Abstract
Introduction
Physical inactivity is common in asthma and is recognised as an important modifiable risk for poor clinical outcomes such as impaired asthma control and health-related quality of life (HRQoL). Despite evidence supporting the role of physical activity in reducing the risk of these outcomes, little is known about optimal interventions for increasing physical activity in those with severe disease. This systematic review and meta-analysis evaluates the effectiveness of interventions in increasing physical activity in severe asthma.
Methods
MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, Embase, PubMed, Informit, SPORTDiscus and Cochrane databases were searched up to September 2021 for physical activity-based intervention studies that assessed physical activity outcomes (e.g. steps per day, time spent undertaking physical activity) in adults with severe asthma. Data on asthma-related (e.g. asthma control) and health-related outcomes (e.g. HRQoL) were assessed as secondary outcomes. The revised Cochrane Risk of Bias tool was used to assess risk of bias. Random-effects meta-analyses synthesised data where possible.
Results
Four randomised controlled trials (all 12 weeks in duration) including 176 adults with moderate-to-severe asthma were included. An increase in physical activity was reported with a moderate-vigorous intensity aerobic and resistance training intervention (steps per day and time spent undertaking physical activity), and an unsupervised pedometer-based intervention (steps per day). Meta-analyses showed that physical activity interventions had an overall positive effect on steps per day (mean difference (MD) 1588, 95% CI 399–2778; p=0.009, I2=23), asthma control (MD −0.65, 95% CI −0.95–−0.35; p<0.0001, I2=0%) and HRQoL (MD 0.56, 95% CI 0.10–1.01; p=0.02, I2=16%) compared to control.
Conclusion
While there is some evidence supporting the effectiveness of interventions in improving physical activity in adults with severe asthma, higher-quality, large-scale studies of longer duration are needed to determine the optimal intervention.
Short abstract
The evidence regarding the effectiveness of interventions in improving physical activity, exercise capacity, asthma control and quality of life in adults with moderate-to-severe asthma is promising; however, further research in this area is needed https://bit.ly/3OJLcSM
Introduction
Asthma is a complex heterogeneous disease in which individual variability in clinical presentation, disease severity and therapeutic response is common [1, 2]. To add to this complexity, behaviours/risk factors and extrapulmonary comorbidities, also referred to as “treatable traits”, that are associated with adverse clinical outcomes are common in asthma [3–5]. Physical inactivity is one proposed treatable trait that is recognised as an important modifiable risk factor for impaired respiratory functioning, asthma control, quality of life (QoL) and mental health, increased disease severity and exacerbation risk and, ultimately, increased healthcare use [6]. Compared to the general population, people with asthma spend significantly less time undertaking moderate-to-vigorous physical activity (MVPA) and achieve fewer steps per day [7]. Physical inactivity is particularly evident in those with severe asthma [7, 8], who, despite constituting only 3–8% of the overall asthma population, are responsible for much of the economic, mortality and morbidity-related burden associated with this disease [9, 10]. Observational studies suggest that a fear of provoking asthma symptoms by participating in physical activity may in part explain these findings [11, 12], as individuals with increased disease severity are more likely to believe that exercise should be avoided [13]. However, interestingly, it has also been demonstrated that patients with severe asthma rate physical activity as one of the most important outcomes they want to achieve following any asthma treatment [14].
Previous reviews have not only demonstrated the safety of physical activity interventions in adults with asthma [15], but have also highlighted the promising role of physical activity as a nonpharmacological strategy to complement existing asthma management approaches [8, 15–17]; these reviews have focused on the general asthma population. Furthermore, while current asthma management guidelines recommend that people with asthma undertake physical activity [18], there are currently no clearly defined physical activity prescription guidelines for asthma, and particularly so for people with severe disease. In fact, the Global Initiative for Asthma (GINA) specifically states that there “is insufficient evidence to recommend one form of physical activity over another” [18].
Tyson et al. [19] published a systematic review examining the effects of interventions on physical activity, sedentary behaviour and health outcomes in people with asthma. However, as the intervention components were similar across all studies regardless of their effectiveness, a definitive conclusion regarding the optimal intervention for this population could not be drawn. In addition, Tyson et al. [19] excluded pulmonary rehabilitation interventions, which have been shown to improve physical activity levels in other chronic respiratory diseases [20], and included all asthma severities, which makes it difficult to determine if the findings are applicable to a severe asthma cohort who experience more disease burden. To our knowledge, the effectiveness of interventions in increasing physical activity in people with severe asthma explicitly has not yet been systematically reviewed. This systematic review and meta-analysis aimed to evaluate the effectiveness of interventions aiming to increase physical activity in adults with severe asthma on physical activity outcomes, and to explore the optimal type, intensity, duration and frequency needed to improve physical activity, as well as health- and asthma-related outcomes in severe asthma.
Methods
Protocol and registration
This review was guided by the Cochrane Handbook for Systematic Reviews of Interventions [21] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. It has been registered at www.crd.york.ac.uk/PROSPERO with identifier number CRD42021210968.
Data sources and search strategy
MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, Embase, PubMed (non-MEDLINE), Informit (Health Collection), Cochrane Library and SPORTDiscus databases were searched from inception to 10 September 2021 for relevant English-language publications. The search strategy covered all relevant terms relating to “physical activity”, “exercise” and “asthma”, and was adapted for each database (supplementary figures S1–S6). In addition, we performed hand-searching of reference lists and cited reference searches of the included articles.
Eligibility and exclusion criteria
Studies were included if they met the following a priori defined inclusion criteria:
Participants: adults aged ≥18 years with physician-diagnosed severe asthma defined according to the GINA criteria; asthma that remains uncontrolled despite adherence with maximised optimised therapy and the management of contributory factors, or that worsens when high-dose therapy is decreased [18]. Studies involving patients with moderate-to-severe asthma were also included.
Interventions: physical activity interventions of ≥2 weeks in duration, including walking, running, cycling, swimming or other aerobic and low-intensity exercise (i.e. yoga), weight-bearing exercise, pulmonary rehabilitation and interval training; interventions with a physical activity component aimed at increasing activity in daily life (including multi-arm trials where one arm is physical activity based); interventions utilising wearable technology (i.e. pedometers) to track or support/encourage physical activity; and movement-based interventions (supervised or unsupervised) with physical activity counselling.
Comparator/control: usual care or sham intervention (i.e. stretching or breathing exercises).
Outcomes: to be eligible, studies had to report on at least one physical activity outcome (i.e. time spent undertaking physical activity, steps per day) or sedentary time.
Study design: randomised controlled trials (RCTs), quasi-experimental RCTs, cohort, longitudinal, case–control, pilot or observational cross-sectional studies.
Studies involving patients with no asthma diagnosis, mild asthma, or moderate asthma only, non-English language publications, no control/comparison arm, review articles, notes, editorials, scientific congress abstracts and qualitative studies were excluded.
Study selection
Two independent reviewers (R.F. McLoughlin, V.L. Clark) performed the screening process against the a priori inclusion criteria using the web-based tool Covidence (www.covidence.org). Discrepancies were resolved by consensus, with persistent disagreements resolved by a third reviewer (V.M. McDonald).
Data extraction
Data extraction was performed by two independent reviewers (R.F. McLoughlin, P.D. Urroz) using customised data extraction templates in Covidence. Missing data were requested from study authors via email. To describe the profile of the included articles, the following data were extracted: authors, journal, year of publication, setting (country), study design, sample size, participant characteristics (i.e. age, sex, asthma severity), intervention details (i.e. the type of physical activity, mode of delivery, frequency, intensity and duration of sessions), control conditions, study duration and follow-up time points.
To examine the effect of the intervention on physical activity (primary outcome), values of the following outcome variables (i.e. mean, standard deviation and sample size in each group) observed at baseline, the end of the intervention period, and any post-intervention follow-ups were extracted: time spent undertaking physical activity (total and by physical activity intensity), steps per day and sedentary time.
Also extracted were data on asthma-related outcomes (i.e. health-related quality of life (HRQoL), asthma control, lung function, exacerbation rates, asthma symptom-free days, biomarkers of airway (i.e. sputum eosinophils/neutrophils, fractional exhaled nitric oxide (FeNO)) and systemic (i.e. serum C-reactive protein) inflammation), and health-related outcomes (i.e. body mass index (BMI), body composition, anxiety and depression scores, exercise capacity and skeletal muscle strength).
Assessment of risk of bias in included studies
Two reviewers (R.F. McLoughlin, P.D. Urroz) independently assessed risk of bias using the revised Cochrane Risk of Bias tool for RCTs (RoB2; 22 August 2019 version) [22], with discrepancies resolved through discussion. RoB2 is a result-based tool which is structured into five domains: randomisation process, deviations from intended interventions, missing outcome data, outcome measurement and selection of the reported result. Each domain was judged as “low risk”, “some concerns”, or “high risk”, resulting in an overall bias judgement for the specific result being assessed. Given the number of outcomes of interest in this review, we conducted risk of bias assessments (effect of assignment to the intervention) for results included in the meta-analyses only. The Cochrane robvis web-application was used to create risk-of-bias plots [23].
Method of analysis
Results of individual studies were tabulated (supplementary tables S1–S3). Meta-analysis was performed using Review Manager (RevMan; version 5.3). Effect sizes were expressed as mean differences (MD) with 95% confidence intervals estimated from the mean±sd post-intervention (or change) scores from each study. In the case of different scales for the same outcome measure (e.g. exercise capacity), the effect size was expressed as the standardised mean difference (SMD), with the magnitude classified as small (≤0.2), moderate (0.5) or large (≥0.8) [24]. When the standard deviation for an outcome was not reported, it was estimated from the 95% confidence interval related to the pertinent number of participants. Using generic inverse-variance analysis, we compared the pooled effect sizes for physical activity interventions versus control using random-effects models, which allows for anticipated differences in treatment effects between studies. Analyses were repeated using a fixed-effects model to test the robustness of our findings (supplementary table S4)
Heterogeneity was examined using the Chi-squared test (p<0.1 indicative of significant heterogeneity) and the I2 parameter (heterogeneity interpretation: low 0–40%; moderate 30–60%; substantial 50–90%; considerable 75–100%). Data that could not be assessed by meta-analyses were qualitatively summarised.
Results
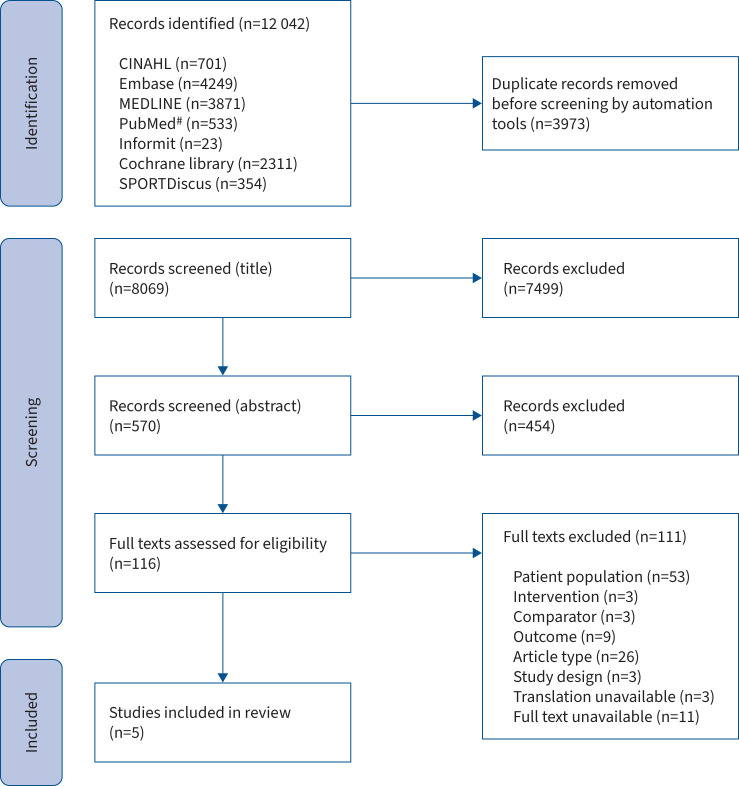
A total of 12 042 articles were identified, of which 3973 were duplicates (figure 1). Of the remaining 8069 articles, 7499 were excluded based on title and a further 454 based on abstract. 116 full-text articles were retrieved and assessed for eligibility, of which five met the a priori defined criteria for inclusion in the review. The main reason for full-text exclusion was incorrect patient population (i.e. participants with mild asthma, or asthma severity not specified), followed by incorrect article type.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of articles for inclusion. CINAHL: Cumulative Index to Nursing and Allied Health Literature. #: non-MEDLINE search.
Study and participant characteristics
Four RCTs which gave rise to five publications were identified [25–29]. Characteristics of the included RCTs are summarised in table 1. Three studies were two-arm parallel-group RCTs, and one was a three-arm parallel-group RCT comparing two interventions (high-intensity pulmonary rehabilitation only or high-intensity pulmonary rehabilitation with an e-health self-management support programme (PR+SMS)) to a single control group. All RCTs were 12-weeks in duration, with post-intervention follow-ups conducted in all but one study [26]. Coelho et al. [25] reported outcomes at 24–28 weeks post-randomisation, while Türk et al. [29] conducted assessments every 3 months for a year. Freitas and co-workers [27, 28] conducted follow-up assessments of body weight only at 6 and 12 months post-randomisation. Three studies were conducted in Brazil [25–28], and one in the Netherlands [29]. All studies were published in the past 5 years.
TABLE 1.
Characteristics of included studies
| First author, year [reference] (duration, design, country) | Intervention participants | Control participants | Intervention characteristics # | Control conditions |
| Freitas, 2017/2018 [27, 28]¶ (12 weeks, 2-arm p-RCT, Brazil) | n=26 moderate-to-severe asthma; age 45.9±7.7 years; female 96%; BMI 38.1±2.8 kg·m−2 | n=25 moderate-to-severe asthma; age 48.5±9.6 years; female 100%; BMI 37.2±2.1 kg·m−2 | F: 2 sessions per week I: 50–75% of peak V′O2 T: 60 min per session T: aerobic and resistance training M: supervised, individual sessions |
Sham exercise (stretching and breathing) |
| Coelho, 2018 [26] (12 weeks, 2-arm p-RCT, Brazil) | n=20 moderate-to-severe asthma; age 45.0±19.0 years; female: 90%; BMI 27.1±6.5 kg·m−2 | n=17 moderate-to-severe asthma; age 47.0±14.0 years; female 82%; BMI 30.3±7.4 kg·m−2 | F: 5 sessions per week (minimum) I: moderate T: 30 min per session (minimum) T: pedometer-based programme (daily step targets calculated bi-weekly; average daily steps over the previous week plus 1000 steps) M: unsupervised, individualised step-based PA prescription, encouraged to walk at moderate intensity ≥5 days per week for ≥30 min·day−1 |
Participants encouraged to walk at moderate intensity ≥5 days per week for ≥30 min per day |
| Evaristo, 2020 [27] (12 weeks, 2-arm p-RCT, Brazil) | n=29 moderate-to-severe asthma; age 49.8±9.7 years; female 76%; BMI 28.4±3.2 kg·m−2 | n=25 moderate-to-severe asthma; age 50.6±9.2 years; female 68%; BMI 27.5±4.7 kg·m−2 | F: 2 sessions per week I: 60–80% heart rate recovery T: 49 min per session T: aerobic training M: supervised, group sessions of 4–7 participants |
Breathing exercise programme (based on pranayama yoga breathing technique) |
| Türk, 2020 [30] (12 weeks, 3-arm p-RCT, the Netherlands) | PR only: n=14 moderate-to-severe asthma; age 41.6±9.7 years; female 71%; BMI 36.7±4.8 kg·m−2 PR+SMS: n=7 moderate–severe asthma; age 41.6±12.5 years; female: 57%; BMI 36.8±5.0 kg·m−2 |
n=10 moderate-to-severe asthma; age 41.9±8.6 years; female 90%; BMI 35.2±3.9 kg·m−2 | F: 3 sessions per week I: 90% of V′O2 max T: 40–60 min per session T: HIIT (body weight exercises) M: supervised, individual sessions |
Participants were encouraged to lose weight and exercise |
p-RCT: parallel-group randomised controlled trial; BMI: body mass index; V′O2: oxygen uptake; PA: physical activity; PR: pulmonary rehabilitation; SMS: internet-based self-management programme; HIIT: high-intensity interval training. #: physical activity/exercise components of the interventions are summarised according to the mode of delivery of the physical activity/exercise intervention (M) and the FITT principles: frequency of sessions (F), intensity of sessions (I), duration of each session (T), type of physical activity/exercise prescribed (T); ¶: Freitas et al. (2017) [27] and Freitas et al. (2018) [28] report different outcomes from the same RCT.
Overall, the four RCTs involved 176 physically inactive adults with moderate-to-severe asthma (sample size ranged from 37 to 54). No studies included participants with severe asthma only. Most participants were female (82%) and had comorbid overweight/obesity (BMI >25 kg·m−2; mean BMI ranged from 27.1 to 38.1 kg·m−2). Mean age of participants ranged from 41.6 to 50.6 years.
Intervention characteristics
Interventions varied methodologically in terms of the mode of delivery, frequency, intensity, duration and type of physical activity, as summarised in table 1. Freitas and co-workers [27, 28] used a supervised training program comprised of aerobic and resistance exercises and physical activity recommendations; Evaristo et al. [26] studied the effect of supervised aerobic exercise only; and both intervention arms of the RCT by Türk et al. [29] incorporated supervised high-intensity interval training (HITT) into a pulmonary rehabilitation programme. Coelho et al. [25] was the only study to examine the effect of an unsupervised intervention on physical activity. This study used a pedometer-based physical activity programme, whereby participants were prescribed individualised daily step targets calculated biweekly and were encouraged to walk at moderate intensity for 30 min ≥5 days per week.
Frequency of physical activity sessions ranged from two to five per week and were 30–60 min in duration. The intensity of the prescribed physical activity ranged from MVPA (based on 50–75% of peak oxygen uptake (V′O2) [27, 28]) to high-intensity physical activity (based on 90% of V′O2max or a score of ≥7 on the 10-grade Borg scale) [29].
In addition to the physical activity component, all studies included some form of nutritional [27–29], educational [25–28] and/or behavioural change programme [27–29]. In the study by Freitas and co-workers [27, 28], all participants, regardless of their group allocation, received a weight-loss programme (individualised hypocaloric diet supported by nutrition counselling and behaviour change techniques) and 6 h of education focusing on asthma management, the benefits of physical activity and the current physical activity recommendations. Coelho et al. [25] also provided all participants with a 1-h education session on asthma management and the benefits of exercise/physical activity, while Evaristo et al. [26] implemented a 4-h educational programme on asthma management only. Nutritional intervention and psychological sessions focusing on behavioural change and motivation strategies were provided to participants in both intervention arms (pulmonary rehabilitation only and PR+SMS) in the study by Türk et al. [29]. In addition, the PR+SMS group received the e-health self-management programme “PatientCoach”, which facilitated goal setting and provided participants with tailored information, self-management education modules and e-consultations with healthcare professionals [29].
Comparator characteristics
Comparator/control groups varied across studies. Freitas and co-workers [27, 28] used a sham intervention comprising stretching and breathing exercises; the control group in Evaristo et al. [26] received a breathing exercise programme; while control participants in both Türk et al. [29] and Coelho et al. [25] were provided with general advice/encouragement to exercise, which is consistent with usual care.
Risk-of-bias assessment
Risk-of-bias assessments were conducted for results in the following outcome categories: physical activity (steps per day), HRQoL (Asthma Quality of Life Questionnaire (AQLQ)), asthma control (Asthma Control Questionnaire (ACQ) score and asthma symptom-free days) and exercise capacity (V′O2). A summary of the risk-of-bias assessments for each outcome is presented alongside the respective meta-analysis forest plot (figures 2 and 3). There was either “some concern” or “high overall” risk of bias for all outcome measures. This was largely driven by concerns of bias arising from the measurements of the outcomes (some concerns in 69% of outcomes), as the nature of the interventions precluded the blinding of participants, which has the potential to impact patient-reported outcomes. This was of particular concern in the study by Coelho et al. [25], as participants were required to record their daily step counts measured using pedometers. In addition, there were some concerns of bias arising due to missing data (56% of outcomes) and due to deviations from intended interventions (63% of outcomes), as limited information was provided as to whether deviations arose due to trial context.
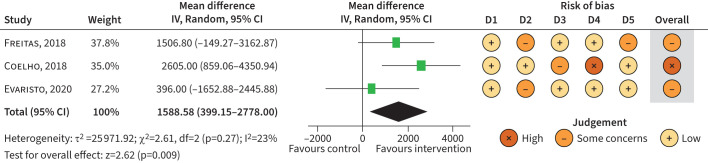
FIGURE 2.
Meta-analysis of randomised controlled trials examining the effect of physical activity interventions versus control on steps per day (post-intervention). IV: inverse variance; df: degrees of freedom; D1: bias arising from the randomisation process; D2: bias due to deviations from the intended intervention; D3: bias due to missing outcome data; D4: bias in measurement of the outcome; D5: bias in the selection of the reported result.
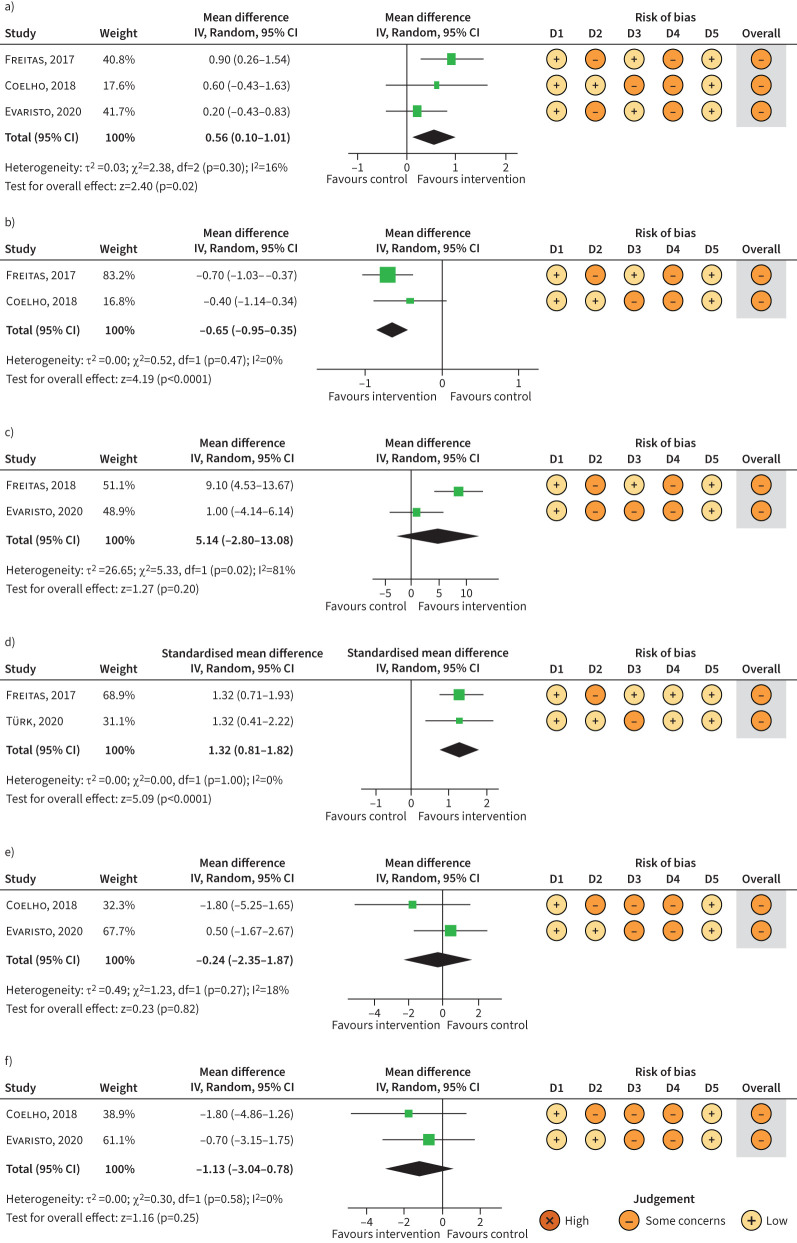
FIGURE 3.
Meta-analysis of randomised controlled trials examining the effect of physical activity interventions versus control on secondary outcomes of interest: a) asthma-related quality of life (Asthma Quality of Life Questionnaire) (post-intervention); b) asthma control (Asthma Control Questionnaire) (post-intervention); c) asthma control (asthma symptom-free days) (post-intervention); d) exercise capacity (oxygen uptake) (change from baseline); e) anxiety scores (post-intervention); and f) depression scores (post-intervention). IV: inverse variance; df: degrees of freedom; D1: bias arising from the randomisation process; D2: bias due to deviations from the intended intervention; D3: bias due to missing outcome data; D4: bias in measurement of the outcome; D5: bias in the selection of the reported result.
Physical activity outcomes
Physical activity outcomes were objectively measured using a device in all studies, three of which used accelerometers [26–29] and one used pedometers [25]. Outcomes included steps per day, which was measured in all studies [25–27, 29], physical activity level (PAL) [29] and time spent performing light-intensity physical activity and MVPA [28] (table 2). Only one study measured physical activity as the primary outcome [25].
TABLE 2.
Summary of physical activity, asthma-related and health-related outcomes reported in the intervention group compared to control in the included studies
| Freitas, 2017/2018 [28, 29] | Coelho, 2018 [26] | Evaristo, 2020 [27] | Türk, 2020 [30] | |
| Physical activity outcomes | ||||
| Daily step count (steps per day) | ↑ | ↑ | ↔ | ↔# |
| Light-intensity physical activity (min per day) | ↑ | |||
| MVPA (min per day) | ↑ | |||
| Sedentary time (min per day) | ↔ | |||
| Physical activity level | ↔ | |||
| Asthma-related outcomes | ||||
| HRQoL (AQLQ) | ↑ | ↔ | ↔ | ↔ |
| Asthma Control Questionnaire score | ↓ | ↔ | ↔ | ↔# |
| Lung function | ↑ | ↑ | ||
| Exacerbation (rates) | ↓ | ↔# | ||
| Airway inflammation | ↓ | ↔ | ↔ | |
| Systemic inflammation | ↓ | ↓ | ||
| Asthma symptom-free days | ↑ | ↔ | ||
| Health-related outcomes | ||||
| Weight | ↓ | ↓ | ||
| Body mass index | ↓ | ↓# | ||
| Waist circumference | ↓ | ↔ | ||
| Skeletal muscle strength | ↑ | |||
| Exercise capacity | ↑ | ↑ | ↔ | ↑# |
| Anxiety/depression scores | ↓¶ | ↔ | ↔ |
Blank cells indicate that the outcome was not measured. MVPA: moderate-to-vigorous physical activity; HRQoL: health-related quality of life; AQLQ: Asthma Quality of Life Questionnaire. ↑: significant increase in the intervention group compared to control; ↔: no significant difference between the intervention group and the control group; ↓: significant decrease in the intervention group compared to control. #: statistically significant positive effect in variable between intervention (pulmonary rehabilitation only group) and control at 12-months follow-up; ¶: significant increase in the proportion of participants without symptoms of depression in the intervention group compared to control.
Of the four included studies, two reported a significant beneficial effect on at least one physical activity outcome in favour of the intervention [25, 27, 28]. Freitas et al. [27] reported a greater increase in steps per day (p<0.0001), and time spent performing MVPA (p<0.001) and light-intensity physical activity (p=0.03) from baseline to post-intervention in the intervention group compared to control [28], with a higher percentage of participants in the intervention group achieving the recommendation of 10 000 steps per day (41.7% versus 4.3%, p=0.019). Coelho et al. [25] reported an increase in physical activity (steps per day) from baseline to post-intervention in participants who received a pedometer-based programme, with a difference of 2488 steps (average adjusted difference, p=0.005) reported between groups post-intervention. However, this increase in physical activity was not sustained after the intervention had ended, with the difference in steps per day between groups no longer significant 3–4 months post-intervention.
Türk et al. [29] reported no significant between-group differences in physical activity (steps per day) post-intervention. However, there was a significant within-group improvement in physical activity (steps per day and PAL) in the PR+SMS group from baseline to post-intervention. Additionally, 9 months after the intervention had ended, the pulmonary rehabilitation only group were reported to be undertaking significantly more steps per day than the control group [29]. Evaristo et al. [26] also reported no significant between-group difference in steps per day post-intervention, with both groups increasing their step count by 2000 steps per day, reaching the recommendation of 10 000 steps per day.
Random-effects meta-analysis (n=3) [25, 26, 28] showed an overall significant (z=2.63, p=0.009, I2=23; n=142) MD of 1588 (95% CI 399–2778) steps per day post-intervention between groups in favour of physical activity interventions (figure 2). However, the magnitude of effect differed by intervention type, ranging from small (SMD 0.1, 95% CI −0.43–0.64) in Evaristo et al. [26], to large (SMD 0.91, 95% CI 0.23–1.59) in Coelho et al. [25] (supplementary figure S7). Türk et al. [29] reported only change from baseline data, and therefore could not be included in the meta-analyses.
Asthma-related outcomes
The effect of physical activity on asthma-related outcomes was reported in the included studies (table 2).
Health-related quality of life
Asthma-related QoL was measured in all four studies using the AQLQ. While only one study reported a significant improvement following physical activity compared to control, random-effects meta-analysis (n=3) [25–27] demonstrated an overall positive effect of physical activity on asthma-related QoL post-intervention (MD 0.56, 95% CI 0.10–1.01; n=142, p=0.02, I2=16%) (figure 3a). However, the proportion of participants that had a clinically significant improvement in AQLQ score (≥0.5 units) did not significantly differ between the intervention and control group in any of the included studies.
Asthma control
A variety of asthma control outcome measures were reported: four studies used the ACQ [25–27, 29]; two reported the number of asthma symptom-free days [26, 28] and two reported exacerbation rates [27, 29]. Of the included studies, only Freitas and co-workers [27, 28] observed a significant improvement in asthma control measures (ACQ score, asthma symptom-free days and exacerbation rates) with physical activity compared to control. In addition, a higher percentage of participants in the intervention group (69%) achieved a clinically significant improvement in ACQ score (defined as a change or decrease in ACQ score of >0.5 points) post-intervention compared to control (36%, p=0.03) [27], with the percentage of participants who experienced no exacerbations during follow-up higher in the intervention group (53% versus 20%, p=0.03) [28]. Significant positive within-group changes in ACQ score were reported by Evaristo et al. [26] and Türk et al. [29] (in both intervention arms); between-groups differences were not significant in either study.
Random-effects meta-analysis (n=2) [25, 27] showed a significant positive treatment effect on ACQ score post-intervention in favour of physical activity (MD −0.65, 95% CI −0.95–−0.35; n=88, p<0.0001, I2=0%) (figure 3b), with an overall nonsignificant (z=1.27, p=0.20, I2=83%) MD of 5.14 (95% CI −2.80–13.08) asthma symptom-free days in favour of physical activity (figure 3c).
Lung function
Two studies examined the effect of physical activity on lung function [27, 29]. Türk et al. [29] reported an improvement in percentage functional residual capacity and expiratory reserve volume (ERV) following pulmonary rehabilitation only compared to control, while Freitas et al. [27] reported significant improvements in forced expiratory volume in 1 s, forced vital capacity and ERV in the intervention group only. It was not possible to conduct meta-analyses.
Systemic and airway inflammation
Airway inflammation was examined in three studies [26, 27, 29], one of which reported a group-by-time interaction effect, with a decrease in FeNO observed in the intervention group only [27]. No significant within- or between-group differences in airway inflammation (FeNO, sputum neutrophils and eosinophils) were reported in the other two studies [26, 29]. Two studies reported a significant reduction in systemic inflammation in the intervention group compared to control [27, 29].
Health-related outcomes
A summary of health-related outcomes reported in the included studies are presented in table 2.
Anthropometrics and body composition
Two studies examined the effect of physical activity on anthropometric measures and body composition, both of which had a focus on weight loss in addition to improving physical activity [27, 29]. Both studies reported greater reductions in body weight, BMI and fat mass in participants who received the intervention compared to control [27, 29]. In addition, Freitas et al. [27] reported a reduction in waist circumference, while preserving lean muscle mass.
Skeletal muscle strength
Freitas et al. [27] reported significant improvements in skeletal muscle strength in participants who received a combined aerobic and resistance training programme compared to control.
Exercise capacity
Various methods were used to measure exercise capacity (i.e. 6-min walk test [25, 29], incremental shuttle walk test [26], work rate [27] and the cardiopulmonary exercise test [27]). Freitas et al. [27], Coelho et al. [25] and Türk et al. [29] reported a significant improvement in exercise capacity in the intervention group compared to control, while no significant group effect was reported by Evaristo et al. [26]. The random-effects model (n=2) [27, 29] showed a large positive effect of physical activity intervention on exercise capacity (V′O2max) (SMD 1.32, 95% CI 0.81–1.82; n=75, p<0.00001, I2=0) (figure 3d).
Anxiety and depression
Three studies examined the effect of physical activity on anxiety and depression measured using the Hospital Anxiety and Depression Scale (HADS) [25, 26, 28], one of which reported the proportion of participants in each group with symptoms of anxiety (HADS-A score >9) or depression (HADS-D score >9) [28], with the other two reporting anxiety and depression scores [25, 26]. In the study by Freitas et al. [28], while there was a significant increase in the proportion of participants without symptoms of depression in the intervention group compared to control post-intervention, there was no significant difference in the proportion of participants with symptoms of anxiety between groups. No within- or between-group changes in anxiety or depression scores were reported in the studies by Coelho et al. [25] and Evaristo et al. [26], and the random-effects meta-analyses (n=2) [25, 26] showed no significant mean differences in anxiety (MD −0.24, 95% CI −2.35–1.87; n=91, p=0.82, I2=18) (figure 3e) or depression scores (MD −1.13, 95% CI −3.04–0.78; n=91, p=0.25, I2=0) between groups post-intervention (figure 3f).
Discussion
This review identified four unique studies examining the effectiveness of physical activity interventions in increasing physical activity outcomes in people with moderate-to-severe asthma [25–29]. While this systematic review and meta-analysis provides promising evidence regarding the potential of physical activity interventions to increase physical activity and improve asthma control, HRQoL and exercise capacity in those with moderate-to-severe disease, there is insufficient evidence to draw a definitive conclusion regarding the optimal physical activity prescription. This paucity of evidence highlights an important research gap that needs to be addressed in order to inform the development of asthma-specific physical activity guidelines.
Of the identified studies only two reported a significant positive effect on physical activity in favour of the intervention compared to control [25, 27, 28], one of which used an unsupervised pedometer-based programme, while the other used a supervised aerobic and resistance training programme. Given the methodological heterogeneity between these studies regarding the physical activity type, mode of delivery, frequency, intensity, duration of sessions and study outcome type reported (i.e. post-intervention versus change-from-baseline data), direct comparison of the effectiveness of these interventions is difficult. However, the effect size of the interventions was different, suggesting that physical activity prescription may be a crucial factor. Nonetheless, these studies provide preliminary evidence regarding the types of interventions that may be most effective in increasing physical activity in this population.
Having a step goal has been identified as an important predictor of increased physical activity [30], with pedometer-based interventions effective in achieving both short- and long-term physical activity increases in the general population [31]. In Coelho et al. [25], individuals with moderate-to-severe asthma who received a 12-week unsupervised pedometer-based programme significantly increased their daily step count compared to those who were encouraged to take daily 30-min walks only. While this provides evidence regarding the short-term benefits of a step-based intervention in this population, between-group differences in physical activity were no longer significant 12–16 weeks post-intervention, indicating that the intervention did not lead to a sustained improvement in physical activity behaviour. These findings mirror the evidence for the short- [32] versus long-term effectiveness [33] of pedometer-based interventions in increasing physical activity in the COPD population. In COPD, it has been suggested that multidimensional and possibly repeated intervention targeting not only physical activity but also exercise capacity and HRQoL may be needed to achieve sustained improvements in physical activity [33]. Whether a similar approach is required in the severe asthma population warrants further investigation.
Conversely, Freitas and co-workers [27, 28] demonstrated the short-term effectiveness of supervised exercise training (two 60-min sessions of moderate-vigorous intensity aerobic and resistance exercises per week) combined with a weight-loss programme, in increasing physical activity (steps per day and time spent undertaking MVPA and light-intensity physical activity) in obese adults with moderate-to-severe asthma compared to a weight-loss programme alone. This is consistent with the findings of the recent review by Tyson et al. [19]. In this review, effective physical activity interventions in the general asthma population typically comprised aerobic exercise and/or resistance/strength training of 30–60 min in duration, two to three times a week [8]. Although not significant compared to control, Evaristo et al. [26] also demonstrated that aerobic training alone can increase physical activity in individuals with moderate-to-severe asthma. In this study, both the intervention and the control group, who received breathing exercises, increased their step count by 2000 steps per day, reaching the recommendation of 10 000 steps per day. There is evidence that breathing exercises improve both symptom management and ventilatory control [34], which may subsequently increase an individual's confidence in undertaking physical activity. Indeed, in a study by Hiles et al. [35], individuals with severe asthma reported increased confidence and motivation to be active after receiving a yoga and mindfulness intervention which focused on controlling breathing, increasing movement and meditation. However, no increases in physical activity were reported following this intervention, which the authors suggest may have been due to the participants prioritising relaxation activities or displacing more active exercise with the low-intensity yoga [35]. Further studies are needed to explore the benefits of aerobic training for increasing physical activity in this population, with breathing exercises a potentially important intervention component to include.
The final study identified by this review examined the effects of a pulmonary rehabilitation programme using HIIT with and without an e-health self-management programme (PR+SMS versus pulmonary rehabilitation only), compared to usual care. Compared to lower intensity exercise that requires longer duration sessions, HIIT is less time consuming and has been suggested to be more effective in eliciting a physiological training response [36]. However, few studies using HIIT have been conducted in the asthma population due to concerns of provoking exercised induced bronchoconstriction [37]. While Türk et al. [29] demonstrated the feasibility of HIIT in individuals with moderate-to-severe asthma, no significant between-group changes in physical activity were observed after 12 weeks. However, there was a within-group improvement in daily steps and PAL in the PR+SMS group post-intervention, which was sustained post-intervention; providing some evidence for the use of HIIT to increase physical activity in this population. It is also interesting to note that at the 12-month post-randomisation visit, which was conducted 9 months after the intervention had ended, daily steps were significantly higher in the pulmonary rehabilitation only group compared to control [29]. It is unclear whether this increase in physical activity post-intervention was related to the pulmonary rehabilitation intervention; however, it could be related to the weight loss achieved in the intervention period, leading to an increase in physical activity participation post-intervention. Further research exploring the long-term benefits of HIIT is warranted.
The second aim of this review was to examine the effect of physical activity interventions on asthma- and health-related outcomes. The findings of our meta-analyses demonstrated an overall positive effect of physical activity interventions on exercise capacity, asthma control and HRQoL in individuals with moderate-to-severe asthma, which complements previous reviews [15, 16, 19]. Interestingly, of the two studies we identified that reported significant improvements in physical activity in favour of the intervention, only Freitas and co-workers [27, 28] reported a significant improvement in clinical outcomes. In contrast, Coelho et al. [25] reported no improvements in any of the clinical outcomes measured (asthma control, HRQoL or psychological parameters) following an unsupervised pedometer-based programme, despite achieving a greater mean difference in daily steps compared to control (MD 2605 versus MD 1506 in Freitas et al. [28]).
A possible explanation is that the intensity of the physical activity performed in the study by Coelho et al. [25] may not have been sufficient to induce a training effect. In COPD, it is suggested that a minimum intensity of 60% of the peak exercise capacity (moderate intensity) is needed to elicit a physiological training effect [38]. Although participants in the study by Coelho et al. [25] were advised to walk at moderate intensity, as this was unsupervised and exercise intensity was not assessed, it is unclear whether participants were adherent. Furthermore, the improvement in exercise capacity observed in Coelho et al. [25] (14.2 m improvement in 6-min walk distance), while statistically significant, did not reach clinical significance (minimal clinically important difference 26 m [39]). Improving cardiorespiratory fitness is one mechanism by which regular physical activity has been proposed to improve clinical outcomes in asthma [40–42]. Indeed, Freitas et al. [27] reported an association between improvements in asthma control and QoL and an improvement in cardiorespiratory fitness. It is also important to note that the intervention group in Freitas et al. [27] also received a weight-loss programme and achieved a significant reduction in weight compared to control, which likely contributed to these clinical improvements [43, 44]. Indeed, in a COPD population, targeting obesity using resistance training and caloric restriction has been shown to not only improve body weight and composition, but also clinical outcomes including health status, strength, dyspnoea and exercise and functional capacity [45]. Nonetheless, the findings from Coelho et al. [25] suggest that to achieve improvements in clinical asthma outcomes, it is important to consider not only the amount of physical activity, but the intensity at which it is undertaken.
While our meta-analysis demonstrated an overall positive effect of physical activity interventions on exercise capacity (V′O2), it is important to note that both studies included in this analysis used an intervention focused on weight loss in addition to improving physical activity [27, 29]. This may in part explain the effect observed as the outcome is often dependent on weight. In Türk et al. [29], while the pulmonary rehabilitation only group showed an improvement in exercise capacity (V′O2max), as well as a reduction in weight and BMI, change in BMI was not significantly associated with an improvement in V′O2max. This may indicate that an improvement in exercise capacity was achieved irrespective of the amount of weight loss. Furthermore, in the study by Freitas et al. [27], while both groups significantly reduced their weight, only the exercise group showed a significant improvement in V′O2, reported in both mL·kg−1·min−1 and mL·min−1. This suggests that improved exercise capacity was not dependent on weight, and that the exercise intervention was effective in improving exercise capacity.
People with severe asthma are less likely to participate in physical activity compared to healthy controls [7]. The reasons for physical inactivity in severe asthma are likely to be heterogeneous and complex. However, due to the adverse clinical implications of physical inactivity in severe asthma [6] and the observed benefits of targeting physical inactivity [15, 16, 19], there is an urgent need to develop interventions aimed at addressing this behaviour. Furthermore, in people with severe asthma, physical inactivity has been shown to cluster with obesity, increased sedentary time and increased symptoms of anxiety and/or depression [46]. Given the clustering of these characteristics, and the additive deleterious clinical impacts observed when they coexist in this population [46], the bundling of interventions that specifically target these characteristics has the potential to have a significant clinical impact. Further research exploring the benefits of multicomponent interventions targeting these other interrelated characteristics is warranted.
Strengths and limitations
Key strengths of this review include the use of a structured protocol, a robust search strategy and the involvement of two reviewers to independently conduct each step of the systematic review process. However, there are several potential limitations that warrant consideration. For instance, we included only English-language publications and therefore studies may have been missed, with publication bias also an important factor to consider. Another potential limitation is that we focused solely on physical activity-based interventions. Recent data indicate that comprehensive behaviour change intervention is also effective in increasing physical activity (steps per day and time spent undertaking MVPA) and improving asthma control, anxiety symptoms and sleep quality in adults with moderate-to-severe asthma [47]. This raises the possibility that behaviour change counselling alone may be sufficient in increasing physical activity in this population. Further research into the use of behaviour change counselling as either an alternative or complementary approach to physical activity-based interventions for increasing physical activity is warranted.
When interpreting the findings of this review, it is also important to consider the limitations of the included studies. Most studies were conducted in Brazil and involved primarily females with comorbid overweight or obesity, thus potentially limiting the generalisability of the findings. However, this high proportion of females is representative of the severe asthma population [48], with obesity also prevalent in this population [49]. Furthermore, although we acknowledge the inherent difficulty in blinding participants to physical activity interventions, risk of bias arising from deviations from intended interventions and from the measurement of patient-reported outcomes was identified to be of particular concern. Other common methodological limitations relate to the use of small sample sizes and short intervention periods, with no studies examining the maintenance of physical activity for >12 months. Nonetheless, these studies demonstrate the feasibility and short-term effectiveness of different physical activity interventions in this population and may help to inform larger scale and longer duration trials. Finally, confounding is an important issue to consider, as other intervention components (i.e. education/behaviour change techniques) and factors (i.e. weight reduction), may have contributed to the improvements in physical activity observed. However, it is difficult to ascertain whether weight reduction preceded the improvements in physical activity observed in the included studies. For instance, in Freitas et al. [27], while there was both a significant increase in physical activity outcomes (i.e. steps per day and time spent undertaking physical activity) and a reduction in body weight from baseline to post-intervention in the intervention group compared to control, it is difficult to determine which occurred first from the data provided. Conversely, in Türk et al. [29], while there was a significant decrease in body weight from baseline to post-intervention in the pulmonary rehabilitation only group compared to control, a significant difference in physical activity outcomes (steps per day) between groups was not observed until 9 months after the intervention had ended. Nonetheless, as severe asthma is a complex heterogeneous disease, it is possible that individualised, multicomponent interventions that combine physical activity prescription with interventions such as physical activity counselling, goal setting and self-monitoring [50, 51], and that address underlying barriers to physical activity such as obesity, are needed.
Conclusion
This review provides promising evidence regarding the feasibility and effectiveness of interventions in improving physical activity, exercise capacity, asthma control and HRQoL in adults with moderate-to-severe asthma. While we are unable to draw a definitive conclusion or offer specific recommendations regarding the optimal physical activity intervention for the severe asthma population specifically, interventions identified by this review showed that short-term improvements in physical activity outcomes regardless of the physical activity prescription used. This suggests that future studies should focus on the continued maintenance of physical activity for example via physical activity goals, such as step counts, or bundling other enabling interventions, such as behaviour change counselling.
Physical inactivity has a significant negative impact on people with asthma, particularly those with severe disease. There is therefore a critical need to develop, test and implement interventions that lead to sustained improvements in physical activity in this population, using high-quality, larger scale studies.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00546-2022.Supplement (584KB, pdf)
Shareable PDF
Footnotes
Author contributions: R.F. McLoughlin contributed to the protocol design, data review and analysis and manuscript preparation; V.L. Clark contributed to the protocol design, data review and analysis and manuscript preparation; P.D. Urroz contributed to the protocol design, data review and analysis and manuscript preparation; P.G. Gibson contributed to the protocol design, data review and manuscript preparation; V.M. McDonald conceived the research question, contributed to the protocol design, data review and manuscript preparation. All authors approved the final manuscript before submitting for publication.
This study is registered at www.crd.york.ac.uk/PROSPERO with identifier number CRD42021210968.
Data availability: Data underlying the findings described in this manuscript are available from the authors upon request.
Conflict of interest: R.F. McLoughlin, V.L. Clark and P.D. Urroz have nothing to disclose. P.G. Gibson reports lecture honoraria from GSK, outside the submitted work. V.M. McDonald reports grants from GSK, AstraZeneca, NHMRC, Ramaciotti Foundation, MRFF and JHH Charitable Trust, outside the submitted work; and also reports the following leadership roles: Co-Director NHMRC Centre of Research Excellence in Treatable Traits, Co-Director NHMRC Centre of Research Excellence in Severe Asthma, Co-Director Priority Research Centre for Healthy Lungs, Co-Director Virus, Vaccines, Immumology and Asthma HMRI programme, Head of Research, School of Nursing and Midwifery, University of Newcastle and COPD-X Guideline.
Support statement: This research was supported by the NHMRC Centre of Research Excellence in Asthma Treatable Traits, and the Hunter Medical Research Institute, Newcastle, Australia. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Gibson PG, McDonald VM, Marks GB. Asthma in the older adult. Lancet 2010; 374: 803–813. doi: 10.1016/S0140-6736(10)61087-2 [DOI] [PubMed] [Google Scholar]
- 2.Gibson PG, McDonald VM. Asthma–COPD overlap 2015: now we are six. Thorax 2015; 70: 683–691. doi: 10.1136/thoraxjnl-2014-206740 [DOI] [PubMed] [Google Scholar]
- 3.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Extrapulmonary associations of health status in severe asthma and bronchiectasis: comorbidities and functional outcomes. Respir Med 2019; 154: 93–101. doi: 10.1016/j.rmed.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 4.McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology 2019; 24: 37–47. doi: 10.1111/resp.13389 [DOI] [PubMed] [Google Scholar]
- 5.Hiles SA, Gibson PG, Agusti A, et al. Treatable traits that predict health status and treatment response in airway disease. J Allergy Clin Immunol Pract 2021; 9: 1255–1264. doi: 10.1016/j.jaip.2020.09.046 [DOI] [PubMed] [Google Scholar]
- 6.Avallone KM, McLeish AC. Asthma and aerobic exercise: a review of the empirical literature. J Asthma 2013; 50: 109–116. doi: 10.3109/02770903.2012.759963 [DOI] [PubMed] [Google Scholar]
- 7.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol 2018; 6: 814–822. doi: 10.1016/j.jaip.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 8.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. A systematic review of associations of physical activity and sedentary time with asthma outcomes. J Allergy Clin Immunol Pract 2018; 6: 1968–1981. doi: 10.1016/j.jaip.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 10.Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med 2018; 28: 31. doi: 10.1038/s41533-018-0098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons JP, Craig TJ, Stoloff SW, et al. Impact of exercise-related respiratory symptoms in adults with asthma: Exercise-Induced Bronchospasm Landmark National Survey. Allergy Asthma Proc 2011; 32: 431–437. doi: 10.2500/aap.2011.32.3501 [DOI] [PubMed] [Google Scholar]
- 13.Mancuso CA, Sayles W, Robbins L, et al. Barriers and facilitators to healthy physical activity in asthma patients. J Asthma 2006; 43: 137–143. doi: 10.1080/02770900500498584 [DOI] [PubMed] [Google Scholar]
- 14.Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res 2021; 7: 00497–2020. doi: 10.1183/23120541.00497-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuder MM, Clark M, Cooley C, et al. A systematic review of the effect of physical activity on asthma outcomes. J Allergy Clin Immunol Pract 2021; 9: 3407–3421. doi: 10.1016/j.jaip.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen ESH, Pitzner-Fabricius A, Toennesen LL, et al. Effect of aerobic exercise training on asthma in adults: a systematic review and meta-analysis. Eur Respir J 2020; 56: 2000146. doi: 10.1183/13993003.00146-2020 [DOI] [PubMed] [Google Scholar]
- 17.Panagiotou M, Koulouris NG, Rovina N. Physical activity: a missing link in asthma care. J Clin Med 2020; 9: 706. doi: 10.3390/jcm9030706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: www.ginasthma.org
- 19.Tyson L, Hardeman W, Marquette M, et al. A systematic review of the characteristics of interventions that promote physical activity in adults with asthma. J Health Psychol 2022; 27: 2777–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantoani LC, Rubio N, McKinstry B, et al. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J 2016; 48: 69–81. doi: 10.1183/13993003.01744-2015 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. 2021. Available from: www.training.cochrane.org/handbook
- 22.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 23.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12: 55–61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for Behavioural Sciences. 2nd Edn. Hillsdale, NJ, Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 25.Coelho CM, Reboredo MM, Valle FM, et al. Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: a randomized controlled trial. J Sports Sci 2018; 36: 1186–1193. doi: 10.1080/02640414.2017.1364402 [DOI] [PubMed] [Google Scholar]
- 26.Evaristo KB, Mendes FAR, Saccomani MG, et al. Effects of aerobic training versus breathing exercises on asthma control: a randomized trial. J Allergy Clin Immunol Pract 2020; 8: 2989–2996. doi: 10.1016/j.jaip.2020.06.042 [DOI] [PubMed] [Google Scholar]
- 27.Freitas PD, Ferreira PG, Silva AG, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 32–42. doi: 10.1164/rccm.201603-0446OC [DOI] [PubMed] [Google Scholar]
- 28.Freitas PD, Silva AG, Ferreira PG, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Med Sci Sports Exerc 2018; 50: 1367–1376. doi: 10.1249/MSS.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 29.Türk Y, Theel W, van Huisstede A, et al. Short-term and long-term effect of a high-intensity pulmonary rehabilitation programme in obese patients with asthma: a randomised controlled trial. Eur Respir J 2020; 56: 1901820. doi: 10.1183/13993003.01820-2019 [DOI] [PubMed] [Google Scholar]
- 30.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296–2304. doi: 10.1001/jama.298.19.2296 [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry UAR, Wahlich C, Fortescue R, et al. The effects of step-count monitoring interventions on physical activity: systematic review and meta-analysis of community-based randomised controlled trials in adults. Int J Behav Nutr Phys Act 2020; 17: 129. doi: 10.1186/s12966-020-01020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong M, Winnard A, Chynkiamis N, et al. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: a systematic review and meta-analysis. Eur Respir Rev 2019; 28: 190039. doi: 10.1183/16000617.0039-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohlbrenner D, Sievi NA, Senn O, et al. Long-term effects of pedometer-based physical activity coaching in severe COPD: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2020; 15: 2837–2846. doi: 10.2147/COPD.S279293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Bruton A. Breathing exercises for asthma. Breathe 2014; 10: 312–322. doi: 10.1183/20734735.008414 [DOI] [Google Scholar]
- 35.Hiles SA, Urroz PD, Gibson PG, et al. A feasibility randomised controlled trial of Novel Activity Management in severe ASthma-Tailored Exercise (NAMASTE): yoga and mindfulness. BMC Pulm Med 2021; 21: 71. doi: 10.1186/s12890-021-01436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012; 590: 1077–1084. doi: 10.1113/jphysiol.2011.224725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013; 187: 1016–1027. doi: 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- 38.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173: 1390–1413. doi: 10.1164/rccm.200508-1211ST [DOI] [PubMed] [Google Scholar]
- 39.Zampogna E, Ambrosino N, Centis R, et al. Minimal clinically important difference of the 6-min walking test in patients with asthma. Int J Tuberc Lung Dis 2021; 25: 215–221. doi: 10.5588/ijtld.20.0928 [DOI] [PubMed] [Google Scholar]
- 40.Andersen JR, Natvig GK, Aadland E, et al. Associations between health-related quality of life, cardiorespiratory fitness, muscle strength, physical activity and waist circumference in 10-year-old children: the ASK study. Qual Life Res 2017; 26: 3421–3428. doi: 10.1007/s11136-017-1634-1 [DOI] [PubMed] [Google Scholar]
- 41.Heikkinen SAM, Quansah R, Jaakkola JJK, et al. Effects of regular exercise on adult asthma. Eur J Epidemiol 2012; 27: 397–407. doi: 10.1007/s10654-012-9684-8 [DOI] [PubMed] [Google Scholar]
- 42.Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev 2013: CD001116. doi: 10.1002/14651858.CD001116.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okoniewski W, Lu KD, Forno E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc 2019; 16: 613–625. doi: 10.1513/AnnalsATS.201810-651SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juel CT-B, Ali Z, Nilas L, et al. Asthma and obesity: does weight loss improve asthma control? A systematic review. J Asthma Allergy 2012; 5: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald VM, Gibson PG, Scott HA, et al. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016; 21: 875–882. doi: 10.1111/resp.12746 [DOI] [PubMed] [Google Scholar]
- 46.Freitas PD, Xavier RF, McDonald VM, et al. Identification of asthma phenotypes based on extrapulmonary treatable traits. Eur Respir J 2021; 57: 2000240. doi: 10.1183/13993003.00240-2020 [DOI] [PubMed] [Google Scholar]
- 47.Freitas PD, Passos NFP, Carvalho-Pinto RM, et al. A behavior change intervention aimed at increasing physical activity improves clinical control in adults with asthma: a randomized controlled trial. Chest 2021; 159: 46–57. doi: 10.1016/j.chest.2020.08.2113 [DOI] [PubMed] [Google Scholar]
- 48.Postma DS. Gender differences in asthma development and progression. Gend Med 2007; 4: Suppl. B, S133–S146. doi: 10.1016/S1550-8579(07)80054-4 [DOI] [PubMed] [Google Scholar]
- 49.McLoughlin RF, McDonald VM. The management of extrapulmonary comorbidities and treatable traits; obesity, physical inactivity, anxiety, and depression, in adults with asthma. Front Allergy 2021; 2: 735030. doi: 10.3389/falgy.2021.735030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller-Riemenschneider F, Reinhold T, Nocon M, et al. Long-term effectiveness of interventions promoting physical activity: a systematic review. Prev Med 2008; 47: 354–368. doi: 10.1016/j.ypmed.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 51.Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis 2016; 11: 3121–3136. doi: 10.2147/COPD.S121263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00546-2022.Supplement (584KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00546-2022.Shareable (484.2KB, pdf)