 21). We therefore tested IFN-γ production by splenic lymphocytes in an antigen-specific fashion. When splenocytes were cultured with UV-inactivated MoPn EBs in vitro, IFN-γ and IL-10 were detected in the culture supernatants of all groups tested. Splenocytes from mice immunized with MOMP DNA priming followed by MOMP ISCOM boosting produced significantly higher levels of IFN-γ than did those from mice given MOMP ISCOM immunization alone or primed with the empty vector pcDNA3 (Fig. 3A). In contrast, IL-10 production was comparable among all immunized groups (Fig. 3B).
FIG. 3.
Cytokine production by antigen-stimulated spleen cell cultures. BALB/c mice (five per group) were immunized as described in the legend to Fig. 1 and killed 10 days after challenge infection with MoPn. Spleen cells were incubated with 2 × 105 IFU of UV-killed MoPn EBs in RPMI-1640 with 10% fetal calf serum and 5 × 10−5 M 2-mercaptoethanol in 5% CO2 at 37°C. The supernatant was collected after 96 h of incubation. IFN-γ (A) and IL-10 (B) in the supernatant were tested by ELISA. The data are means ± SEM. ∗∗, P < 0.01 compared with N, IS, and pc/IS.
Protective immunity.
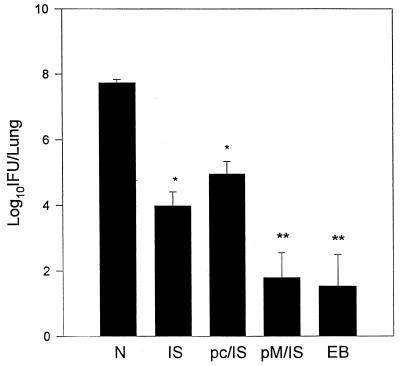
Protective efficacy was evaluated by challenging mice with 1,000 IFU of C. trachomatis MoPn via the respiratory route. A direct measurement of protection is determined by testing the in vivo growth of chlamydia following challenge infection. In this infection model, day 10 postchallenge is the time of peak chlamydial growth and thus was chosen for comparison of lung clearance among the various groups of mice (21). Partial protection from C. trachomatis infection was observed in the mice immunized with MOMP ISCOM (Fig. 4). Mice primed with MOMP DNA followed by MOMP ISCOM boosting had significantly fewer chlamydia organisms in the lung than those given MOMP ISCOM immunization alone. Importantly, the priming-boosting strategy approximated the level of protective immunity observed in the mice immunized with live chlamydial EBs. The enhancing effect of priming with DNA was specific to MOMP DNA because priming with the empty vector pcDNA3 failed to increase immunity among mice boosted with MOMP ISCOM.
FIG. 4.
Protection against MoPn infection. BALB/c mice were immunized as described in the legend to Fig. 1. The mice were challenged with 1,000 IFU of MoPn EBs 14 days after boosting and killed on day 10 postinfection. Chlamydial growth in the lungs was analyzed by quantitative tissue culture. The data are means ± SEM. ∗∗, P < 0.001 compared with N and P < 0.01 compared with IS and pc/IS; ∗, P < 0.01 compared with N; P < 0.01 when pM/IS is compared with IS or pc/IS.
DISCUSSION
A vaccine against C. trachomatis infection has proven difficult to develop. Early vaccine studies showed that whole inactivated bacterial cells administered intramuscularly were partially protective in human and primate trials. Primate vaccine trials and human experimental infection studies suggested that C. trachomatis immunity was in part strain specific, and the identification of MOMP as the strain-specific antigen of C. trachomatis quickly focused vaccine efforts on this protein. In general, use of MOMP or peptide epitopes derived from it as a vaccine has engendered variable or no protective immunity in a variety of animal model systems. The best results were observed when MOMP was used as a structurally intact molecule, suggesting that conformationally intact antigenic sites on the molecule are important for protective immunity.
We previously reported that MOMP DNA administered parenterally was also partially effective in inducing protective immunity against lung infection with MoPn. The result has been confirmed by some investigators and has been extended to protection against Chlamydia psittaci as well as Chlamydia pneumoniae infection (13, 17, 18). MOMP DNA vaccination was observed to induce variable protection from experiment to experiment, possibly due to the stochastic nature of cellular uptake and expression of the plasmid vector (Zhang et al., unpublished observations). Because DNA priming followed by protein boosting has been demonstrated to be a promising approach for vaccination against other pathogens, we evaluated this approach for C. trachomatis vaccine design. MOMP DNA was administered in a plasmid vector that we had used successfully before, and MOMP protein was incorporated into lipid vesicles as ISCOM. ISCOM was chosen as a delivery vehicle because of the likelihood that the MOMP would retain its conformational structure in the lipid bilayer of the ISCOM and because ISCOMs deliver antigen intracellularly (11).
The results reported in this study show that the strategy of MOMP DNA priming followed by MOMP ISCOM protein boosting is able to strongly enhance the immune responses and protection against C. trachomatis MoPn pulmonary infection. In particular, MOMP DNA priming followed by MOMP ISCOM boosting increased protection about 1,000-fold and virtually prevented infection in most of the mice. The magnitude of protection induced by this approach approximates that induced by viable EB immunization, the most efficient vaccination approach known for prevention of C. trachomatis infection (22). DTH responses, which correlate with protective T-cell responses, were also induced in primed-boosted animals and were much stronger than those elicited by MOMP ISCOM immunization alone. The results paralleled the MoPn-specific IFN-γ production by spleen cells. Given the extreme importance of IFN-γ in host defense against C. trachomatis infection, this response is particularly important (3, 6, 25). An interesting finding in this study is the enhancement of IgA antibody response against chlamydial EBs in the group immunized with MOMP DNA priming and MOMP ISCOM boosting. Since mucosal IgA is considered an important component to host defense against chlamydial infection, this result suggests an additional advantage to the combination vaccination approach. A previous study reported that IFN-γ receptor knockout mice failed to clear C. trachomatis infection despite a significant IgA response (8), but it may be that the coexistence of IFN-γ and IgA responses as induced by DNA priming-protein boosting is particularly important to protective immunity against chlamydial infection.
DNA immunization is known to preferentially activate T-cell responses (23, 24), and we speculate that sensitized T-cell clones are substantially expanded by MOMP ISCOM boosting. In this way, DNA priming promotes the initiation of a T-cell immune response, while protein boosting expands the specific response to chlamydial MOMP. Overall, the results suggest that MOMP DNA priming followed by MOMP ISCOM boosting efficiently enhanced humoral and cellular immune responses and protection against C. trachomatis MoPn infection.
In summary, a DNA priming-protein boosting protocol which has proven effective for other pathogens is also effective at eliciting stronger immune responses and protection against C. trachomatis lung infection. This approach may prove useful for chlamydial vaccine design.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada, Canadian Bacterial Diseases Network, and Aventis Pasteur.
We thank K. Sokoll for helpful discussions..
REFERENCES
- 1.Bavoil P M, Hsia R C, Rank R G. Prospects for a vaccine against chlamydia genital disease. I. Microbiology and pathogenesis. Bull Inst Pasteur. 1996;94:5. [Google Scholar]
- 2.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 3.Byrne G I, Lehmann J K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad D, Liljeqvist S, Stahl S, Hansson M, Perlmann P, Ahlborg N, Berzins K. Characterization of antibody response to a Plasmodium falciparum blood-stage antigen induced by a DNA prime/protein boost immunization protocol. Scand J Immunol. 1999;49:506–514. doi: 10.1046/j.1365-3083.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 5.Ho J L, He S, Hu A, Geng J, Basile F G, Almeida M G, Saito A Y, Laurence J, Johson W D., Jr Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-infected patient's mononuclear cells and cell line: an in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med. 1995;181:1493–1505. [PMC free article] [PubMed] [Google Scholar]
- 6.Igietseme J U. The molecular mechanism of T-cell control of chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Igietseme J U D, Magee M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by chlamydia-specific T lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson M, Schön K, Ward M, Lycke N. Genital tract infection with chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Batter V, Alary M. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morein B, Villacres-Eriksson M, Lovgren-Bengtsson K. ISCOM, a delivery system for parenteral and mucosal vaccination. Dev Biol Stand. 1998;92:33–39. [PubMed] [Google Scholar]
- 12.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdin, A. D., P. Dunn, R. Sodoyer, J. Wang, J. Caterini, R. C. Brunham, L. Aujame, and R. Oomen. 2000. Use of a mouse lung model to identify antigens protective against Chlamydia pneumoniae lung infection. J. Infect. Dis., in press. [DOI] [PubMed]
- 14.Schatcher J. Chlamydial infections. N Engl J Med. 1978;298:540–548. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- 15.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 16.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanrompay D, Cox E, Mast J, Goddeeris B, Volckaert G. High-level expression of Chlamydia psittaci major outer membrane protein in Cos cells and in skeletal muscles of turkeys. Infect Immun. 1998;66:5494–5550. doi: 10.1128/iai.66.11.5494-5500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanrompay D, Cox E, Vandenbussche F, Volckaert G, Goddeeris B. Protection of turkeys against Chlamydia psittaci challenge by gene gun-based DNA immunization. Vaccine. 1999;17:2628–2635. doi: 10.1016/s0264-410x(99)00053-5. [DOI] [PubMed] [Google Scholar]
- 19.West S K, Rapoza P, Munoz B, Katala S, Taylor H R. Epidemiology of ocular chlamydial infection in a trachoma-hyperendemic area. J Infect Dis. 1991;163:752–756. doi: 10.1093/infdis/163.4.752. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Brunham R C. T lymphocyte immunity in host defense against Chlamydia trachomatis and its implication for vaccine development. Can J Infect Dis. 1998;9:99–109. doi: 10.1155/1998/395297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Hay-Glass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-γ responses correlated with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]
- 22.Zhang D, Yang X, Lu H, Zhong G, Brunham R C. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlated with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production and with dendritic cell-like maturation. Infect Immun. 1999;67:1606–1613. doi: 10.1128/iai.67.4.1606-1613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D-J, Yang X, Berry J, Shen C, McClarty G, Brunham R C. DNA vaccination with the major outer-membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D-J, Yang X, Shen C, Brunham R C. Characterization of immune responses following intramuscular DNA immunization with the MOMP gene of Chlamydia trachomatis mouse pneumonitis strain. Immunology. 1999;96:314–321. doi: 10.1046/j.1365-2567.1999.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong G, Peterson E M, Czaniechi C W, Schreiber R D, de la Maza L M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infection. Infect Immun. 1989;57:152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]