Abstract
Objectives:
To define the incidence and characteristics of influenza-associated neurologic complications in a cohort of children hospitalized at a tertiary care pediatric hospital with laboratory-confirmed influenza, and to identify associated clinical, epidemiologic, and virologic factors.
Study design:
Historical cohort study of children 0.5-18.0 years-old hospitalized between 2010-2017 with laboratory-confirmed influenza. Children with immune compromise or a positive test due to recent receipt of live virus vaccine or recently resolved illness were excluded. Influenza-associated neurologic complications were defined as new-onset neurologic signs/symptoms during acute influenza illness without another clear etiology.
Results:
At least one influenza-associated neurologic complication was identified in 10.8% (95% CI 9.1-12.6%, n=131 of 1,217) of hospitalizations with laboratory-confirmed influenza. Seizures (n=97) and encephalopathy (n=44) were the most commonly identified influenza-associated neurologic complication, although an additional 20 hospitalizations had other influenza-associated neurologic complications. Hospitalizations with influenza-associated neurologic complications were similar in age and influenza type (A/B) to those without. Children with a pre-existing neurologic diagnosis (n=326) had a higher proportion of influenza-associated neurologic complications compared with those without (22.7% vs 6.4%, p<0.001). Presence of a pre-existing neurologic diagnosis (aOR 4.6, p<0.001), lack of seasonal influenza vaccination (aOR 1.6, p=0.020), and age ≤5 years (aOR 1.6, p=0.017) were independently associated with influenza-associated neurologic complications.
Conclusions:
Influenza-associated neurologic complications are common in children hospitalized with influenza, particularly those with pre-existing neurologic diagnoses. A better understanding of the epidemiology and factors associated with influenza-associated neurologic complications will direct future investigation into potential neuropathologic mechanisms and mitigating strategies. Vaccination is recommended and may help prevent influenza-associated neurologic complications in children.
Influenza is a major cause of acute respiratory tract illness in children, infecting approximately 10% of all children in the United States annually 1, and hospitalizing up to one in 140 children 2. Influenza-associated neurologic complications are common, occurring in up to 7-10% of children hospitalized with influenza 3,4. The most commonly reported influenza-associated neurologic complication is seizure, followed by influenza-associated encephalopathy or encephalitis 3,4. Other less common, but potentially more severe, influenza-associated neurologic complications include acute demyelinating syndromes, ischemic and hemorrhagic stroke, cerebral edema, Reye syndrome, and Guillain-Barré syndrome (GBS) 3-7. Precise estimates of the incidence, detailed clinical characterization, and risk factors associated with these influenza-associated neurologic complications have been difficult to determine due to small sample sizes, variable influenza types examined, and heterogeneous study designs 8. However, a better understanding of the spectrum of influenza-associated neurologic complications and associated features is critically important to develop testable hypotheses about neuropathogenesis, and design potential prevention and mitigation strategies.
We therefore aimed to define the frequency and characteristics of influenza-associated neurologic complications in children hospitalized at a tertiary care pediatric hospital with laboratory-confirmed influenza, and to identify associated clinical, epidemiologic, and virologic factors.
METHODS
Study Design and Setting
We performed an historical cohort study of children with laboratory-confirmed (polymerase chain reaction [PCR] positive) influenza hospitalized at the Children’s Hospital of Philadelphia (CHOP), a large academic tertiary care children’s hospital that had 466 beds and over 28,000 inpatient admissions in 2017. All data collection was approved by the CHOP institutional review board (12/20/17).
Data Collection
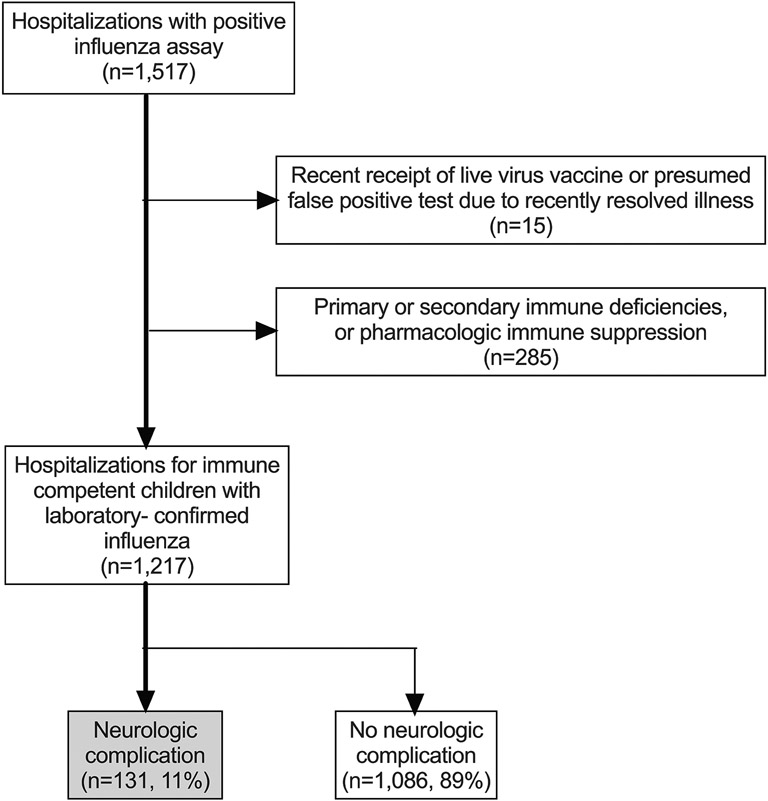
We queried the electronic medical record (EMR) for any child age 0.5-18.0 years hospitalized at CHOP between August 1, 2010, and June 30, 2017, with laboratory-confirmed influenza. Children less than 0.5 years old were not included because of a concern that they may have a unique set of risk factors for influenza-associated neurologic complications, given a relatively immature immune system, and that they may be hospitalized for reasons differing than those in older children (eg, observation for isolated fever). Some children were hospitalized with laboratory-confirmed influenza multiple times during this study time period, contributing multiple data points over time to the analysis. Medical record number, demographic, and clinical data were extracted from the EMR. We then manually reviewed the EMR to confirm accuracy of extracted data and to abstract additional clinical data onto a standardized collection form. Children with no symptoms of active infection but positive influenza testing due to a recent receipt of live vaccine or recently resolved illness during the same season were excluded. Children with documented primary and secondary immune deficiencies, and those on immunosuppressant medications (including chronic steroids), were excluded.
Study Definitions
Laboratory-confirmed influenza was defined by a positive PCR test performed at our institution. Multiplex PCR panel testing for influenza virus types A and B, and other respiratory viral pathogens were performed using laboratory developed, in-house, real-time, qualitative assays on nasopharyngeal swab specimens. Nosocomial infection was defined when first positive influenza testing was sent ≥3 days after admission. Date of influenza onset was defined as either the first day of typical influenza symptoms, or the first day of fever if the child had ongoing upper respiratory symptoms for greater than one week prior to presentation. Hospitalization was defined as either admission to an inpatient unit or observation in the Emergency Department Extended Care Unit. Concurrent infections were defined by positive PCR, direct immunofluorescence assay, culture, or by antibiotic administration for a clinically diagnosed bacterial infection (eg, otitis media). Influenza season was defined as August 1 through July 31; when seasons are referred to by year in the Results, the year designated refers to the year at the start of the season (eg, anything between August 1, 2010 – July 31, 2011 was counted as the 2010 season). Influenza vaccine was defined as received if administration was recorded at our institution or if caregivers reported the child received annual vaccine during a given influenza season; data regarding type of vaccine (eg, intranasal versus injectable) was not consistently available. If EMR stated “immunizations up to date” without specific reference to influenza vaccine, this variable was defined as “not received.” If a child received only one of two recommended doses for first time vaccination, they were defined as “received.”
Influenza-associated neurologic complications were documented only when there was no alternative explanation to neurologic signs or symptoms other than influenza infection. Influenza-associated seizures were defined as any clinical or electrographic seizure occurring in a child without a known seizure disorder, or seizures above baseline frequency in a child with a known seizure disorder. Febrile seizures were defined as a seizure occurring concurrent with body temperature ≥38.0°C, in a typically-developing child 0.5-6.0 years old without history of a neurologic disorder. Status epilepticus was defined as a single seizure (febrile or afebrile) lasting longer than 5 minutes, a cluster of seizures requiring anticonvulsant therapy to abort the cluster, or EMR documentation of status epilepticus. Encephalopathy was defined as documented parental or clinician concern for persistent altered mental status, confusion, or irritability out of proportion to medical illness. Pleocytosis was defined as cerebrospinal fluid (CSF) white blood cells (WBC) >7 cells/μL or the serum WBC multiplied by the ratio of CSF/serum red blood cells (RBC) in CSF specimens with elevated RBC. Meningitis/encephalitis were defined as presence of pleocytosis, magnetic resonance imaging (MRI) findings consistent with meningitis or encephalitis (e.g. leptomeningeal enhancement, T2 hyperintense, gadolinium enhancing lesions, others), or a clinical diagnosis of meningitis by the treating team when lumbar puncture and MRI were unable to be performed, in the absence of alternative explanations (eg, a concurrent central nervous system infection). Pre-existing neurologic diagnoses recorded included epilepsy, current/ongoing developmental delay and/or intellectual disability (not including isolated expressive speech delay or specific learning disorders, such as dyslexia, reading comprehension deficits, others), central nervous system (CNS) causes of weakness, congenital structural brain differences, cerebrovascular disease, anoxic brain injury, neuromuscular disorders, and others.
Data Analysis
Data were analyzed using STATA version 14.2 (Stata Corp, College Station, Texas). Non-parametric methods were used because not all continuous variables (such as age) were normally distributed, and to minimize the effect of outliers with statistical associations. Continuous variables were described using medians and interquartile range (IQR), and inter-group differences compared using the Wilcoxon rank-sum test. Categorical variables were described using counts and frequencies, and inter-group differences compared using the χ2 test. Statistical significance was determined a priori as a two-tailed p-value < 0.05.
Our a priori analytic plan included examining the impact of pre-existing neurologic diagnoses on the outcome of influenza-associated neurologic complications. Multivariable logistic regression models were built to determine the relationship between influenza-associated neurologic complications and associated factors overall and stratified by presence of a pre-existing neurologic diagnosis. Initial models were constructed using age, sex, race, and other candidate predictor variables with a p-value <0.2 on univariable analysis (Table 1). Stepwise elimination was then performed based on model-fit measured by Akaike’s Information Criteria (AIC). Variables that did not retain statistical significance in the adjusted model but whose elimination worsened overall model fit were retained as confounders. Presence of a pre-existing neurologic diagnosis was explored a priori as a potential effect modification term in the relationship between influenza-associated neurologic complications and other candidate predictor variables.
Table 1:
Demographics, Clinical, and Virologic Features of Hospitalizations with Laboratory Confirmed Influenza, 2010-2017.
| All | By Neurologic Complication | |||
|---|---|---|---|---|
| Characteristica | n=1,217 | No Neurologic Complication (n=1,086) |
Neurologic Complication (n=131) |
p-valueb |
| Demographics | ||||
| Age (years) | 5.5 (2.2, 9.8) | 5.6 (2.3, 9.8) | 4.7(1.9, 9.3) | 0.17 |
| Male sex | 641 (53) | 569 (52) | 72 (55) | 0.58 |
| African American | 644 (53) | 587 (54) | 57 (44) | 0.027 |
| Hispanic or Latino ethnicity | 139 (11) | 126 (12) | 13 (10) | 0.24 |
| Comorbidities | ||||
| Pre-existing neurologic diagnosis | 326 (27) | 252 (23) | 74 (56) | <0.001 |
| Co-infection at the time of influenza | 400 (35) | 354 (33) | 47 (36) | 0.87 |
| Influenza Data | ||||
| Influenza vaccine up to date | 608 (50) | 551 (51) | 57 (44) | 0.12 |
| Influenza strain: A | 805 (66) | 727 (67) | 78 (60) | 0.09 |
| B | 412 (33) | 359 (33) | 53 (40) | |
| Hospital acquired (nosocomial) influenza | 46 (4) | 40 (4) | 6 (5) | 0.61 |
| Treatment | ||||
| Oseltamivir | 920 (76) | 808 (74) | 112 (86) | 0.005 |
| Steroids | 444 (36) | 406 (37) | 38 (29) | 0.06 |
| Hospital Course | ||||
| Length of Stay (days) | 2 (1, 4) | 2 (1, 3) | 3 (2, 8) | <0.001 |
| Time from influenza symptom onset to neurologic symptom onset (days) | 1 (0, 3) | |||
| Time from neurologic symptom onset to confirmed influenza infection (days) | 1 (1, 2) | |||
| EDECU admission | 94 (8) | 92 (9) | 2 (2) | 0.005 |
| PICU or CICU admission | 243 (20) | 178 (16) | 65 (49) | <0.001 |
| Neurologic morbidity at discharge | 15 (11) | |||
| Mortality | 4 (0.3) | 4 (0.4) | 0 (0) | 0.49 |
Categorical variables are described using n (%). Continuous variables are described using median (interquartile range)
p-values to compare characteristics between children that had neurological complications and children that did not have neurologic complications and were calculated using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Abbreviations: EDECU, Emergency Department Extended Care Unit; PICU, Pediatric Intensive Care Unit; CICU, Cardiac Intensive Care Unit; EEG, Electroencephalogram
To calculate population incidence of children hospitalized with influenza-associated neurologic complication, we defined a neighborhood cohort using 13 contiguous zip-code areas (19023, 19032, 19050, 19082, 19103, 19104, 19106, 19131, 19139, 19143, 19145, 19146, 19153) from which ≥85% of all children <18 years of age are admitted to CHOP for common respiratory and gastrointestinal conditions 9. Data from US Census Factfinder (2017 American Community Survey) were used to estimate the number of overall and age-specific, child-years of observation during the study period (www.census.gov). In order to calculate a population incidence of influenza-associated neurologic complication, we estimated the prevalence of epilepsy in children to be 0.7%, consistent with prior reports 10.
RESULTS
Demographic and clinical characteristics of the cohort
We identified 1,217 hospitalizations for 1,156 unique children during the study period (Figure 1: online, Table 1). The median age at influenza symptom onset was 5.6 years (IQR 2.2, 9.8). Approximately one-quarter (23.2%, 95% CI 21-26%) of hospitalizations were in children two years-old or younger. Only 50% (95% CI 47-53%) of the cohort had documented receipt of that season’s annual influenza vaccine prior to hospitalization.
Figure 1 online:
Flow diagram of study population.
Prevalence and characteristics of influenza-associated neurologic complications:
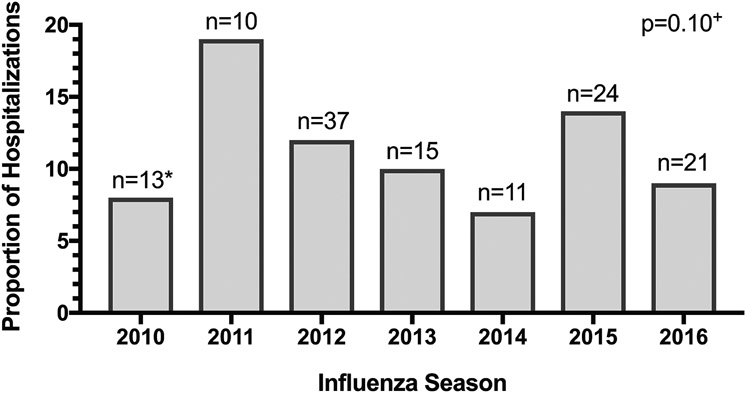
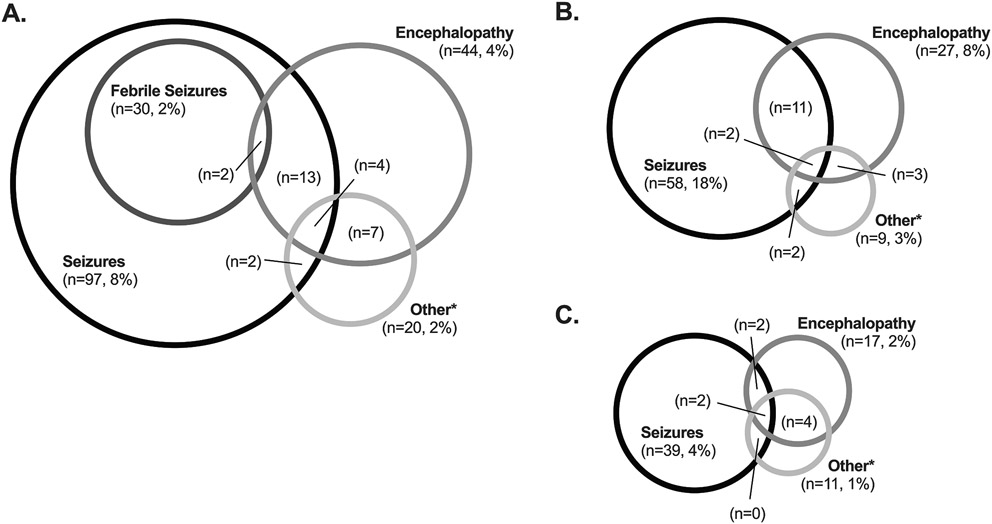
Nearly 11% of both hospitalizations (131/1,217, 10.8%, 95% CI 9.1-12.6%) and unique children (126/1,156, 10.9%) with acute laboratory-confirmed influenza had an influenza-associated neurologic complication. The proportion of hospitalizations with an influenza-associated neurologic complication did not vary significantly by season (p=0.10; Figure 2: online). Seizures (n=97, 74%) were the most common influenza-associated neurologic complication, followed by encephalopathy (n=44, 34%), ataxia (n=8, 6%), and meningitis/encephalitis (n=8, 6%). Other influenza-associated neurologic complications, such as cerebral edema (n=3), arterial ischemic stroke (n=1; cerebellar), posterior reversible encephalopathy syndrome (PRES; n=1), and others were less common (Table 2). Some hospitalizations included more than one influenza-associated neurologic complication (Figure 3A). Onset of neurologic symptoms began a median of one day after influenza symptom onset.
Figure 2 online:
Proportion (y-axis) and number (*) of hospitalizations with an influenza-associated neurologic complication by season. +p-value represents difference in proportion by year.
Table 2:
Frequency of Neurologic Complications
| Complication | Frequencya | Age, years | Days between influenza onset and IANC onset |
Presence of pre-existing neurologic disorderc |
Not back to neurologic baseline at dischargec |
|---|---|---|---|---|---|
| Any neurologic complicationb | 131 (100) | 4.7 (1.9, 9.3) | 1 (0, 3) | 74 (56) | 15 (11) |
| Seizures | 97 (74) | 4.3 (1.7, 8.2) | 1 (0,2) | 58 (60) | 9 (7) |
| Febrile seizures | 29 (22) | 1.6 (1.2, 2.4) | 1 (0,2) | 1 (3) | 1 (33) |
| Status epilepticus | 50 (38) | 3.3 (1.6, 6.4) | 0.5 (0, 2) | 32 (64) | 4 (8) |
| Encephalopathy | 44 (34) | 7.0 (3.1, 11.3) | 2 (0, 4) | 27 (61) | 8 (18) |
| Any neurologic complication other than seizure or encephalopathy | 20 (15) | 7.3 (3.7, 12.4) | 2.5 (1, 4) | 9 (45) | 8 (40) |
| Meningitis/encephalitis | 8 (6) | 11.6 (4.6, 15.2) | 0.5 (0, 2.5) | 5 (63)d | 4 (50) |
| Ataxia | 8 (6) | 7.7 (2.4, 11.9) | 2.5 (1, 3) | 4 (50) | 2 (25) |
| Cerebral edema | 3 (2) | 6.4 (2.8, 14) | 1 (0, 2) | 2 (67) | 3 (100) |
| Arterial ischemic stroke | 1 (1) | 5.7 | 7 | 0 | 0 |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) flare | 1 (1) | 12.1 | 11 | 1 | 1 |
| Clonus | 1 (1) | 4.7 | 11 | 0 | 0 |
| Demyelinating disorder | 1 (1) | 6.9 | 16 | 0 | 1 |
| Motor deficit | 1 (1) | 2.8 | 2 | 0 | 1 |
| Mutism | 1 (1) | 2.8 | 2 | 0 | 1 |
| Posterior reversible encephalopathy syndrome (PRES) | 1 (1) | 7.4 | 5 | 0 | 1 |
Some hospitalizations had ≥1 neurologic complication, so percentages do not add up to 100%
Categorical variables are described using n (%). Continuous variables are described using median (interquartile range)
Percentages calculated with total number of each neurologic complication as denominator; some children had more than one neurologic complication.
None of the children with a pre-existing neurologic disorder had a cerebrospinal fluid diversion device (e.g. ventriculoperitoneal shunt) or any identified co-infections.
Figure 3:
Frequency of neurologic complications. A. Among the overall cohort (n=1,217); B. Among those with a pre-existing neurologic diagnosis (n=326); C. Among those without a pre-existing neurologic diagnosis (n=876). Of the seizures (n=39) in panel C, 29 were febrile seizures.
Among the hospitalizations that had seizure, status epilepticus occurred in more than half (50/97, 52%). Most status epilepticus was convulsive (42/50, 84%), and anticonvulsant therapy was required by 39 (78%) to abort the ongoing seizure. Among the hospitalizations with meningitis/encephalitis, four were diagnosed based on pleocytosis (CSF WBC ranged 10-39 cells per μL), three based on MRI changes, and one who was not clinically stable for lumbar puncture or MRI, based on a clinical diagnosis by the treating team. None of the eight hospitalizations with ataxia had an associated MRI finding. Among the three hospitalizations with cerebral edema, two had diffuse fulminant edema requiring hyperosmolar therapy, and one had right-sided cerebellar edema. All three of these children had concurrent encephalopathy and a diagnosis of encephalitis based on MRI findings. The one patient with PRES presented clinically with encephalopathy and seizures. PRES, in this case, was thought to be secondary to an influenza-induced hypertension. Notably, there were no cases of Guillain-Barré syndrome (GBS) or Reye syndrome in our cohort. No child in this cohort with an influenza-associated neurologic complication died, but 15 had not yet returned to pre-admission neurologic baseline by the time of hospital discharge.
Estimated incidence of influenza-associated neurologic complications in neighborhood cohort
To estimate a local population incidence of influenza-associated neurologic complications, we examined influenza-associated neurologic complications in our neighborhood cohort. Based on a calculated 754,091 child-years of observation, we estimated that the incidence of influenza-associated neurologic complications (n=27) in this cohort was 3.6 per 100,000 child-years. We also calculated the age-specific incidence of influenza-associated neurologic complications in our neighborhood cohort, and found that children less than 5-years-old had the highest incidence of influenza-associated neurologic complication (n=15; 7.7 per 100,000 child-years).
To estimate a local population incidence of influenza-associated neurologic complications among children with epilepsy, a common pre-existing neurologic diagnosis, we examined influenza-associated neurologic complications among the estimated number of children with epilepsy in our neighborhood cohort. Based on a calculated 5,279 child-years of observation (0.7% of 754,091), we estimated that the incidence of influenza-associated neurologic complications (n=5) among local children with epilepsy was 94.7 per 100,000 child-years, 26-fold higher compared with the overall neighborhood cohort.
Risk factors for influenza-associated neurologic complications
On univariable analysis, there was no association between age, sex, influenza type, lack of seasonal influenza vaccine, community versus nosocomial infection, or the presence of a co-infection and influenza-associated neurologic complication in the overall cohort (Table 1). There was a smaller proportion of Black children among the hospitalizations with an influenza-associated neurologic complication, compared with those without (44% vs 54%, p=0.027), a difference likely driven by higher rates of sickle cell disease among Black children, and our institutional practice of admitting children with influenza for observation in the setting of sickle cell disease. When children with sickle cell disease were removed from analysis (n=147, 138 of whom were Black), there was no longer a difference in influenza-associated neurologic complication by race (p=0.29). Hospitalizations with an influenza-associated neurologic complication, compared with those without, were more likely to have a history of a pre-existing neurologic diagnosis (56% vs. 23.2%, p<0.001).
Oseltamivir was used more commonly in hospitalizations with an influenza-associated neurologic complication compared with those without (86% vs 74.4%, p=0.005; Table 1). However, only 8% of hospitalizations that had an influenza-associated neurologic complication and received oseltamivir did so prior to onset of neurologic symptoms, making it difficult to determine if oseltamivir use influenced risk of influenza-associated neurologic complication. Corticosteroids may have been used slightly less often in hospitalizations with an influenza-associated neurologic complication compared with those without (29% vs 37.4%, p=0.06). However, similar to oseltamivir timing, only 32% of hospitalizations that had an influenza-associated neurologic complication and received corticosteroids did so prior to onset of neurologic symptoms, making determination of whether corticosteroid use influenced risk of influenza-associated neurologic complication difficult. Corticosteroid use was much more common among children with asthma than those without (OR 17.0, 95% CI 12.5, 23.1). Neither oseltamivir nor corticosteroid use were included in multivariable model building given these data on timing of use.
On multivariable analysis, independent factors associated with an influenza-associated neurologic complication included presence of a pre-existing neurologic diagnosis (adjusted odds ratio [aOR] 4.6, p<0.001), age ≤5 years old (aOR 1.6, p=0.017), and lack of seasonal influenza vaccine (aOR 1.6, p=0.020), when adjusting for influenza type (A/B) in the overall cohort (Table 3A).
Table 3:
Multivariate Analysis of Factors Associated with Influenza-Related Neurologic Complications
| Adjusted OR | 95% Confidence Interval | p-value | |
|---|---|---|---|
| A: Among the entire cohort (n=1,217) | |||
| Presence of a pre-existing neurologic diagnosis | 4.6 | 3.2-6.7 | <0.001 |
| No annual influenza vaccinea | 1.6 | 1.1-2.3 | 0.020 |
| Age ≤5 years oldb | 1.6 | 1.1-2.3 | 0.017 |
| Influenza strain Bc | 1.4 | 0.9-2.0 | 0.10 |
| B: Among children without pre-existing neurologic diagnosis (PND; n=326) | |||
| No annual influenza vaccine | 2.1 | 1.2-3.7 | 0.013 |
| Age ≤5 years old | 3.1 | 1.7-5.7 | <0.001 |
| Influenza strain B | 0.8 | 0.5-1.6 | 0.60 |
| C: Among children with pre-existing neurologic diagnosis (PND; n=891) | |||
| No annual influenza vaccine | 1.3 | 0.7-2.1 | 0.39 |
| Age ≤5 years old | 0.9 | 0.5-1.6 | 0.75 |
| Influenza strain B | 2.0 | 1.2-3.4 | 0.012 |
Effect modification of the presence of a pre-existing neurologic diagnosis was examined a priori:
p=0.27
p=0.002
p=0.017
Evaluation for referral bias (sensitivity analysis)
To explore the possibility that our overall cohort data were influenced by a referral bias, we performed a sensitivity analysis of all influenza-associated neurologic complication prevalence estimates using children from our neighborhood cohort only (n=320/1,217). We found a similar proportion of influenza-associated neurologic complications among hospitalizations from the neighborhood cohort, compared with those outside the neighborhood cohort (27/320, 8.5% vs 104/897, 11.6%; p=0.12). Seizures were again most common (19/27, 70%), followed by encephalopathy (7/27, 26%), and others (4/27, 15%), suggesting that our results were not significantly impacted by a referral bias.
Analysis of influenza-associated neurologic complications, stratified by pre-existing neurologic diagnosis
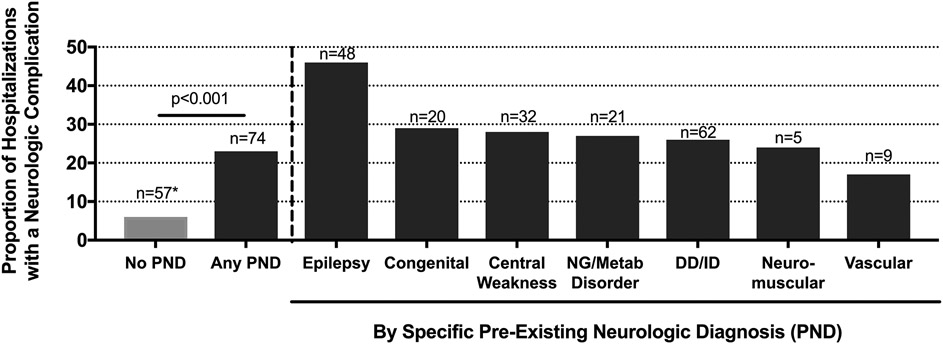
About a quarter of the overall cohort hospitalizations were for children with pre-existing neurologic diagnoses (n=326/1,217, 26.7%, 95% CI 24-29%). Developmental delay and/or intellectual disability were the most common pre-existing neurologic diagnoses (236/326, 72.4%), followed by epilepsy (104/326, 31.9%). Among hospitalizations that had at least one pre-existing neurologic diagnosis, 63% had more than one pre-existing neurologic diagnosis (median 2, [range 1-6]). Compared with hospitalizations without a pre-existing neurologic diagnosis, hospitalizations with a pre-existing neurologic diagnosis were more often admitted to the intensive care unit (31.6% vs 15.7%, p<0.001), had a longer length of stay (median 3 days [IQR 2, 6] vs 2 days [IQR 1, 3], p<0.001), and had a higher incidence of influenza-associated neurologic complications (22.7% vs 6.4%, p<0.001; Figure 4 online). Seizures (17.8% vs 4.4%, p< 0.001) and encephalopathy (8.3% vs 1.9%, p< 0.001) in particular were more common in hospitalizations with a pre-existing neurologic diagnosis, but other influenza-associated neurologic complications occurred in comparable proportions to those without (2.8% vs 1.2%, p=0.088) (Figures 3B, 3C). Among those with influenza-associated neurologic complications, 51% (38/74) of those with pre-existing neurologic diagnoses were vaccinated for influenza, and 33% (19/57) without a pre-existing neurologic diagnosis were vaccinated.
Figure 4 online:
Proportion (y-axis) and number (*) of hospitalizations with an influenza-associated neurologic complication by pre-existing neurologic disorder. PND (pre-existing neurological diagnosis), NG (neurogenetic), metab (metabolic), DD (developmental delay), ID (intellectual disability).
The potential effect modification of the presence of a pre-existing neurologic diagnosis in our multivariable analysis was examined a priori. Effect modification terms between pre-existing neurologic diagnosis and younger age (≤5 years old, p=0.002) and influenza strain (p=0.017) were significant, but lack of seasonal influenza vaccine (p=0.27) was not. Multivariable analysis stratified on the presence of pre-existing neurologic diagnoses (Table 3B, 3C), demonstrated that the independent factors associated with the presence of an influenza-associated neurologic complication among hospitalizations without a pre-existing neurologic diagnosis were lack of seasonal influenza vaccine (aOR 2.1, 95% CI 1.2-3.7, p=0.013) and age ≤5 years old (aOR 3.1, 95% CI 1.7-5.7, p<0.001), when controlling for influenza type (A/B). Among hospitalizations with a pre-existing neurologic diagnosis, influenza type B (aOR 2.0, 95% CI 1.2-3.4, p=0.012) was the only independent factor associated with the presence of influenza-associated neurologic complications.
DISCUSSION
Influenza-associated neurologic complications were common in this large sample of children hospitalized at a single institution with laboratory-confirmed influenza, particularly among children with a pre-existing neurologic diagnosis. While seizures and encephalopathy occurred most frequently, other influenza-associated neurologic complications occurred in 2% (20/1,217) of the overall cohort. Independent factors associated with occurrence of an influenza-associated neurologic complication differed among hospitalizations with and without a pre-existing neurologic diagnosis, suggesting that the pathophysiology of some influenza-associated neurologic complications may vary between children with and without a pre-existing neurologic diagnosis. The prevalence of influenza-associated neurologic complications was similar in hospitalizations from our neighborhood cohort compared with our overall cohort, suggesting our findings should be generalizable outside a tertiary care referral hospital.
The influenza-associated neurologic complications observed in this study were heterogeneous. While seizures and encephalopathy are well described in the literature 3,4,9,11-13, other complications of influenza that we observed, such as arterial ischemic stroke, ataxia, PRES, and cerebral edema, have been less frequently described. Any type of recent infection has been demonstrated to increase risk of arterial ischemic strokes in children by 6-fold 14, which may account for the one arterial ischemic stroke that we observed. Case reports exist of influenza-triggered mild encephalopathy with a reversible splenial lesion (MERS) resulting in ataxia 15. While we did not have any children with MERS, we did have eight children with ataxia without neuroimaging correlate, a phenotype that has not previously been reported. Case reports of PRES in adults, but not in children, with influenza have rarely been reported 16. Multiple prior groups have reported cerebral edema in the context of acute influenza infection 17,18, but these each have been single case reports, one of which described a patient with an interleukin-10 receptor mutation thought to have increased the systemic inflammatory response. The lack of Reye Syndrome in this cohort likely reflects the dropping number of cases since the Federal Drug Administration (FDA) placed a warning on aspirin in 1980 19,20.
The neuropathophysiology of influenza-associated neurologic complications remains poorly understood, but may be related to systemic cytokine release with secondary central nervous system (CNS) dysfunction 18,21-24 and specific host genetic susceptibilities 18,25. Influenza is not thought to infect the CNS, and has not typically been found in brain tissue or CSF 26, so the pathophysiology behind our eight cases of meningitis and encephalitis remains unknown. Additionally, influenza has been associated with a higher incidence of febrile seizures, and of repeated seizures in the same febrile episode, than other febrile illnesses of childhood27; the reasons for this are unknown. Whether we could mitigate or prevent influenza-associated neurologic complications using anti-inflammatory or antiviral therapies is unknown. Unfortunately, only a small proportion of children in this cohort received corticosteroids or oseltamivir prior to onset of neurologic complications, limiting our ability to address this question.
Our finding that prevalence and factors associated with influenza-associated neurologic complications were fundamentally different among children with and without pre-existing neurologic diagnoses in this cohort is clinically significant. While younger age was associated with influenza-associated neurologic complication in hospitalizations without a pre-existing neurologic diagnosis, this association was not observed among hospitalizations with pre-existing neurologic diagnoses. This difference may be because common influenza-associated neurologic complications (seizure and encephalopathy) in children with a pre-existing neurologic diagnosis can occur when underlying neurologic dysfunction is exacerbated by infection (eg, a breakthrough seizure in the context of illness in a child with epilepsy), despite age. While vaccination status was not statistically significant in stratified models among children without a pre-existing neurologic diagnosis (Table 3C, p=0.39), the effect modification term between vaccination status and pre-existing neurologic diagnosis in the overall cohort was not significant (p=0.27). We interpret these data to suggest that influenza-associated neurologic complication risk conferred by lack of vaccine is likely applicable to all children, as suggested in the non-stratified overall model (Table 3A). This benefit of vaccine is analogous to the reduced risk of death from influenza conferred by vaccine 28. Unfortunately, only 50% of overall hospitalizations and 55.8% of hospitalizations with a pre-existing neurologic diagnosis were vaccinated, demonstrating opportunities for improvement in both overall and targeted vaccination strategies. For unimmunized children with a pre-existing neurologic diagnosis, the role of post-exposure influenza antiviral chemoprophylaxis could also be considered to prevent infection in this very vulnerable population 29.
Our study had several limitations. First, the study was conducted at a single, pediatric tertiary care center, which may introduce referral bias. However, a sensitivity analysis of hospitalizations from neighborhood zip codes only generated similar estimates of influenza-associated neurologic complication prevalence, supporting the generalizability of our findings. Second, our study was retrospective, and thus, subtle details may have been lost or influenza-associated neurologic complications misclassified. For example, it is difficult to define accurately encephalopathy retrospectively, particularly in a young child or a child with developmental disabilities. Future prospective studies of influenza-associated neurologic complications would address this limitation. Third, children presented to our institution at variable times in their acute illness, frequently after onset of their influenza-associated neurologic complication. Therefore, it is not possible to determine if oseltamivir and/or corticosteroids may have prevented influenza-associated neurologic complications in this cohort. Randomized controlled clinical trials would address these limitations, but are logistically difficult in a rare disorder. Fourth, not all children with influenza are hospitalized, and among those who are, influenza-associated neurologic complications may drive the indication for admission, limiting generalizability outside the hospital of those factors identified with influenza-associated neurologic complication. However, understanding these factors in hospitalized children specifically remains valuable. Fifth, we aimed to estimate a local population incidence of influenza-associated neurologic complication among children with epilepsy, a common pre-existing neurologic diagnosis. However, these data are based on local population prevalence estimates of epilepsy, so are gross estimates only. Finally, we elected to look specifically for neurologic complications during the hospitalization with acute laboratory-confirmed influenza infection. We may have therefore missed later neurologic complications that occurred as an outpatient, or during a subsequent admission, such as Guillain-Barré syndrome.
In conclusion, influenza-associated neurologic complications were common in this large cohort of children hospitalized with laboratory-confirmed influenza, although their pathophysiology is not well understood. The presence of a pre-existing neurologic diagnosis was significantly independently associated with influenza-associated neurologic complications, but influenza-associated neurologic complications occurred in children without pre-existing neurologic diagnoses as well, particularly those who were younger. Vaccination to prevent illness remains critically important for all children, and may additionally protect against influenza-associated neurologic complications in the context of illness. Future study of complication-specific and population-specific influenza-associated neurologic complication pathogenesis will drive development of prevention and mitigation strategies.
Funding Source:
NIH (NINDS) K23 NS094069 (McGuire)
Role of Funder:
The funder did not participate in the work.
Abbreviations:
- ADEM
acute disseminated encephalomyelitis
- GBS
Guillain-Barré Syndrome
- CHOP
Children’s Hospital of Philadelphia
- EMR
electronic medical record
- PCR
polymerase chain reaction
- CSF
cerebrospinal fluid
- MRI
magnetic resonance imaging
- IQR
interquartile range
- PRES
posterior reversible encephalopathy syndrome
- MERS
mild encephalopathy with reversible splenial lesion
- FDA
Federal Drug Administration
- CNS
central nervous system
Footnotes
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure Statement: Ms. Frankl, Ms. Harrison, Dr. Swami, and Dr. McGuire have no financial relationships relevant to this article to disclose. Dr. Coffin serves on a Merck DSMB, unrelated to influenza.
Data Statement:
A deidentified data set is available upon request
REFERENCES
- 1.Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the united states. Clin Infect Dis. 2018;66:1511–1518. doi: 10.1093/cid/cix1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Influenza (flu) including seasonal, avian, swine, pandemic, and other [Internet]. 2019. Jul 22 [updated 2019]. Available from: https://www.cdc.gov/flu/index.htm.
- 3.Britton PN, Blyth CC, Macartney K, Dale RC, Li-Kim-Moy J, Khandaker G, et al. The spectrum and burden of influenza-associated neurological disease in children: Combined encephalitis and influenza sentinel site surveillance from Australia, 2013-2015. Clin Infect Dis. 2017;65:653–660. doi: 10.1093/cid/cix412. [DOI] [PubMed] [Google Scholar]
- 4.Newland JG, Laurich VM, Rosenquist AW, Heydon K, Licht DJ, Keren R, et al. Neurologic complications in children hospitalized with influenza: Characteristics, incidence, and risk factors. J Pediatr. 2007;150:306–310. doi: 10.1016/j.jpeds.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Toovey S. Influenza-associated central nervous system dysfunction: A literature review. Travel Med Infect Dis. 2008;6:114–124. doi: 10.1016/j.tmaid.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Muhammad Ismail HI, Teh CM, Lee YL, National Paediatric H1N1 Study Group. Neurologic manifestations and complications of pandemic influenza A H1N1 in Malaysian children: What have we learnt from the ordeal? Brain Dev. 2015;37:120–129. doi: 10.1016/j.braindev.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Vianello FA, Osnaghi S, Laicini EA, Milani GP, Tardini G, Cappellari AM, et al. Optic neuritis associated with influenza B virus meningoencephalitis. J Clin Virol. 2014;61:463–465. doi: 10.1016/j.jcv.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrand JJ. Neurologic complications of influenza. Semin Pediatr Neurol. 2012;19:96–100. doi: 10.1016/j.spen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Coffin SE, Zaoutis TE, Rosenquist AB, Heydon K, Herrera G, Bridges CB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics. 2007;119:740–748. doi: 10.1542/peds.2006-2679. [DOI] [PubMed] [Google Scholar]
- 10.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 11.Surana P, Tang S, McDougall M, Tong CY, Menson E, Lim M. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr. 2011;170:1007–1015. doi: 10.1007/s00431-010-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landau YE, Grisaru-Soen G, Reif S, Fattal-Valevski A. Pediatric neurologic complications associated with influenza A H1N1. Pediatr Neurol. 2011;44:47–51. doi: 10.1016/j.pediatrneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Calitri C, Gabiano C, Garazzino S, Pinon M, Zoppo M, Cuozzo M, et al. Clinical features of hospitalised children with 2009 H1N1 influenza virus infection. Eur J Pediatr. 2010;169:1511–1515. doi: 10.1007/s00431-010-1255-y. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton HJ, Hills NK, Elkind MS, Dowling MM, Wintermark M, Glaser CA, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015;85:1459–1466. doi: 10.1212/WNL.0000000000002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ka A, Britton P, Troedson C, Webster R, Procopis P, Ging Joanne, et al. Mild encephalopathy with reversible splenial lesion: An important differential of encephalitis. Eur J Paediatr Neurol. 2015;19:377–382. doi: 10.1016/j.ejpn.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Bartynski WS, Upadhyaya AR, Boardman JF. Posterior reversible encephalopathy syndrome and cerebral vasculopathy associated with influenza A infection: Report of a case and review of the literature. J Comput Assist Tomogr. 2009;33:917–922. doi: 10.1097/RCT.0b013e3181993a43. [DOI] [PubMed] [Google Scholar]
- 17.Albaker A, Soder C, Top KA. Acute encephalopathy associated with influenza infection: Case report and review of the literature. Paediatr Child Health. 2019;24:122–124. doi: 10.1093/pch/pxy085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishige T, Igarashi Y, Hatori R, Tatsuki M, Sasahara Y, Takizawa T, et al. IL-10RA mutation as a risk factor of severe influenza-associated encephalopathy: A case report. Pediatrics. 2018;141:e20173548. doi: 10.1542/peds.2017-3548. [DOI] [PubMed] [Google Scholar]
- 19.Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB. Reye's syndrome in the United States from 1981 through 1997. N Engl J Med. 1999;340:1377–1382. doi: 10.1056/NEJM199905063401801. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, (CDC). Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:773–778. [PubMed] [Google Scholar]
- 21.Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl. 2007;186:45–56. [PubMed] [Google Scholar]
- 22.Kawada J, Kimura H, Ito Y, Hara S, Iriyama M, Yoshikawa T, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis. 2003;188:690–698. doi: 10.1086/377101 [DOI] [PubMed] [Google Scholar]
- 23.Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev. 2009;31:731–738. doi: 10.1016/j.braindev.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Aiba H, Mochizuki M, Kimura M, Hojo H. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology. 2001;57:295–299. doi: 10.1212/wnl.57.2.295. [DOI] [PubMed] [Google Scholar]
- 25.López-Laso E, Mateos-González ME, Pérez-Navero JL, Camino-León R, Briones P, Neilson DE. Infection-triggered familial or recurrent acute necrotizing encephalopathy. An Pediatr (Barc). 2009;71(3):235–239. doi: 10.1016/j.anpedi.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Ichiyama T, Kimura H, Shibata M, Ishiwada N, Kuroki H, et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58:420–425. doi: . [DOI] [PubMed] [Google Scholar]
- 27.Chiu Susan S., Tse Catherine Y. C., Lau Yu Lung and Peiris Malik. Influenza A Infection Is an Important Cause of Febrile Seizures. Pediatrics 2001;108;e63. doi: 10.1542/peds.108.4.e63 [DOI] [PubMed] [Google Scholar]
- 28.Flannery B, Reynolds SB, Blanton L, Santibanez TA, O’Halloran A, Lu P, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244. doi: 10.1542/peds.2016-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Influenza antiviral medications: Summary for clinicians ∣ CDC [Internet]. 2019. Jul 11 (updated 2019). Available at: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A deidentified data set is available upon request