Abstract
Machine-washable and antimicrobial substances are the demand for the current wool fabrics. The characteristic of wool fabric is studied with novel self-made azetidinium cationic polyurethane dispersions (PUDs), which used dihydroxyalkyl glycidylamine as the chain extender. Dihydroxyalkyl glycidylamine was synthesized by ring opening with epichlorohydrin and diisopropanolamine. Influences of the cationic PUDs on antibacterial properties, felting shrinkage, and yellowness index of wool fabric are studied. The results show that the antifelting feature of wool fabric with the cationic PUDs is better than the others. It is also found that the wool fabric has a clear antibacterial property. The optimal process has been concluded as follows: the compound PUDs of 50 g/L and curing at 130 °C 5.0 min. The finished fabric has low felting shrinkage after washing for 20 times at 60 °C.
1. Introduction
Wool is a kind of natural fiber with soft luster and excellent heat preservation.1 The fabrics, which are composed of wool fibers, would provide good skin affinity and natural glossy and thermal performance.2,3 Consequently, wool fabrics are widely used in high-grade clothing.4−7 However, the disadvantage of wool fabric is obvious. It has a higher tendency to pill, and the fabric shrinkage happens during the washing process, especially with a washing machine.8−10 Polyurethane dispersions (PUDs) have been recognized by International Wool Secretariat (IWS) as finishing agents for wool fabrics.11,12 The most widely used finishing agent on the market is Synthappret BAP (Bayer), which is the typical structure of sodium bisulfite blocked PUDs.13,14 The antishrinkage feature of sodium bisulfite blocked PUDs is effective; however, it produces high-pH sewage or residues such as sodium bisulfite during treatment, resulting in environmental and health threats.
This research adopted an innovative synthesis method to obtain novel azetidinium cationic PUDs with good antishrinkage ability and antibacterial ability. First, diisopropanolamine (DIPA) reacted with epichlorohydrin (EPI) and yielded diisopropanol glycidyl amine, which was used as a chain extender to introduce the glycidyl amine structure into waterborne polyurethane. The azetidine ion is used not only as a hydrophilic group to solubilize macromolecules but also as a cross-linking functional group to react with amine groups, carboxyl groups, and phenol groups to enhance adhesion to wool fibers.15,16 Next, glycidylamine isomerized into azetidine in acidic conditions and formed a new type of cationic waterborne polyurethane containing azetidine. In the process of reaction, the quaternary ammonium salt which resulted from azetidine entered the cell to modify the protein and destroy the cell structure of the bacteria, ultimately killing it.17−19 Finally, the effects of this new type of polyurethane on wool finishing agents were explored.
2. Experimental Section
2.1. Materials
EPI (Aladdin, China), DIPA (Macklin, China), sodium hydroxide particles (NaOH, Sinopharm Chemical Reagent Co., Ltd., China), and isopropanol (IPA, Macklin, China) were used as raw materials for the synthesis of dihydroxyalkyl glycidylamine. Isophorone diisocyanate (IPDI, Aladdin, China), polyether glycol (PPG: number-average molecular weight (Mn) = 2000 g mol–1, Haishihua Inc., China), 1,4-butanediol (BDO, Sinopharm Chemical Reagent Co., Ltd., China), acetone (Sinopharm Chemical Reagent Co., Ltd., China), and acetic acid (Sinopharm Chemical Reagent Co., Ltd., China) were used as diisocyanate, the polymer glycol, the chain extender, the solvent, and the neutralizing agent, respectively. Other materials obtained were wool fabrics (80s, Qinghe Ruifengxiang Cashmere Factory, China), sodium dichloroisocyanurate (DCCNa, Usolf, China), TF-163A (Zhejiang Transfar Chemical Group Co., Ltd., China), RY-1107 (Jiangxi Derongtai New Material Co., Ltd., China), and Goon815 (Dong Guan Goon Silicon Technology Co., Ltd., China). All other chemicals were used as received.
2.2. Characterization
The structure of dihydroxyalkyl glycidylamine was observed with liquid chromatography–mass spectrometry (LC–MS) and 1H nuclear magnetic resonance (1H NMR). LC–MS was performed on a Thermo Scientific LTQ Orbitrap XL orbitrap high-resolution combined mass spectrometer using N,N-dimethylformamide as a solvent with positive ions as the irradiation source. 1H NMR was obtained at room temperature on AVANCE II 400 MHz spectrometers in DMSO-d6 using tetramethylsilane as an internal standard.
The morphology of PUDs, the microgel particle size, and particle size distribution were measured using dynamic light scattering (DLS) on a Zetasizer Nano series ZS 90 with diluted to 1.85 wt % in distilled water. Fourier transform infrared spectroscopy (FT-IR) spectra were obtained using a Bruker VERTEX 80 with a HYPERION 2000 Infrared microscope. The stability of PUDs was characterized by centrifugation at 3000 rpm for 15 min at room temperature and then was observed to evaluate whether precipitation occurred. By thermogravimetry (TG) analysis, the decomposition temperature of PUD film was measured on a Netzsch TG 209 F3 from room temperature to 600.0 °C. Differential scanning calorimetry (DSC) thermal analysis of PUD film was performed on a TA Instruments DSC Q20 from −40 to 100.0 °C with a heating rate of 20.00 °C/min.
The properties of finishing agent coating film through the study of mechanical and water absorption mechanical properties were tested by the film with a standard dumbbell shape according to ISO 527 2-1993 (E) on a Landmark LDX-200 electronic universal testing machine. The tensile strength was measured, and the tensile speed was 300 mm min–1. Water absorption was tested by the weighting method. At room temperature, the weights of films before and after 24 h of immersion in deionized water were compared. The water absorption can be calculated according to eq 2.1. The weight of original film was W1, and the weight of film after soaking for 24 h was W2. Each kind of film was measured three times, and the average value was taken
| 2.1 |
The finishing process of the raw material as a finishing agent was determined by felting shrinkage and yellowing index. The wool fabric after the double-dip-double-nip process was folded into a double-layer sample (300 mm × 400 mm); the three sides were sewn firmly, and three sets of marks were made in the warp and weft directions of the fabric. The sample was put into a Meling MB90-660BILG standard washing machine, washed with detergent Whitecat washing powder, followed by drying. Scanning electron microscopy (SEM) was used to observe the surface shape of the wool fibers before and after washing. The test temperature was 4 °C, and the voltage was 3.0 kV. Through felting shrinkage tests, the antifelt shrinkage of the new finishing agent was studied. The area felting shrinkage rate of the wool fabric was measured according to textile industry standard FZ/T 70009-2012. The felting shrinkage (FS) rate was calculated using eq 2.2, where SW is the shrinkage rate in the weft direction (%) and SL is the shrinkage rate in the warp direction (%). The average of three data sets was reported
| 2.2 |
The yellowing index was tested using a Caipu Techology ColorMeter Pro Spectrophotometer. By testing the three stimulus values (X, Y, and Z) of the finished wool fabric (as per standard GB 8424-2001), the yellowing index Y1 of the fabric was calculated using eq 2.3
| 2.3 |
The antibacterial properties of the film were tested, with bacteria selected from typical Gram-negative Escherichia coli (E. coli, represented by EC below). The film was cut into 3 cm by 3 cm pieces, washed three times with deionized water, and sterilized under UV light for 30 min before use. A 33 g sample of nutrient agar solid powder was placed into a conical flask to which 1 L of deionized water was added and stirred using a clean glass rod to dissolve the powder. The rubber band was closed, and the product was steam-sterilized for 2 h as a liquid medium for use. The liquid medium was introduced into the test tube and allowed to solidify. The inoculation loop took the bacteria on the medium for inoculation, which were inoculated in a constant temperature incubator at 37 °C for 24 h. Next, normal saline was added to the test tube, and the inoculation loop took an appropriate amount of bacteria for placement in the test tube; the strains were dispersed using a dispersant and placed in a shaker at 28 °C for 8 h to disperse the strains uniformly for EC bacterial suspension. A 20 mL liquid culture medium sample was placed into two disposable Petri dishes. After cooling and solidification, 100 μL of EC bacterial suspension was added to each Petri dish and spread evenly with a coating rod. No tested sample was added to one of the Petri dishes and served as the blank. The cut film was placed in the center position of the other Petri dish, the lid was closed, the incubator was set at 37 °C for 24 h, and the zone of inhibition was observed.
2.3. Synthesis of Dihydroxyalkyl Glycidylamine
The synthetic procedure was similar to the previous report (see Scheme 1).15,20
Scheme 1. Synthesis of Dihydroxyalkyl Glycidylamine.
EPI (0.23 mol) was dissolved in IPA in the range of 28–30 °C, followed by addition of DIPA (0.20 mol) within 5.0 h. The reaction was carried out at a temperature less than 30 °C. Subsequently, 50 wt % NaOH was added to the reaction mixture within 2 h at 45 °C; stirring was continued for an additional 1.0 h. After subsidence, the precipitate was removed by filtration, the filtrate was taken, filtration was continued, and the operation was repeated three times. Then, the final filtrate was placed in a flask, and the solvent and excess EPI were removed by rotary evaporation. The final product was obtained by drying in a vacuum oven for 24.0 h at 80 °C. The structure of dihydroxyalkyl glycidylamine was confirmed by LC–MS spectra and 1H NMR.
2.4. Synthesis of Azetidinium Cationic PUDs
After a series of experiments, we found that when the ratio of isocyanate group (NCO) to active hydroxyl group (OH) was 0.97, the proportion of dihydroxyalkyl glycidylamine was 10 wt %, the ratio of BDO to PPG was 14%, and the ratio of neutralization degree was 100%, and stabilized and low-particle PUDs were obtained.20 The azetidinium cationic PUDs were synthesized through the following procedure (see Scheme 2).
Scheme 2. Synthesis Route of Azetidinium Cationic PUDs.
PPG was charged under stirring, and IPDI was then slowly added into the diol mixed for 2 h at 80 °C. Next, the chain extenders BDO and dihydroxyalkyl glycidylamine were added into the mixture at the same temperature for 2.5 h until the theoretical NCO content of the prepolymer was reached, which was estimated using the amine equivalent method, through adjusting the viscosity by acetone. Then, distilled water was added into the neutralized PU by using acetic acid as a neutralizing agent. The PUDs of 25 wt % solids were obtained after removal of acetone by rotary evaporation. The structure of azetidinium cationic PUDs was confirmed by FI-IR.
2.5. Preparation of Wool Fabric Finishing Agent using the Azetidinium Cationic PUDs
The wool fabrics were prepared with the azetidinium cationic PUDs. The liquor ratio was 1:20, and the pH value was 6.0. The wool fabrics prepared through double-dip–double-nip with pick-up was 70%. Specifically, the wool fabrics were immersed in distilled water at room temperature about 10.0 min. Then, the wool fabrics were moved into a padding machine. Subsequently, the PUD finishing agent was applied to the wool fabrics for 25.0 min at room temperature, followed by use of the padding machine to complete double-dip–double-nip. The obtained product was pre-baked at 80 °C 3 min and then dried at a higher temperature.
3. Results and Discussion
3.1. Design of the Azetidinium Cationic PUDs
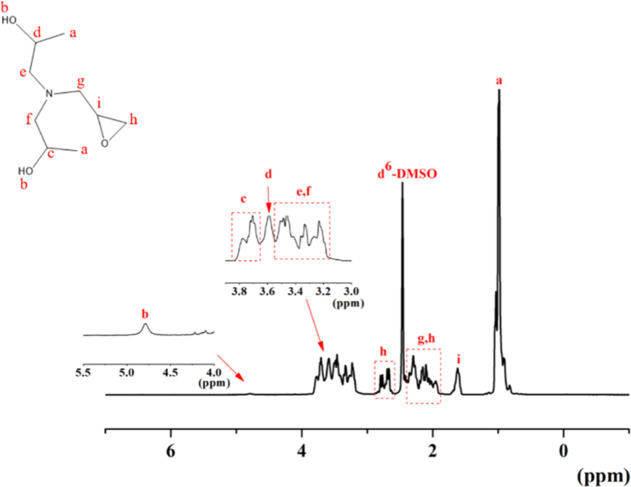
The preparation of the azetidinium cationic PUDs was based on dihydroxyalkyl glycidylamine as a chain extender. Therefore, we first tested the structure of the hydrophilic chain extender as described in Figure 1 by HNMR. We can see the methylidyne proton signals in the dihydroxyalkyl glycidylamine backbone proton signals at δ = 0.97 ppm. The proton signal at δ = 1.62 ppm is ascribed to methine protons on the epoxy structure of dihydroxyalkyl glycidylamine. The two protons of methylene on the epoxy group were cracked by methylene protons and oxygen atoms and affected by the strong electronegativity of oxygen heteroatoms. The shielding effect reduced because the proton closer to oxygen atoms. Therefore, the signal peak of the proton appeared in the low field. Therefore, the multiple peaks at δ = 2.79 and 2.68 ppm indicated the proton in methylene that was closer to the oxygen atom. Another proton peak of methylene on the epoxy group was 2.09 ppm. The three methylene proton peaks linked to the nitrogen atom, affected by protons on the adjacent carbon, were broken into multiple peaks at δ = 3.47, 3.23, and 2.30 ppm. The peak of methine proton, which was linked to hydroxyl terminated near the epoxy group, was at δ = 3.70 ppm. The other methine proton signal peak, which was linked with another hydroxyl terminated, was at δ = 3.59 ppm. The signal of the terminal of the hydroxyl terminated at δ = 4.79 is marked in Figure 1.
Figure 1.
1H NMR spectrum of glycine DIPA.
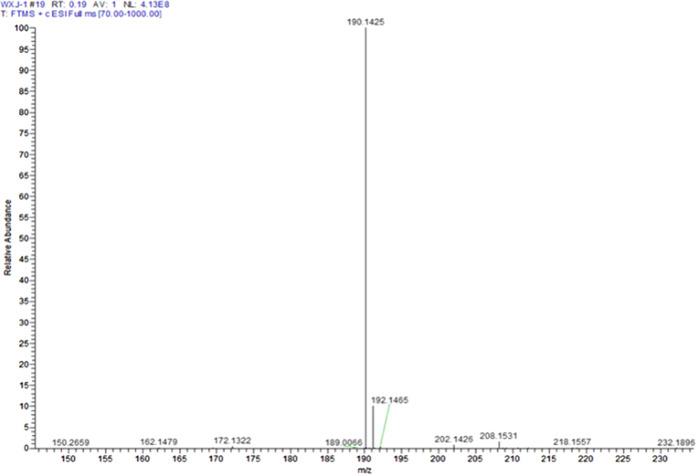
To further verify the molecules of dihydroxyalkyl glycidylamine, its LC–MS spectra were measured, as seen in Figure 2.20 From Figure 2, the molecular ion peak of the product was 190 and its molecular weight was 189, which was consistent with the analysis of 1H NMR. These results accorded with the molecular weight of dihydroxyalkyl glycidylamine designed theoretically, demonstrating the successful synthesis of dihydroxyalkyl glycidylamine.
Figure 2.
LC–MS spectrum of the chain extender of glycine DIPA.
From a superficial point of view, we got stable PUDs as shown in Figure 3a. There was no precipitation at all after centrifugation at 3000 rpm for 15 min at room temperature. According to DLS analysis (Figure 3b), the average diameter of formed PUD particles was 43 nm, and the particle size distribution was small. Therefore, dihydroxyalkyl glycidylamine as a hydrophilic chain extender was used up according to this method as shown in Scheme 2.
Figure 3.
(a) Appearance of PUDs after centrifugation at 3000 rpm for 15 min at room temperature. (b) Particle size distribution of PUDs by DLS analysis.
The PUDs were confirmed by FT-IR spectroscopy (see Figure 4). Compared with the cationic PUDs synthesized by N,N-dimethyl-2-(dimethylol) butylamine as hydrophilic chain extenders,21 the stretching vibrations of −CO– at 1095 cm–1 became broadened, and a peak in the region of 3400 cm–1 appeared, which means that there was hydroxyl generation after neutralization using dihydroxyalkyl glycidylamine as a hydrophilic chain extender. Additionally, the intensity of the peak at 1634 cm–1 was increased because the asymmetric stretching vibration intensity of carbonyl groups in carboxylates increased, which means that the electron cloud density around carbonyl groups decreased, with the spectrum of equilibrium reaction on dihydroxyalkyl glycidylamine under acidic and alkaline conditions.22 Other characteristic peaks of polyurethane were consistent with the studies of other scholars.21,23 Therefore, using the method in this paper stable cationic PUDs could be successfully synthesized.
Figure 4.

FI-IR spectrum of the azetidinium cationic PUD film.
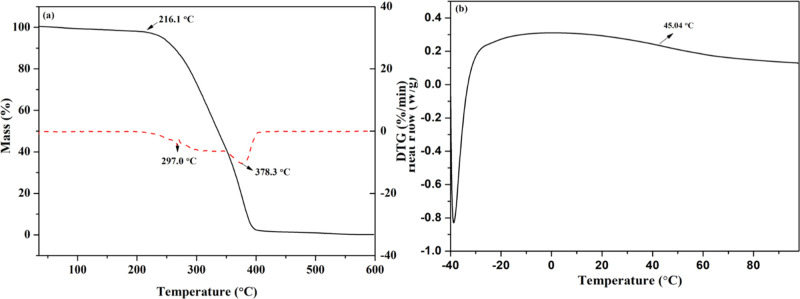
The thermal properties of the azetidinium cationic PUDs film are shown in Figure 5. As shown in Figure 5a, the initial decomposition temperature of this PUD film is 216 °C. The glass-transition temperature is 45 °C, as seen in Figure 5b. Therefore, the azetidinium cationic PUD film has good heat resistance.
Figure 5.
Thermal analysis of the azetidinium cationic PUD film. (a) TGA and DTG spectra. (b) DSC spectrum.
3.2. Performance of the Azetidinium Cationic PUDs
As a raw material of wool antishrinkage agent approved by IWS, PUDs play an important role in the finishing of wool fabric. In this paper, the application technology of PUDs in wool finishing is studied. In order to further explore the possibility of the application of the azetidinium cationic PUDs in the field of wool finishing agent, the water absorption and tensile strength of the azetidinium cationic PUD film are tested and compared with the corresponding properties of the commercial product Synthappret BAP, as shown in Table 1.
Table 1. Performance Comparison between PUDs Filmed and Synthappret BAP24.
| model | tensile strength (MPa) | water absorption (%) |
|---|---|---|
| the azetidinium cationic PUDs | 2.0 | 14.98 |
| Synthappret BAP (100 °C) | 36.02 | 44.09 |
| Synthappret BAP (80 °C) | 33.47 | 42.75 |
| Synthappret BAP (60 °C) | 31.08 | 40.11 |
From Table 1, we find that the azetidinium cationic PUD film is softer than Synthappret BAP, so it has less effect on the hardness of the fabric. At the same time, this azetidinium cationic PUDs have low water absorption and can reduce the damage of water penetration to the protective coating during washing. With the increase of film forming temperature, the tensile strength and water absorption of Synthappret BAP film are increased. This is because for thermal reaction finishing agents, such as Synthappret BAP, the increase of temperature is beneficial for the cross-linking reaction of the film, so it can be formed as a more perfect film, which is characterized by the increase of the tensile strength of the film.
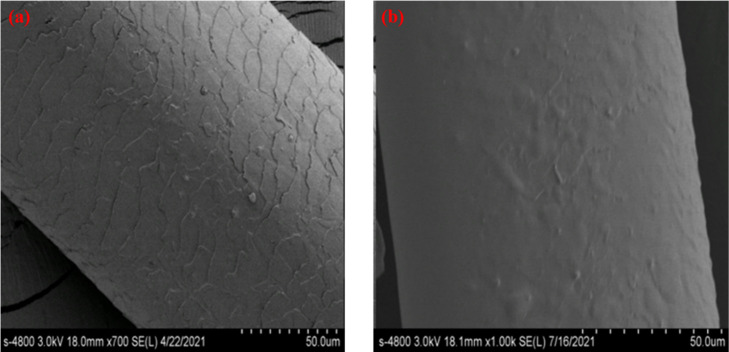
A comparison with the SEM spectrum of washed wool fibers, which were added the finishing agent before and after, is shown in Figure 6. It could be seen that the scale layers of wool fibers were covered by the coating formed by polyurethane after using finishing agents. The wool fiber still maintained this firm covering state after washing, which reflected that azacyclobutane could react with carboxyl and amino groups to form chemical cross-linking branches on the surface of the substrate to strengthen the coating in this PUDs. Therefore, as the raw material of the finishing agent, these PUDs had both film-forming and chemical cross-linking properties of polymer so as to prevent the movement of wool fiber and protect the deformation of wool fiber. It was determined that this kind of waterborne polyurethane products could be used for wool antishrinkage and antipilling.
Figure 6.
(a) SEM image of the washed wool fiber. (b) SEM image of the washed wool fiber treated with the finishing agent.
According to previous studies, Synthappret BAP was a thermal reactive polyurethane, and its antishrinkage effect on wool varies greatly under different curing methods.25 At the same time, the actual antishrinkage effect of Synthappret BAP was greatly affected by other auxiliaries such as pH regulators and surfactants.26,27 In this paper, the azetidinium cationic PUDs were non-thermal reactive resins, so they are not affected by the curing mode. Due to the fact that the protective effect is achieved by coating wool fiber with good film-forming properties, these PUDs as finishing agents are less sensitive to the environment. Therefore, the azetidinium cationic PUDs synthesized in this experiment have significant advantages in the field of wool treatment.
Considering the unique antibacterial properties of nitrogen heterocyclic quaternary ammonium salt, we made a preliminary study on the antibacterial properties of the film. Figure 7 shows the antibacterial results of 1% azetidinium cationic PUD film measured by the coating method. It can be seen intuitively in Figure 7a,e that the area of white colonies visible in Figure 7e decreases. In other words, the culture medium put into the PUD film produced an obvious bacteriostatic zone around the film, but there was no bacteriostatic zone in the blank sample after 24.0 h.
Figure 7.
Coating method to test the antibacterial effect of 1% azetidinium cationic PUD film. (a) Colony image of the blank sample. (b) Blank sample with enhanced color difference. (c) Enlarged map of the colony statistical region of blank samples. (d) Statistical diagram of colony color difference intensity distribution of blank samples. (e) Colony image of the sample. (f) Sample with enhanced color difference. (g) Enlarged map of the colony statistical region of the sample. (h) Statistical diagram of colony color difference intensity distribution of the sample.
We use the color difference to distinguish the colony area of the white spot from the aseptic area and get Figure 7b,f. According to the proportion of intensity, the colonies in a part of the area were selected for statistics (see Figure 7c,g). The colony number of the culture medium with polyurethane film in this area was 85, while that of the blank sample was 224. Therefore, the newly synthesized waterborne polyurethane has a good inhibitory effect on EC bacteria.
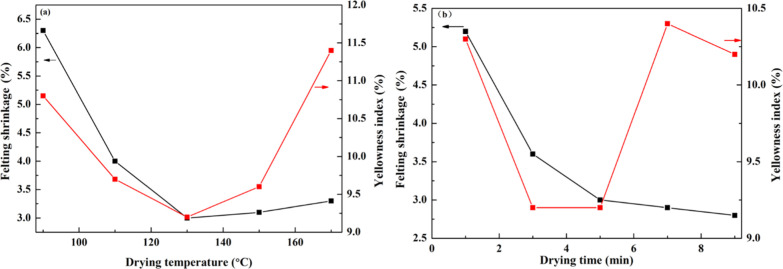
3.3. Antishrinking Properties of the Wool Fabric Finishing Agent
In order to confirm the best use method of applying finishing agents, we explored the dosage of PUDs in the finishing agent by felting shrinkage (see Figure 8) and studied the drying time and the drying temperature according to the test results of felting shrinkage and yellowing index of wool fabric after soaping, as shown in Figure 9. First of all, we changed the concentration of PUDs in the finishing solution, keeping the drying temperature and drying time unchanged, 150 °C and 5 min, respectively. In Figure 8, with an increase of PUDs in the finishing agent, the felting shrinkage of wool fabrics was decreased. When the finishing agent contained PUDs more than 30 g/L, the felting shrinkage of wool fabric was less than 8%, which conformed to the machine-washable IWS Standard (IWS TM 31). When there were more PUDs in the finishing agent, the thicker the elastic film with a cross-linking structure formed outside the wool scale layer, the greater the drag force to the migration of the wool fiber to its root, which reduces the felting shrinkage of the wool fabric. Especially, the concentration of PUDs was 50 g/L, the felting shrinkage decreased significantly. At that time, the felting shrinkage of wool fabric was less than 3%. When the PUD content in the finishing agent was more than 50 g/L, the movement of the wool scale layer had been greatly limited. The content of PUDs continued to increase, although the thickness and strength of the film formed on the wool surface of the protective coating increased; the activity space of wool fiber can be prevented and the proportion of uncoated wool fiber becomes less, so the felting shrinkage of wool fabric has a small change when the finishing agent increases the concentration of PUDs. Considering the effect of felting shrinkage and the cost of the finishing agent, the mass concentration of PUDs was determined to be 50%.
Figure 8.
Effect of finishing agents with different PUD contents on shrink-proof finishing of wool fabric.
Figure 9.
Variation diagram on felting shrinkage and the yellowing index of wool fabric: (a) the concentration of PUDs was 50 g/L; the drying time was 5.0 min. (b) The concentration of PUDs was 50 g/L; the drying temperature was 130 °C.
Next, we prepared the finishing agent by PUDs with a concentration of 50 g/L to treat wool fabric and further explored the drying time and drying temperature according to felting shrinkage and yellowing index. In Figure 9a, when the concentration of PUDs was 50 g/L and the drying time was 5.0 min, drying temperatures were changed to test the felting shrinkage and yellowing index of wool fabric in order to explore the best drying temperature. As shown in Figure 9a, with the increase of drying temperature, the felting shrinkage and the yellowing index of wool fabric were decreased continuously before 130 °C. However, when the temperature exceeds 150 °C, the felting shrinkage was increased instead. The yellowing index was increased at 130 °C. The reason for this trend was that a too high temperature would damage wool fiber, and the structure of PUDs might break as well. However, the initial decomposition temperature of this PUD film is 216 °C, as shown in Figure 5a. Under the conditions of high temperature, the increase of area shrinkage and yellowing index is due to the damage of wool fiber. Therefore, the curing temperature is 130 °C.
In Figure 9b, with the increase of drying time, the felting shrinkage had been decreased under a drying temperature of 130 °C. From the variation of yellowing index, it could be seen that when the drying time increased from 1.0 to 3.0 min, the yellowing index decreased quickly. When the drying time exceeds 5 min, the yellowing index increases obviously again. The reason for this phenomenon is that the actual drying degree of PUD coating increases with the extension of drying time and the density of protective film formed on the wool fiber also increases. In addition, the number of nitrogen heterocycles that cross-linked with wool fiber had increased; therefore, the strength of preventing the deformation wool fiber had been increased. However, if the drying time was too long, the wool fiber belongs to animal protein, which was seriously damaged for a long time at high temperature, resulting in the increase of yellowing index. Therefore, the best curing time was 5.0 min.
Therefore, the application of azetidinium cationic PUDs as a finishing agent for wool fabric should be adopted to choose 50 g/L concentration, 5.0 min curing time, and 130 °C curing temperature.
The antishrinkage properties and yellowing index of the two types of wool finishing agents in the market are compared (show in Table 2). Among them, DCCNa and TF-163A are scale-stripping wool finishing agents, which use DCCNa and a biological enzyme as phosphorus stripping agents to destroy the scale layer on the wool surface to achieve the antiwrinkling effect. RY-1107 and Goon815 belong to another kind of antishrinkage method, which utilize special quaternary ammonium salt and special silicone to enhance the adhesion strength between wool and wool fiber, respectively, so as to form a coating on the wool surface to achieve the purpose of phosphorus sheet wrapping wool antishrinkage agent.
Table 2. Performance Comparison between the Finishing Agent and Commercial Goods.
| shrinkage
(%) |
three stimulus values |
||||||
|---|---|---|---|---|---|---|---|
| type | SL | Sw | FS | X | Y | Z | yellowing index Y1 |
| blank | 2.3 | 7.0 | 9.3 | 52.1 | 54.5 | 46.9 | 9.5 |
| finishing agent (PUDs) | 2.4 | 0.6 | 3.0 | 50.7 | 53.0 | 45.8 | 9.2 |
| DCCNa | 2.4 | 1.3 | 3.7 | 54.1 | 57.3 | 46.6 | 13.2 |
| TF-163A | 0.8 | 0.6 | 1.4 | 56.6 | 59.3 | 52.5 | 6.9 |
| RY-1107 | 0.5 | 0.4 | 0.9 | 57.5 | 60.8 | 52.8 | 7.7 |
| Goon815 | 0.8 | 0.9 | 1.7 | 52.0 | 54.5 | 44.8 | 13.3 |
In Table 2, we can see that the finishing agent has superior felting shrinkage to DCCNa and is weaker than that of the other three. Moreover, the weft shrinkage resistance of this finishing agent is better than that of silicone resin type Goon815 and the same as the weft shrinkage resistance of TF-163A. In addition, the yellowing index of the finishing agent is lower than that of the traditional descaling agent DCCNa and silicone resin finishing agent. Therefore, compared with the commercial goods in the market, this finishing agent has antishrinkage performance and a small yellowing index, so it has a significant advantage in the field of wool antishrinkage.
4. Conclusions
A finishing agent for wool fabrics has been successfully prepared by the novel azetidinium cationic PUDs using dihydroxyalkyl glycidylamine as a chain extender. Through the characterization of film softness, water absorption, and coating appearance, we analyzed the applicability of the wool finishing field. Because of its low tensile strength, softness, and low water absorption, the film has an obvious effect on the coating of wool fiber. We performed antishrinking and yellowing index measurements to confirm the addition amount, curing time, and curing temperature of the finishing agent through a series of experiments, which revealed the relationship between antishrinking properties and the application process of the finishing agent. Based on the advantages of these PUDs, the finishing agent can be used directly without pretreatment, reaching the machine-washing standard of shrinkage resistance. Moreover, it has less effect on the environment. In the future, we expect that the novel azetidinium cationic PUDs will realize industrialization so as to commercialize these finishing agents with better performance.
Acknowledgments
The research, including the SEM/LC–MS/1HNMR measurements, was supported by Anhui Univeristy. We acknowledge Dr. Gong for his helpful discussions on antibacterial experiments. We acknowledge Yijiao Zhang and Shuzhen Zhen for their assistance on the DLS, TGA, and DSC measurements.
Author Contributions
The manuscript was completed through contributions of all authors. X.C. analyzed the data and wrote the manuscript. X.C. and X.W. performed the experiments. X.Y. provided the competitive products and application method related to competitive products. W.W. guided the whole process of this experiment and the revision of the manuscript. All authors revised and finally approved the submission.
The authors declare no competing financial interest.
References
- Cao C.; Yang Z.; Zheng S.; Wen S.; Ding W.; Liu A. Study on the microstructure of wool fabric by ftir and sem. Wool Text. J. 2017, 45, 14–17. [Google Scholar]
- Chen Y.; Li Z.. Design and application of wool felt craft in new chinese style fashion. Design and Application of Wool Felt Craft in New Chinese Style Fashion: Online Meeting, 2020; pp 370–375.
- Hassan M. M. Enhanced antistatic and mechanical properties of corona plasma treated wool fabrics treated with 2,3-epoxypropyltrimethylammonium chloride. Ind. Eng. Chem. Res. 2014, 53, 10954–10964. 10.1021/ie500447p. [DOI] [Google Scholar]
- Babu K. M.Chapter 3-natural textile fibres: animal and silk fibres. In Textiles and Fashion; Sinclair R., Ed.; Woodhead Publishing, 2015; pp 57–78. [Google Scholar]
- Chen D.; Tan L.; Liu H.; Hu J.; Li Y.; Tang F. Fabricating superhydrophilic wool fabrics. Langmuir 2010, 26, 4675–4679. 10.1021/la903562h. [DOI] [PubMed] [Google Scholar]
- Patil N. V.; Netravali A. N. Enhancing strength of wool fiber using a soy flour sugar-based “green” cross-linker. ACS Omega 2019, 4, 5392–5401. 10.1021/acsomega.9b00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wang X. A comparative study on the felting propensity of animal fibers. Textil. Res. J. 2007, 77, 957–963. 10.1177/0040517507083517. [DOI] [Google Scholar]
- Wenhong C.; Qicheng Z.; Qinhua Y. Mechanism and application of enzyme treatment on wool. J. Donghua Univ. 2011, 28, 226–232. [Google Scholar]
- Xue Z.; Jin-Xin H.; Yi-Zhen Z. The antimicrobial activity of wool fabrics treated with crosslinking agents and polyhexamethylene biguanide. China Text. 2009, 46–52. [Google Scholar]
- Borghei S. M.; Shahidi S.; Ghoranneviss M.; Abdolahi Z. Investigations into the anti-felting properties of sputtered wool using plasma treatment. Plasma Sci. Technol. 2013, 15, 37. 10.1088/1009-0630/15/1/07. [DOI] [Google Scholar]
- Zhao Q.; Sun G.; Yan K.; Zhou A.; Chen Y. Novel bio-antifelting agent based on waterborne polyurethane and cellulose nanocrystals. Carbohydr. Polym. 2013, 91, 169–174. 10.1016/j.carbpol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Hu C.; Jin Y. Wash-and-wear finishing of silk fabrics with a water-soluble polyurethane. Textil. Res. J. 2002, 72, 1009–1012. [Google Scholar]
- Cook J. R.; Fleischfresser B. E. Shrinkproofing wool with synthappret bap formulations containing urea. J. Text. Inst. 1985, 76, 122–127. 10.1080/00405008508658494. [DOI] [Google Scholar]
- Gehui W.; Weiyuan Z.; R P.; D P. Effect of commercial synthappret bap treatment on the tailorabilitv of light-weight worsted wool fabrics. J. Donghua Univ. 2001, 18, 101–104. [Google Scholar]
- Xiaohua L.; Qinghuan S.; Yongzhou T.; Weiqiang S.; Gang D. Synthesis and characterization of glycidyl diethanolamine. Henan Sci. 2005, 349–351. [Google Scholar]
- Zhong Z.; Sun X. S.; Wang D. Isoelectric ph of polyamide–epichlorohydrin modified soy protein improved water resistance and adhesion properties. J. Appl. Polym. Sci. 2007, 103, 2261–2270. 10.1002/app.25388. [DOI] [Google Scholar]
- Shahid-Ul-Islam; Shahid M.; Mohammad F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—a review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. 10.1021/ie303627x. [DOI] [Google Scholar]
- Li S.; Guo Z.; Zhang H.; Li X.; Li W.; Liu P.; Ren Y.; Li X. Abc triblock copolymers antibacterial materials consisting of fluoropolymer and polyethylene glycol antifouling block and quaternary ammonium salt sterilization block. ACS Appl. Bio Mater. 2021, 4, 3166–3177. 10.1021/acsabm.0c01571. [DOI] [PubMed] [Google Scholar]
- Ohta Y.; Kondo Y.; Kawada K.; Teranaka T.; Yoshino N. Synthesis and antibacterial activity of quaternary ammonium salt-type antibacterial agents with a phosphate group. J. Oleo Sci. 2008, 57, 445–452. 10.5650/jos.57.445. [DOI] [PubMed] [Google Scholar]
- Xujian W.; Yijiao Z.; Wusheng W. Synthesis of novel azetidinium cationic waterborne polyurethane dispersions. Paint Coat. Ind. 2022, 7, 48–53. [Google Scholar]
- Miao Z.; Long Y.; Yingshuang Z.; Wusong G.; Xujian W.; Wusheng W. Synthesis of cationic aqueous polyurethane dispersion with laterally pendant tertiary amine groups. Paint Coat. Ind. 2020, 50, 1–5. [Google Scholar]
- Chattopadhyay S.; Keul H.; Moeller M. Functional polymers bearing reactive azetidinium groups: synthesis and characterization. Macromol. Chem. Phys. 2012, 213, 500–512. 10.1002/macp.201100480. [DOI] [Google Scholar]
- Li M.; Liu F.; Li Y.; Qiang X. Synthesis of stable cationic waterborne polyurethane with a high solid content: insight from simulation to experiment. RSC Adv. 2017, 7, 13312–13324. 10.1039/c7ra00647k. [DOI] [Google Scholar]
- Shiyu Y.; Kelu Y.; Yi H. Prepareation and application of polyurethane-silica sol for wool anti-felting finish. Wool Text. J. 2009, 37, 1–5. [Google Scholar]
- Guise G. B.; Jackson M. B. The shrinkproofing of wool with the bisulphite adducts of polyisocyanates. J. Text. Inst. 1973, 64, 665–667. 10.1080/00405007308630317. [DOI] [Google Scholar]
- Kistamah N.; Carr C. M. Novel delayed-cure, durable press, shrink-resist treatment of wool fabrics and garments. Color. Technol. 2020, 136, 305–316. 10.1111/cote.12475. [DOI] [Google Scholar]
- çİftçİ A.; Miih K. Y.; Sagem-Bursa S. Shinkprofing woll with synthappret bap formulations containing urea. Tekst. Muhendis 1987, 1, 201–203. [Google Scholar]