Abstract
We analyzed the CD4 T-cell immunodominance of the response to a model antigen (Ag), MalE, when delivered by an attenuated strain of Salmonella enterica serovar Typhimurium (SL3261*pMalE). Compared to purified MalE Ag administered with adjuvant, the mapping of the peptide-specific proliferative responses showed qualitative differences when we used the Salmonella vehicle. We observed the disappearance of one out of eight MalE peptides' T-cell reactivity upon SL3261*pMalE immunization, but this phenomenon was probably due to a low level of T-cell priming, since it could be overcome by further immunization. The most striking effect of SL3261*pMalE administration was the activation and stimulation of new MalE peptide-specific T-cell responses that were silent after administration of purified Ag with adjuvant. Ag presentation assays performed with MalE-specific T-cell hybridomas showed that infection of Ag-presenting cells by this intracellular attenuated bacterium did not affect the processing and presentation of the different MalE peptides by major histocompatibility complex (MHC) class II molecules and therefore did not account for immunodominance modulation. Thus, immunodominance of the T-cell response to microorganisms is governed not only by the frequency of the available T-cell repertoire or the processing steps in Ag-presenting cells that lead to MHC presentation but also by other parameters probably related to the infectious process and to the bacterial products. Our results indicate that, upon infection by a microorganism, the specificity of the T-cell response induced against its Ags can be much more effective than with purified Ags and that it cannot completely be mimicked by purified Ags administered with adjuvant.
CD4+ T cells recognize peptides presented by major histocompatibility complex (MHC) class II molecules. Class II molecules are mainly specialized to present peptides derived from an exogenous antigen (Ag) internalized by Ag-presenting cells (APC), such as dendritic cells (DC) and macrophages (Mφ) (16). After uptake, Ags are proteolytically degraded into peptides in the endocytic route. Peptides resulting from this process are loaded on newly synthesized class II molecules under the control of DM chaperones (47), in specialized prelysosomal compartments enriched in class II molecules (44, 56, 60). In these compartments the formation of sodium dodecyl sulfate-stable peptide-class II molecule complexes (49) leads to their presentation at the plasma membrane. In addition to synthesis, recycling in early endosomes enables mature class II molecules to acquire processed peptides in these compartments (41, 63). As a result of these Ag processing events and of T-cell repertoire shaping in the thymus, the T-cell response mainly focuses on a few determinants among the potential T-cell determinants contained into the primary structure of a protein Ag (1). This immunodominance phenomenon has been extensively studied mainly with purified proteins administered in adjuvants (51), but little is known about the rules that govern the selection of immunodominant T-cell epitopes in response to live microorganisms (37).
T-cell immunity to intracellular bacteria has to deal with the interaction between bacteria and host APC, namely, DC and Mφ, that are involved in Ag presentation to T cells, which in turn feed back APC for pathogen clearance. Uptake of bacteria by APC is performed by phagocytosis mediated by Fc receptors, complement receptors, or any receptors for the components of the bacterial membrane (57). Actin polymerization in the contact region leads to engulfment of bacteria and formation of phagosomes. Maturation of phagosomes to phagolysosomes enables in most cases the destruction of internalized microorganisms. However, some intracellular bacteria can alter phagosome maturation and its microbicidal properties to create a propitious environment for their survival and replication. For instance, Salmonella enterica serovar Typhimurium enhances acidification of phagosomes (46), whereas Mycobacterium avium prevents a pH decrease (52). Consequently, endocytic resident bacteria can modulate class II molecule trafficking and proteolytic degradation of bacterial Ag and therefore might affect the repertoire of epitopes displayed for T-cell activation. More generally, many mechanisms of immunological escape have been characterized for viruses (4), bacteria (59), or parasites (10) that interfere with the MHC class I or class II pathways and therefore impair the elicitation of a T-cell immune response.
Originally, attenuated strains of serovar Typhimurium were a model for typhoid vaccination (21), but they have since been widely developed as a vehicle for the delivery of pathogen-derived Ags in order to induce immunity against various microorganisms (20, 42, 55, 62). Moreover, recombinant, homologous, attenuated serovar Typhi strains have been subjected to clinical evaluation (18, 26, 54). Salmonellae are facultative intracellular bacteria which can survive in Mφ within a phagolysosome distinct from the degradative pathway (45). However, in vitro, Salmonella can deliver Ag in either the MHC class I or class II pathway of Mφ and DC (38, 53). Indeed, such attenuated Salmonella can efficiently deliver heterologous Ag to induce a Th1 immune response (22), as well as cytotoxic T-lymphocyte responses in vivo (2, 14, 20).
We studied the immunodominance of the CD4+ T-cell response to a model Ag, Escherichia coli MalE protein, expressed by an attenuated aroA strain of serovar Typhimurium with a deletion of its own malE gene (SL3261*) by examining the ability of the Salmonella-APC interaction to modulate the presentation of immunogenic peptides by class II molecules. Comparison of proliferative responses to MalE peptides after immunization with purified MalE in adjuvant or with live SL3261* bacteria expressing MalE (SL3261*pMalE) shows that Salmonella Ag delivery induces a broadened epitope-specific response. In vitro and ex vivo presentation assays indicated that the modulation of Ag processing events is not responsible for this epitope modulation but that the epitope modulation rather involves the level of activation of APC.
MATERIALS AND METHODS
Mice and cell lines.
Six- to ten-week-old female C57BL/6 (H-2b) inbred mice (IFFA Credo, L'Arbresles, France) were used in all experiments. Hypoxanthine-aminopterin-thymidine (HAT)-sensitive A20 and BW5147 (α−β−) cell lines were given by J.-G. Guillet (Institut Cochin de Genetique Moleculaire, Paris, France). L (I-Ab) is a fibroblast line transfected with MHC class II I-Ab genes and was kindly given by M. Viguier (ICGM). LB27.4 (H-2b/d) is a B lymphoma line. LB27.4pMalE corresponds to the LB27.4 cell line transfected with the E. coli malE gene (7). The granulocyte-Mφ colony-stimulating factor-secreting J558 cell line was obtained from P. Marche (CEA, Grenoble, France).
Peptides and MalE protein.
Based on the sequence of MalE (12), a complete set of 385 overlapping 15-mer peptides was synthesized on polyethylene pins according to standard PEPSCAN procedures (17, 58). The final peptides were released from their support under basic conditions and contained a free amino terminus and an amidated C terminus. In addition to this mg scale synthesis, selected regions of the MalE sequence were synthesized at 20 mg scale according to standard synthesis procedures for peptides using Wang resin (p-alkoxybenzylalcohol resin; Bachem, Dubendorf, Switzerland), resulting in peptides with free amino and carboxy termini. The MalE protein was purified by affinity chromatography from E. coli strain ED9 (pPD1) as described previously (31). Purified MalE protein was concentrated by ammonium sulfate precipitation, resuspended in phosphate-buffered saline (PBS), and desalted on Sephadex G25 (Pharmacia Inc.) in PBS. The concentration of the protein was determined by UV absorbance.
Bacterial strains and growth conditions.
The SL3261* strain (previously reported as SL3261ΔMalE) was derived from the aroA serovar Typhimurium strain SL3261 (21) (a gift from B. A. D. Stocker) by introducing a deletion in the serovar Typhimurium malE gene as previously described (30). The E. coli malE gene was expressed constitutively on a multicopy plasmid derived from pBR322 under the control of the ptac promoter, leading to SL3261*pMalE. The MalE protein produced by these bacteria was quantitated using a monoclonal antibody (MAb), 56.5, directed against E. coli MalE (unpublished data). SL3261*pMalE expressed 15.7 ng of E. coli MalE per 106 bacteria. For immunization, bacteria were cultured for 16 h at 37°C, without shaking, in a closed bottle of L broth (33) containing 100 μg of ampicillin per ml. Bacteria were collected by centrifugation and resuspended in PBS at the required concentration. Bacteria were prepared on the day of immunization, and the concentration was estimated by the optical density of the suspension at 600 nm (1 optical density unit = 5 × 108 bacteria/ml). The viable count and the plasmid stability were confirmed by plating suitable dilutions on L agar plates with or without 100 μg of ampicillin/ml.
T-cell proliferation assay.
Mice were immunized subcutaneously (s.c.) or intraperitoneally (i.p.) with the purified MalE protein in adjuvant or i.p. with 107 bacteria. Draining inguinal lymph nodes (LN) or the spleen was removed, and single-cell suspensions were prepared and cultured in HL-1 medium (Hycor Biomedical Inc., Irvine, Calif.) with 2 mM l-glutamine. A total of 106 LN cells/well were plated onto 96-well microtiter plates (TPP, Trasadingen, Switzerland) in duplicate or triplicate with Ag. After 3 days at 37°C, cells were pulsed for 18 h with [3H]thymidine (NEN, Boston, Mass.). Incorporated radioactivity was measured by scintillation counting. Results were expressed as mean counts per minute (or change in counts per minute [Δcpm] when variations in background proliferation between groups were more than twofold) from duplicate or triplicate culture wells. Results are representative of two or three experiments.
Generation of T-cell hybridomas.
Mice were immunized s.c. with 10 μg of the MalE protein emulsified in CFA (Sigma, St. Louis, Mo.). Seven days later, LN were removed, and a single-cell suspension was prepared and cultured in complete medium used in T-cell proliferation assays with 10 μg of MalE per ml. Three days later, viable lymphocytes were isolated by fractionation with Ficoll (Seromed, Berlin, Germany) and fused with an equal number of BW5147 (α−β−) myeloma cells using 0.5 ml of polyethylene glycol 1500 (50%; Boehringer GmbH, Mannheim, Germany). The cell suspension was brought to a final volume of 40 ml with RPMI 1640 (Seromed) supplemented with 20% fetal calf serum, 5 × 10−5 M 2-mercaptoethanol, 2 mM glutamine, and antibiotics. After incubation for at least 2 h at 37°C, feeder HAT-sensitive A20 cells were added to a final concentration of 105/ml. Then cells were plated onto 96-well flat-bottomed microtiter plates with 100 μl/well. Sixteen hours later, 20 μl of 6× HAT (Boehringer) was added to each well. Hybridomas appeared 7 to 15 days later and were assayed for peptide-specific reactivity. For screening, T-cell hybridomas were expanded to near confluence in 24-well plates. Then 100-μl aliquots of resuspended cultures from 1-ml wells were added to L (I-Ab) APC pulsed with 25 μM MalE or with 5 μM concentrations of the following MalE peptides: p100–114, p120–134, p151–165, p207–221, p221–235, p262–276, p296–310, and p341–370. Hybridomas for each peptide specificity were selected.
For p40–54-specific T-cell hybridomas, T lymphocytes were primed in vivo and then restimulated in vitro with the p40–54 peptide before fusion. Screening was performed with the following APC: (i) LB27.4 cells transfected with the E. coli MalE gene, (ii) LB27.4 cells with 25 μM MalE, and (iii) L (I-Ab) cells with 5 μM p40–54. Hybridomas responding to these different APC were further used in the study. The production of interleukin-2 (IL-2) was measured using CTLL cells as described below.
In vitro Ag presentation assay.
Peritoneal exudate cells (PEC) were obtained after washing the peritoneal cavities of C57BL/6 mice injected 4 days before with 2 ml of thioglycolate (Sanofi Diagnostics Pasteur, Marne La Coquette, France). PEC were incubated for 1 h at 37°C, and then plastic adherent cells were used as APC. DC were prepared from C57BL/6 mice mainly as described previously (25). After appropriate depletion steps, bone marrow cells were cultured with a supernatant from J558 cells expressing granulocyte-Mφ colony-stimulating factor for 5 days. About 70% of the cells were DC as determined using anti-CD11c MAb (HL-3 clone; Pharmingen, San Diego, Calif.), while remaining cells were granulocytes (anti-Gr-1 MAb [RB6-8C5 clone]; Pharmingen) that do not express MHC class II molecules. These cells were used as APC. For two to four hours, 2 × 105 PEC or DC were incubated with the MalE protein or with bacteria, and cells were washed twice before fixation with 0.05% glutaraldehyde. In some experiments, PEC were first treated with brefeldin A (BFA; Sigma) or with cytochalasin B (CCB; Sigma). A total of 105 T-cell hybridomas were added to these APC for 24 h, and then 100-μl aliquots of supernatants were cultured with 104 cells of the IL-2-dependent CTLL cell line. Two days later, [3H]thymidine (0.3 μCi/well; AS = 1 Ci/mmol) was added, and the cells were harvested 18 h later with an automated cell harvester. Incorporated thymidine was detected by scintillation counting.
Ex vivo Ag presentation assay.
Mice were immunized s.c. with MalE in adjuvant, and 2 to 5 days later, LN cells were recovered. T cells were depleted from the cell suspension by incubation for 30 min at 37°C with anti-Thy1.2 MAb (clone 30H12 from the American Type Culture Collection) mixed with complement prepared from guinea pig serum (bioMerieux SA, Marcy l'Etoile, France). Cells were then left untreated or fixed with 0.05% glutaraldehyde. After two washes, serial dilution of cells in complete medium were plated, and then 105 T-cell hybridomas were added to these APC for 24 h. IL-2 content in the culture was determined as described above.
ELISPOT.
MalE-specific gamma interferon (IFN-γ)-secreting cells were measured by enzyme-linked immunospot assay (ELISPOT). Whole spleen cells from individual immunized mice were recovered, and serial cell dilutions in complete RPMI medium were plated in ester-cellulose bottom plates (Millipore, St. Quentin en Yvelines, France) previously coated with 4 μg of a capture antibody specific for IFN-γ per ml (clone R4-6A2; Pharmingen). Cells were incubated at 37°C for 48 h in the presence of Ag and tested in duplicate. Afterward, cells were removed by washing the plates twice with H2O-Tween (0.05%) and five times with PBS-Tween (0.05%), and then biotinylated anti-mouse IFN-γ (clone XMG1.2; Pharmingen) was added at 4 μg/ml and incubated for 3 h at room temperature (RT). After extensive washing in PBS-fetal calf serum (0.05%), streptavidin-alkaline phosphatase was added for 3 h at RT. We then added under 100 μl of 5-bromo-4-chloro-3-indolylphosphate–tetrazolium salts substrate (Sigma) and incubated it at RT until blue spots developed. The frequency of IFN-γ-secreting cells was determined by dividing the number of spots counted in each well by the total number of spleen cells plated at a given dilution. The results are expressed as the number of specific cells secreting IFN-γ in response to the restimulating peptide.
RESULTS
Characterization of class II-restricted T-cell response to MalE in C57BL/6 mice.
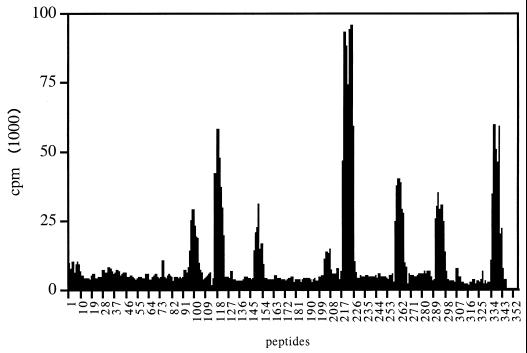
To map the dominant CD4+ T-cell determinants of the MalE protein, we used a set of 15-amino-acid-long synthetic peptides that walk over the MalE protein sequence with a single amino acid step. C57BL/6 mice were immunized with 10 μg of MalE protein in CFA, and proliferation of LN cells from these mice was determined after in vitro stimulation with MalE synthetic peptides. As shown in Fig. 1, eight nonoverlapping T-cell epitopes were characterized in C57BL/6 mice and were defined by their minimal core sequences as 105–111, 124–130, 154–161, 208–218, 226–232, 266–273, 298–305, and 343–350. T-cell hybridomas specific for these eight epitopes (see Fig. 2A for response of these T-cell hybridomas to MalE) were produced using MalE-primed LN T cells, showing that these epitopes represent dominant T-cell determinants. All these T-cell determinants were presented to T cells by the I-Ab molecule since C57BL/6 mice express only this unique MHC class II restriction element. That was further confirmed using an I-Ab transfected cell line as APC to stimulate these T-cell hybridomas in the presence of these MalE peptides (data not shown).
FIG. 1.
Mapping of the dominant T-cell determinants of the MalE protein in C57BL/6 mice. C57BL/6 mice were primed s.c. with 10 μg of MalE protein emulsified in CFA. Ten days later, LN cells were stimulated in vitro with overlapping 15-mer peptides (6.5 μM concentration) covering the entire MalE sequence in a single amino acid step, which is referred as the NH2-terminal amino acid of each peptide. Proliferation was determined by 3H incorporation on day 4.
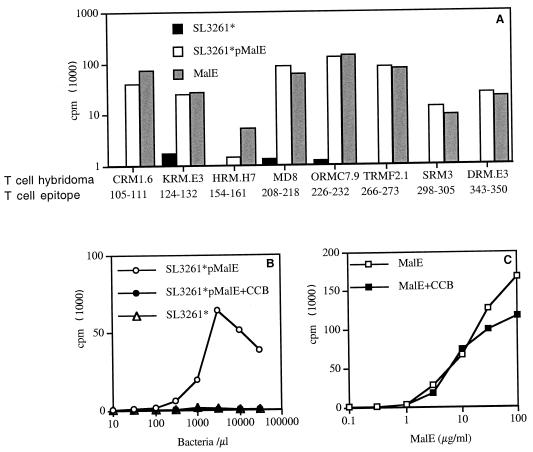
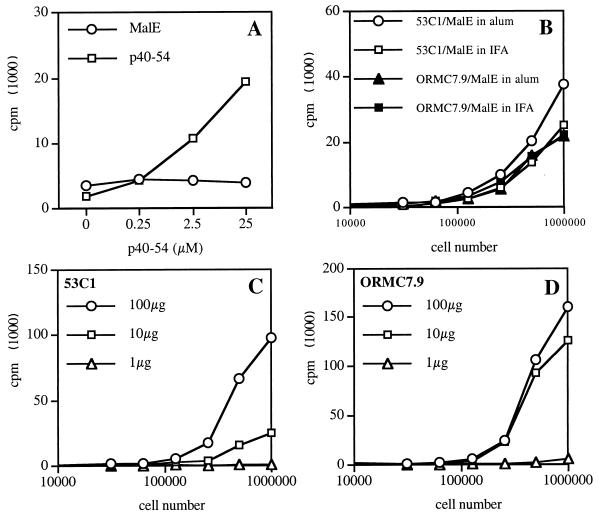
FIG. 2.
Stimulation of MalE-specific T-cell hybridomas using SL3261*pMalE. (A) PEC were incubated for 2 h with either 100 μg of purified MalE protein per ml, 107 SL3261*pMalE bacteria per ml, or 107 control SL3261* bacteria per ml and were then fixed with glutaraldehyde. These APC were used to stimulate the different MalE-specific T-cell hybridomas with the indicated specificity. (B and C) PEC were first incubated with or without 10 μM CCB. Serial dilutions of purified MalE protein or SL3261*pMalE were then added for 2 h. Fixed PEC were then cultured with the KRM.E3 T-cell hybridoma. The IL-2 content in the 24-h supernatant was measured using the CTLL cell line.
In vitro and in vivo analysis of the specificity of the T-cell response induced against MalE delivered by aroA serovar Typhimurium.
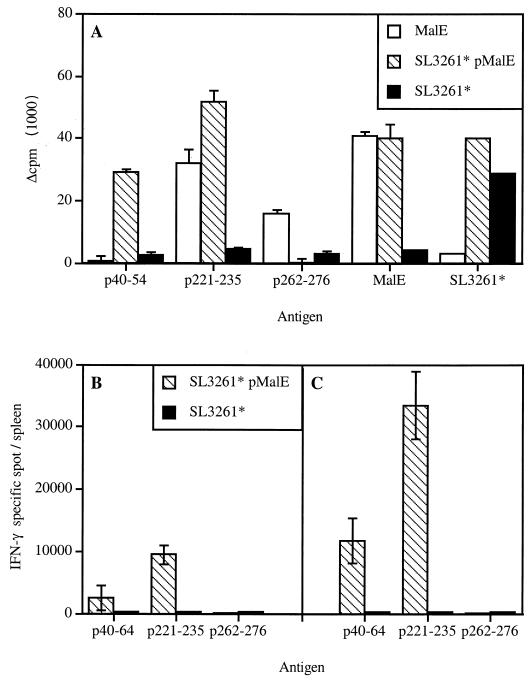
We first looked at the ability of APC infected with SL3261* expressing the MalE Ag to process and present the different MalE determinants to T cells in vitro. As shown in Fig. 2A, all the different T-cell hybridomas raised against the purified MalE protein were efficiently stimulated by PEC incubated with SL3261*pMalE or the MalE protein but not by PEC incubated with the control, SL3261*. However, one can notice that the HRM.H7 T-cell hybridoma that is specific for MalE (154–161) was poorly stimulated by SL3261*pMalE. The stimulation of these T-cell hybridomas was inhibited by CCB in the case of SL3261*pMalE but not in the case of soluble MalE protein, as illustrated in Fig. 2B and C for the KRM.E3 T-cell hybridoma. This indicates that phagocytosis of bacteria is a prerequisite for MalE presentation by the APC and that presentation is not due to soluble material released by the bacteria. These results also indicate that the APC-Salmonella interaction does not modify the processing and presentation by MHC class II molecules of the recombinant MalE Ag and therefore should not alter the subsequent T-cell response. To verify this, we next analyzed the MalE-specific T-cell response induced by SL3261*pMalE. We selected peptides p100–114, p120–134, p151–165, p207–221, p221–235, p262–276, p296–310, and p341–355 among the different MalE peptides to further study these T-cell determinants, since each one contains a single core sequence and strongly stimulates proliferative responses to itself (data not shown). C57BL/6 mice were immunized twice i.p. on days 0 and 21 with SL3261*pMalE or the SL3261* control. Three to four weeks later, spleen cells were restimulated in vitro with the different MalE synthetic peptides (Fig. 3A). Analysis of the peptide specificity of this T-cell response showed that an efficient priming is induced for p100–114, p120–134, p151–165, p207–221, p221–235, p296–310, and p341–355 (seven out of the eight T-cell determinants in Fig. 1) but not for p262–276 or the negative control, p239–253. To further confirm this observation, Salmonella-primed spleen cells were restimulated with overlapping 15-mer peptides spanning the entire MalE sequence but pooled by 12 consecutive peptides (Fig. 3B).
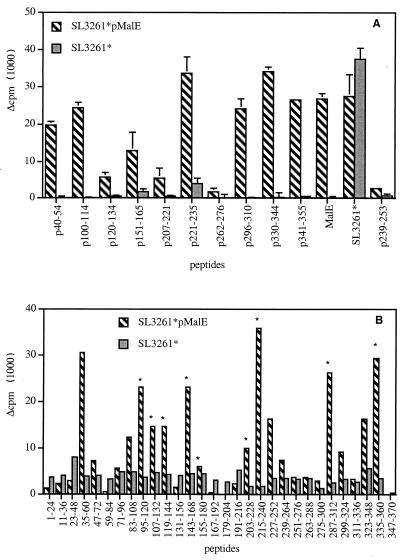
FIG. 3.
Analysis of the MalE-specific T-cell repertoire stimulated in vivo by SL3261*pMalE. C57BL/6 mice (two per group) were i.p. immunized on days 0 and 21 with 106 SL3261*pMalE or control SL3261* bacteria. Spleen cells from each group were pooled and were stimulated in vitro with the indicated Ag. (A) Indicated MalE synthetic peptides were tested individually at 10 μM, purified MalE was tested at 0.25 μM, and SL3261* extract was tested at a dilution corresponding to 106 bacteria/ml. (B) Series of 12 peptides were pooled (2 μM concentration of each peptide) and tested for reactivity. The peptide pools are referred to as the sequence encompassed by peptide series. Proliferation was determined by 3H incorporation on day 4. Asterisks indicate positive proliferative responses corresponding to those depicted in Fig. 1.
Strikingly, significant proliferative responses were observed for 14 peptide pools, whereas only 9 correspond to the MalE T-cell epitopes depicted in Fig. 1. Among the five newly reactive peptide pools, 35–60 and 323–348 were further characterized by fine mapping, showing that they correspond in proliferation assays to 42–52 and 332–338 T-cell sequences, respectively (data not shown). As shown in Fig. 3A, after SL3261*pMalE immunization, a very efficient T-cell proliferative response was induced for these two T-cell epitopes using the p40–54 and the p330–344 peptides. These results seem to indicate that immunization with SL3261*pMalE broadens the spectrum of MalE-specific T-cell responses compared to purified MalE administered in adjuvant. However, no proliferative response to pools 251–276 and 263–288 (Fig. 3B) was observed, confirming the lack of detectable T-cell response to the 266–273 T-cell determinant (p262–276) (Fig. 3A). It should be mentioned that this modification of T-cell repertoire specificities using Salmonella versus Ag in adjuvant does not influence the level of antibody responses, as members of our group previously showed that the MalE-specific antibody was identical in both cases (13).
The appearance of new T-cell reactivities in SL3261*pMalE-primed mice is not tuned by Ag-processing steps in infected APC.
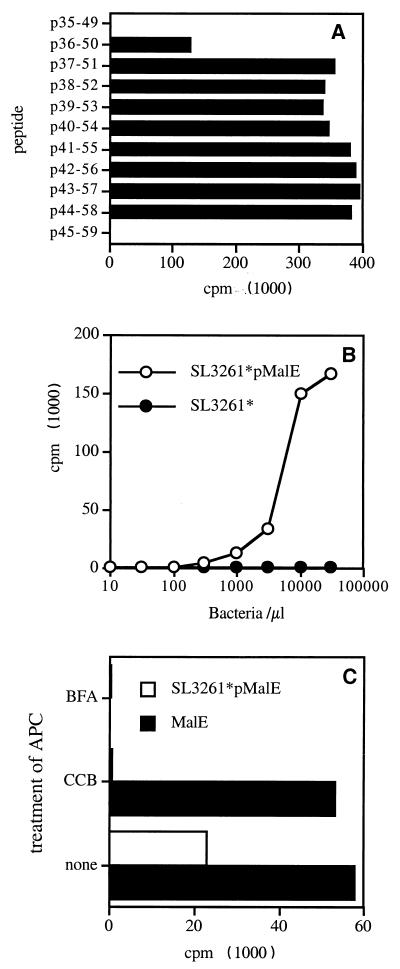
In order to investigate if the appearance of new T-cell reactivities could result from the modification of processing steps in infected APC, we produced specific T-cell hybridomas using the p40–54 synthetic peptide. The minimal core sequence of MalE recognized by a typical T-cell hybridoma, 53C1, is depicted in Fig. 4A and is defined by amino acids 44 to 50. Figure 4B shows the in vitro stimulation of the 53C1 T-cell hybridoma by C57BL/6-derived PEC incubated with SL3261*pMalE or the control, SL3261*. In these conditions, SL3261*pMalE, but not SL3261*, stimulated the p40–54-specific T-cell hybridoma, confirming in vivo results on the ability of SL3261*pMalE-infected mice to elicit a p40–54-specific T-cell response. Figure 4C shows that the presentation of the p40–54 T-cell epitope using SL3261*pMalE was dependent upon Salmonella phagocytosis since it was inhibited by CCB. Unexpectedly, when purified MalE was used as Ag, it also stimulated the p40–54-specific T-cell hybridoma 53C1. It should be noted that only 1 out of 14 p40–54-specific T-cell hybridomas tested was not stimulated by the MalE protein (data not shown), indicating that the in vitro processing and presentation of the p40–54 T-cell epitope from the purified MalE protein represent a general phenomenon. The sensitivity of the p40–54 T-cell epitope to BFA following Salmonella delivery of MalE (Fig. 4C), as well as for the other MalE T-cell epitopes (data not shown), demonstrates that its production uses the classical class II pathway involving nascent class II molecules. This result was further confirmed using PEC derived from invariant chain-deficient mice, which were unable to present the different MalE T-cell epitopes to specific T-cell hybridomas following SL3261*pMalE infection (data not shown).
FIG. 4.
Stimulation of p40–54-specific T-cell hybridoma by purified MalE or SL3261*pMalE. (A) The 53C1 T-cell hybridoma was stimulated by a 10-μM concentration of synthetic peptides corresponding to residues 35 to 60 from MalE in the presence of an I-Ab transfected L cell. (B) PEC were incubated for 2 h with SL3261*pMalE or SL3261*. After fixation these cells were used to stimulate the 53C1 T-cell hybridoma. (C) PEC were incubated with 10 μg of MalE per ml or with 106 SL3261*pMalE bacteria per ml alone, with 10 μM BFA, or with 10 μM CCB. The IL-2 content in the 24-h supernatant was measured using the CTLL cell line.
Following immunization with MalE in adjuvant, we were unable to detect any proliferative response to p40–54 (Fig. 1 and 5A), whereas immunization with p40–54 efficiently primed specific T cells (Fig. 5A). As shown in Fig. 4C, T-cell hybridomas specific for p40–54 were efficiently stimulated by APC pulsed with purified MalE, showing that efficient processing can occur at least in vitro. To clearly demonstrate that the lack of in vivo stimulation of p40–54-specific T cells by MalE did not result from deficient processing, we isolated APC from LN after immunization of mice with purified MalE in adjuvant. These in vivo-pulsed APC were directly used to stimulate the 53C1 (specific for p40–54) and the ORMC7.9 (specific for p221–235) T-cell hybridomas (Fig. 5B, C, and D). Under these conditions, both T-cell hybridomas were efficiently stimulated, showing that MHC class II molecules display the immunodominant p221–235 T-cell epitope, as well as the cryptic p40–54 T-cell epitope. The MalE Ag dose required for ex vivo detection of peptide-MHC complexes formed in vivo was identical for both T-cell epitopes (Fig. 5C and D). Altogether, these results suggest that the modification of intracellular processing events in Salmonella-infected APC is not responsible for the presentation of the p40–54 T-cell epitope, since processing and presentation of p40–54 T-cell epitope are achieved in vitro and in vivo with the purified MalE protein.
FIG. 5.
The crypticity of p40–54 following immunization with purified MalE in adjuvant is not due to inefficient processing in vivo. (A) Mice were s.c. immunized with 10 μg of MalE or p40–54 in CFA. Ten days later, LN cells were restimulated with serial dilutions of the p40–54 peptide. Proliferation was determined by 3H incorporation on day 4. (B, C, and D) Mice were s.c. immunized with MalE in adjuvant. Three days later, T-cell-depleted LN cells were directly used as APC to stimulate T-cell hybridomas specific for p40–54 (53C1) or p221–235 (ORMC7.9). (B) One hundred micrograms of MalE was administered in IFA or alum as indicated. (C and D) Indicated doses of MalE were injected together with IFA.
Epitope modulation cannot be explained by discrepancies in epitope processing efficiency of purified MalE and SL3261*pMalE.
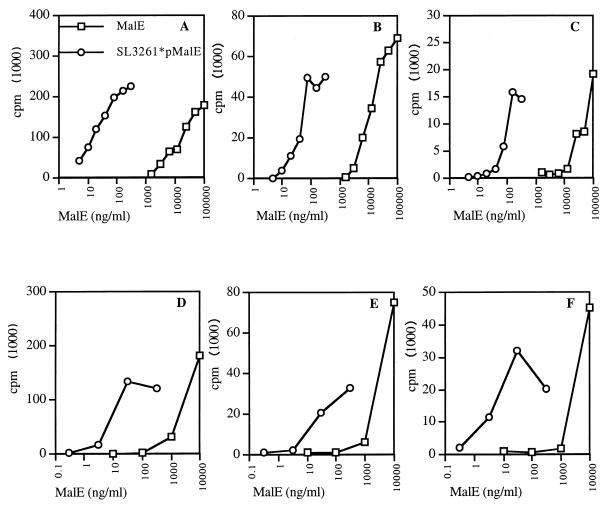
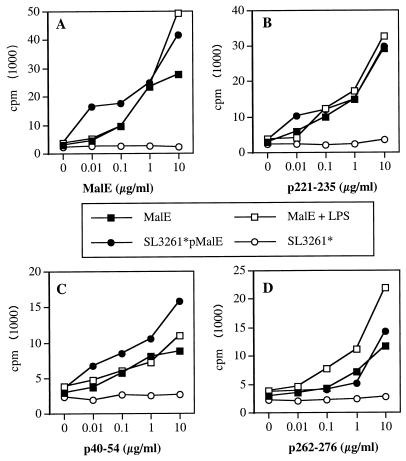
Since it is clear that processing capabilities of infected APC do not account for the discrepancies observed in vivo in the T-cell response, we asked whether the efficiency of the MHC presentation of the different T-cell epitopes could be related to this phenomenon. Ag dose response assays show that the amount of MalE expressed by SL3261*pMalE and necessary to stimulate the ORMC7.9 T-cell hybridoma specific for the immunodominant p221–235 T-cell epitope was 100-fold lower than the amount of purified MalE protein required when PEC were used as APC (Fig. 6A). This calculation is based on the initial amount of MalE per bacterium but does not take into account de novo synthesis of MalE by the bacteria within the APC. The relative increase of MHC presentation for p221–235 was similarly observed with SL3261*pMalE-infected APC for the new p40–54 T-cell epitope (Fig. 6B) as well as for the p262–276 T-cell epitope, which is not immunogenic upon SL3261*pMalE infection (Fig. 6C). Identical results were obtained when DC were used as APC (Fig. 6D, E, and F), showing that DC and Mφ are both able to efficiently process and present MalE T-cell epitopes after Salmonella delivery. Altogether, these results indicate that processing events do not contribute to the modulation of T-cell responses observed in vivo.
FIG. 6.
Salmonella delivery of MalE does not modify the MHC presentation pattern of epitopes by Mφ and DC. PEC (A, B, and C) and DC (D, E, and F) were incubated for 2 and 3 h, respectively, with serial doses of SL3261*pMalE or purified MalE. After fixation these cells were used to stimulate the following T-cell hybridomas: ORM.C7.9 specific for p221–235 (A and D), 53C1 specific for p40–54 (B and E), or TRM.F2.1 specific for p262–276 (C and F). The IL-2 content in the 24-h supernatant was measured using the CTLL cell line.
The route of immunization does not account for broadening of MalE T-cell reactivities induced by SL3261*pMalE.
Since the discrepancies of MalE epitope-specific T-cell responses could be due to the experimental conditions used for monitoring the T-cell responses to purified MalE administered in adjuvant and to MalE delivered by live SL3261*pMalE, we analyzed the influence of the route of immunization on this phenomenon. Purified MalE in CFA was injected s.c., whereas live SL3261*pMalE was injected i.p., and the subsequent T-cell response was analyzed, respectively, in the LN and the spleen. In other words, differences in recruitment of T cells and APC in the spleen and in the LN could contribute to the modulation of the MalE-specific T-cell reactivities.
To address this issue, we compared the T-cell response induced by purified MalE protein administered in adjuvant by following the same experimental procedure used to analyze the response to MalE delivered by attenuated Salmonella. C57BL/6 mice were immunized i.p. with SL3261*pMalE or with purified MalE in alum on days 0 and 21. Three weeks later, the proliferative response to different MalE peptides was monitored in the spleen (Fig. 7A). Under these conditions, as previously shown, specific proliferative responses to the p221–235 and p40–54 peptides were observed after SL3261*pMalE immunization, whereas no response to p262–276 was induced. In contrast, after i.p. immunization with purified MalE in adjuvant, positive proliferative responses were observed for the p221–235 and p262–276 peptides but not for p40–54. This pattern of response was not dependent on the number and route of immunization, the Ag dosage, or the adjuvant used. Virtually identical results were obtained after one (Fig. 1) or two (days 0 and 21 [data not shown]) s.c. injections of 10 or 100 μg of purified MalE in Freund's adjuvant as well as after three i.p. injections (days 0, 21, and 42) of 100 μg of MalE in alum (data not shown).
FIG. 7.
The route of immunization does not account for the modulation of the peptide-specific T-cell priming for purified MalE and MalE delivered by SL3261*. (A) C57BL/6 mice (two per group) were i.p. immunized on days 0 and 21 with 100 μg of MalE in alum or with 106 SL3261*pMalE or control SL3261* bacteria. Spleen cells from each group were pooled and were stimulated in vitro with the indicated Ag and tested for proliferation on day 4. (B and C). Mice were intravenously immunized with a single dose of 106 SL3261*pMalE or control SL3261* bacteria. Three (B) and four (C) weeks later, spleen cells from individual mice (two per group) were recovered and tested for IFN-γ-producing cells by ELISPOT in response to the indicated antigenic stimulation. Each data point corresponds to the mean of the number of positive CD4+ T cells per spleen obtained in two individual mice.
The T-cell proliferative response to MalE peptides after SL3261*pMalE immunization gives a qualitative picture of the specific immune response induced. To analyze quantitatively the initial profile of the in vivo T-cell priming, C57BL/6 mice were intravenously injected with a single dose of SL3261*pMalE that allowed the detection of cytokine-producing cells after peptide restimulation, even though we could not efficiently detect proliferative responses (data not shown). We monitored the secretion of IFN-γ by cells after SL3261*pMalE immunization, since we previously showed that the proliferative response of CD4+ T cells to the MalE protein induced by SL3261*pMalE was associated with production of IL-2 and IFN-γ, but not production of IL-4 and IL-5 (30). As shown in Fig. 7B, 3 and 4 weeks after SL3261*pMalE administration, we were able to detect IFN-γ-secreting cells specific for the p40–64 and the p221–235 peptides but not for the p262–276 peptide (frequency < 10−6). These data confirm that immunization with SL3261*pMalE leads to a very efficient priming of p40–54-specific T cells, since as many as 15,000 IFN-γ-secreting cells per spleen were detected for this determinant after a single immunization. Altogether, these results show that the difference in MalE-specific T-cell responses between purified MalE and SL3261*pMalE is not related to the route of immunization but rather to the use of the Salmonella delivery vector.
Unresponsiveness to MalE 262–276 is overcome by a multiple-immunization scheme.
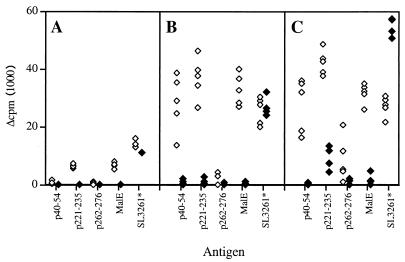
In the basic immunization scheme, two i.p. injections of SL3261*pMalE at days 0 and 21 are required to induce an efficient MalE specific proliferative response (30). Therefore, we examined whether the modulation of MalE epitope-specific T-cell responses was dependent upon the number of immunizations using the i.p. route. As shown in Fig. 8A, a very weak proliferative response specific for MalE, p221–235, or Salmonella extracts was induced after a single immunization with SL3261*pMalE. After a second immunization performed 21 days later, we observed a strong response to the p221–235 and p40–54 peptides but not to the p262–276 peptide (Fig. 8B). Therefore, we tested whether this unresponsiveness could be overcome by further immunization. C57BL/6 mice were immunized i.p. on days 0, 21, and 42, and then the specific proliferative response to the MalE peptides was examined in individual mice. Figure 8C shows that, under these conditions, two mice out of five were able to produce an efficient p262–276-specific response and two others exhibited a very weak response, whereas the response to p40–54 and p221–235 was still high in all mice. This result shows that the priming of p262–276-specific T cells is not totally impaired. However, it occurs at a much lower level with SL3261*pMalE than with purified MalE in adjuvant, in agreement with the capacity of SL3261*pMalE-infected APC to stimulate a p262–276-specific T-cell hybridoma.
FIG. 8.
Multiple immunizations with SL3261*pMalE enable p262–276-specific T-cell priming. A total of 106 SL3261*pMalE (open symbols) or control SL3261* (closed symbols) bacteria were injected i.p. in mice (five per group) on day 0 (A), days 0 and 21 (B), and days 0, 21, and 42 (C). Three weeks after the last immunization, spleen cells from individual mice were stimulated in vitro with the indicated Ag. Proliferation was determined by 3H incorporation on day 4. Each data point corresponds to an individual mouse.
In summary, priming with SL3261*pMalE leads to one major effect: the efficient stimulation of a larger peptide-specific T-cell repertoire which is accompanied by poor T-cell priming for one peptide specificity that can be overcome by hyperimmunization with SL3261*pMalE.
Fully activated APC are able to prime p40–54-specific T cells regardless of the formulation of the Ag.
Finally, we investigated whether fully activated APC pulsed in vitro with Ag were able to overcome epitope modulation in MalE-specific T-cell priming in vivo. Since PEC were able to stimulate all types of MalE-specific T-cell hybridomas when either SL3261*pMalE or free soluble MalE was used, we determined whether these cells would be able to prime any type of MalE-specific T cells. Thioglycolate-induced PEC, containing more than 85% Mφ (with a fully competent APC phenotype [Mac-1high I-Ab(high) CD40+ CD56+ B7.1+ B7.2+] [data not shown]), were pulsed in vitro for 4 h with SL3261*pMalE or SL3261*. Cells were washed to remove free bacteria and i.p. injected in C57BL/6 mice. Peptide-specific proliferative responses measured 2 weeks later in the spleen showed that MalE-specific T cells were primed for the p40–54, p221–235, and p262–276 determinants (Fig. 9). Likewise, when PEC pulsed with free soluble MalE, alone or together with Salmonella lipopolysaccharide, were injected, all these peptide-specific proliferative responses were also observed. Therefore, in agreement with T-cell hybridoma stimulation assays, when fully activated APC are used, no modulation in the specificity of T-cell activation is induced for the different MalE T-cell epitope regardless of the form of the Ag formulation. These results strongly suggest that the modulation in the epitope specificity of the response to MalE results from discrepancies in the activation or maturation state of APC. In other words, adjuvants and Salmonella infection will differ in the activation signals delivered to APC, leading to distinct cosignaling for T-cell activation.
FIG. 9.
In vitro-pulsed APC are able to prime T cells in vivo regardless of the Ag formulation. PEC were incubated for 4 h with 100 μg of MalE per ml alone, with 10 μg of lipopolysaccharide per ml, or with SL3261*pMalE or SL3261*. These cells were then i.p. injected in mice. Two weeks later, spleen cells were restimulated with MalE, p221–235, p40–54, or p262–276. Proliferation was determined by 3H incorporation on day 4.
DISCUSSION
In the present study, we investigated the MHC class II-restricted response to the MalE model Ag delivered by an attenuated aroA strain of serovar Typhimurium (SL3261*). We found that this Salmonella vehicle modulates the epitope specificity of the T-cell repertoire stimulated against the MalE Ag compared to that found while using purified MalE with classical adjuvants. The major effect observed in immunodominance modulation is the broadening of the T-cell epitope response to MalE after live SL3261*pMalE immunization, as measured by the proliferative response of spleen cells. This extension of peptidic reactivity was unexpected since as many as eight T-cell epitopes were already characterized using the purified MalE protein.
The modulation of the immunodominance of the T-cell response using bacteria or a vaccine vehicle was recently reported in three other studies, but all used Mycobacterium tuberculosis (3, 9, 64). In all cases, the bacteria stimulated a T-cell epitope repertoire similar to that stimulated by other antigenic preparations. In the study published by Anderton et al. (3), the number of hsp65-specific T-cell epitopes presented using killed M. tuberculosis was less than that obtained with the use of recombinant hsp65 due to Ag processing efficiency. Indeed, the stimulation of T-cell clones with these two different hsp65 preparations indicated that the amount of Ag in the M. tuberculosis preparation was too low to enable the presentation of poorly immunogenic epitopes.
In this regard, it can be noted that in our study, the amount of MalE Ag delivered by live SL3261*pMalE at the time of immunization was 50- to 500-fold less than the amount of purified MalE used with adjuvant. However, given that we were unable to determine MalE production in infected animals, we cannot exclude the possibility that the amount of Ag delivered by the bacteria is much greater, despite the fact that the AroA− attenuated Salmonella strain has limited replication capacity in vivo. Two other studies examined the CD4 and CD8 T-cell responses induced after M. tuberculosis infection and after DNA vaccination for 38-kDa Ag (64) and Ag85A (9). For Ag85A, the M. tuberculosis immunization induced a narrow epitope specificity compared to DNA immunization, whereas for 38-kDa Ag the epitope number was identical but with only two overlapping specificities between the two immunization schemes. We can also add that mice immunized with Mycobacterium bovis BCG expressing recombinant MalE display the same antigenic T-cell response pattern to MalE as elicited by administration of purified MalE in adjuvant (X. Jiao and R. Lo-Man, unpublished data).
The MHC class II pathway for presentation of Salmonella-delivered Ag is sensitive to chloroquine and BFA and therefore has the same characteristics as the pathway for soluble exogenous protein Ag (61). However, several recent reports indicated that two different class II pathways can be involved in the presentation of different epitopes belonging to the same antigenic molecule (41, 63). Indeed, some determinants are presented by a recycling pool of mature MHC class II molecules in an early endocytic compartment (BFA insensitive), whereas others are presented by BFA-sensitive nascent class II molecules, probably depending on their sensitivity to protease degradation. Here we were unable to see any difference in processing requirements for the different MalE T-cell epitopes using SL3261*pMalE. Another concern is the amount of MalE Ag necessary to obtain the same level of presentation for SL3261*pMalE and purified MalE. The processing and presentation efficiency was increased about 100-fold using SL3261*pMalE, to the same extent for all MalE T-cell epitopes.
The bactericidal role of Mφ makes them a privileged reservoir for bacterial Ag, but the key role of DC in initiating the T-cell response raises the question of the role of Mφ in Ag presentation of bacteria and in the associated T-cell response, even though it was postulated that Mφ might also be able to prime naive T cells (48). T-cell priming following immunization with purified Ag in adjuvant was clearly demonstrated to be mediated by DC (19). If Mφ play a crucial role when bacteria are the Ag vehicle, then it could be postulated that the immune modulation we observed is due to the superior processing capabilities of Mφ to those of DC. However, in vitro presentation studies show that Mφ as well as DC were able to present the different T-cell epitopes to corresponding T-cell hybridomas, demonstrating that no difference exists between these two APC types in processing MalE delivered by SL3261*pMalE. This finding is in agreement with previous reports showing that DC are fully competent in vitro for Ag presentation of bacteria (24) and in particular of Salmonella (53). Nevertheless, Ag processing capabilities of APC can be modulated by cytokines (15, 27). Recently, IL-6 was shown to decrease endosomal and lysosomal pH, enabling the processing and presentation of hen egg lysozyme-derived cryptic T-cell epitopes to T-cell hybridomas (11). Moreover, in this case, the in vitro presentation of cryptic T-cell epitopes was associated with priming for new T-cell specificities in vivo when IL-6-treated and Ag-pulsed DC were injected. Salmonella infection of cells also leads to the acidification of the bacterium-containing phagosome (46), but in our model no processing difference was observed in vitro between the purified MalE protein and SL3261*pMalE. Nevertheless, our data support the idea that processing events do not account for the modulation of T-cell responses we observed in vivo with purified MalE and MalE delivered by Salmonella. Therefore, the epitope modulation induced with SL3261*pMalE is likelier associated with a more general effect of the Salmonella vehicle on the level or phenotype of the T-cell activation.
Indeed, purified Ags mixed with alum or CFA lead to a Th0 or a mixed Th1-Th2 response (8, 50). By contrast, the induction of a strong Th1 response following immunization with Salmonella vaccine strains expressing foreign Ags is nicely illustrated in the leishmania model, where the Th1-Th2 balance is critical for protection (62). Indeed, the IL-12 burst following serovar Typhimurium infection (6, 40) promotes Th1 responses (23) that contribute to the elimination of the bacteria through IFN-γ production. Therefore, our explanation for our results is that purified MalE in adjuvant induces a low and undetectable proliferative response to some MalE epitopes due to a lower lymphokine production associated with the adjuvant stimulation. In contrast, following SL3261*pMalE immunization, these weak T-cell reactivities are strongly enhanced towards a Th1-like proliferative response as a result of IL-12 induced by APC infection. The threshold of activation or differentiation of T cells is under the control of the peptide-MHC complex density, which varies from one peptide to another depending on its affinity for a given MHC molecule (28, 35, 36, 39). Likewise, the costimulatory activity required for T-cell activation is dependent on the T-cell ligand density and is further enhanced by locally produced lymphokines, such as IL-12 (34). Thus, a higher IL-12 production level (maybe in association with other lymphokines, such as IL-18) will lead to MHC-restricted stimulation of T cells requiring higher activation and differentiation thresholds. Therefore, qualitative and quantitative variations in the lymphokine environment in response to the infection process or to the adjuvant would lead to discrepancies in the T-cell response to different immunogenic epitopes displayed by the same APC. We propose that in the case of the Salmonella delivery system and under our experimental conditions, all these costimulatory activities reach a sufficient level to give all the MalE peptides available on APC the capacity to activate the corresponding T-cell repertoire.
This model could also fit well with results from studies showing the modulation of the T-cell epitope repertoire when comparing T-cell response induced by M. tuberculosis infection and by DNA vaccination (9, 64). Indeed, DNA induces a strong Th1 response (5, 29, 32) and therefore could promote low peptide-specific T-cell responses. In contrast, T-cell responses induced by mycobacteria can develop into a Th1 or a mixed Th1-Th2 cytokine profile depending on the bacterial dose (43). This suggests that the balance of lymphokines which are produced (for instance, IL-12 versus IL-10 and IL-4) depending on the infection level (and probably depending on the bacterial products) modulates the activation and differentiation thresholds of T cells. In this respect, attenuated Salmonella seems to be a versatile and powerful vehicle for stimulating a large MHC class II-restricted response, although the results we obtained after parenteral administration of the Salmonella vaccine remain to be confirmed by studies using the natural route of infection, i.e., after oral administration.
In conclusion, the epitope modulation observed in delivery of our model Ag by an attenuated strain of serovar Typhimurium emphasizes the key role played by the antigenic formulation in inducing efficient immune responses. What is true for a live attenuated bacterial vaccine strain might also be true for a pathogenic bacterium, and therefore tuning of T-cell immunodominance to such microorganisms could in some cases be associated with an impaired immunity. Moreover, for vaccine design, the use of purified Ag may not be always suitable for any pathogen if dealing with immunodominance modulation.
REFERENCES
- 1.Adorini L, Appella E, Doria G, Nagy Z A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988;168:2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal A, Kumar S, Jaffe R, Hone D, Gross M, Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990;172:1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderton S M, van der Zee R, Noordzij A, van Eden W. Differential mycobacterial 65-kDa heat shock protein T cell epitope recognition after adjuvant arthritis-inducing or protective immunization protocols. J Immunol. 1994;152:3656–3664. [PubMed] [Google Scholar]
- 4.Beersma M F, Bijlmakers M J, Ploegh H L. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 5.Carson D A, Raz E. Oligonucleotide adjuvants for T helper 1 (Th1)-specific vaccination. J Exp Med. 1997;186:1621–1622. doi: 10.1084/jem.186.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong C, Bost K L, Clements J D. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infect Immun. 1996;64:1154–1160. doi: 10.1128/iai.64.4.1154-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement J M, Jehanno M. Secretion of a bacterial protein by mammalian cells. J Biotechnol. 1995;43:169–181. doi: 10.1016/0168-1656(95)00127-1. [DOI] [PubMed] [Google Scholar]
- 8.Comoy E, Capron E, Thyphronitis G. In vivo induction of type 1 and 2 immune response against protein antigens. Int Immunol. 1997;9:523–531. doi: 10.1093/intimm/9.4.523. [DOI] [PubMed] [Google Scholar]
- 9.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T-P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Souza Leao S, Lang T, Prina E, Hellio R, Antoine J C. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. J Cell Sci. 1995;108:3219–3231. doi: 10.1242/jcs.108.10.3219. [DOI] [PubMed] [Google Scholar]
- 11.Drakesmith H, O'Neil D, Schneider S C, Binks M, Medd P, Sercarz E, Beverley P, Chain B. In vivo priming of T cells against cryptic determinants by dendritic cells exposed to interleukin 6 and native antigen. Proc Natl Acad Sci USA. 1998;95:14903–14908. doi: 10.1073/pnas.95.25.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duplay P, Bedouelle H, Fowler A, Zabin I, Saurin W, Hofnung M. Sequences of the malE gene and of its product, the maltose binding protein of Escherichia coli K12. J Biol Chem. 1984;259:10606–10613. [PubMed] [Google Scholar]
- 13.Fayolle C, O'Callaghan D, Martineau P, Charbit A, Clément J M, Hofnung M, Leclerc C. Genetic control of antibody responses induced against an antigen delivered by recombinant attenuated Salmonella typhimurium. Infect Immun. 1994;62:4310–4319. doi: 10.1128/iai.62.10.4310-4319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J L, Weiss W R, Norris K A, Seifert H S, Kumar S, So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990;4:2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 15.Frosch S, Bonifas U, Reske-Kunz A B. The capacity of bone marrow-derived macrophages to process bovine insulin is regulated by lymphokines. Int Immunol. 1993;5:1551–1558. doi: 10.1093/intimm/5.12.1551. [DOI] [PubMed] [Google Scholar]
- 16.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 17.Geysen H M, Meloen R H, Barteling S J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez C, Hone D, Noriega F R, Tacket C O, Davis J R, Losonsky G, Nataro J P, Hoffman S, Malik A, Nardin E, Sztein M D, Heppner D G, Fouts T R, Isibasi A, Levine M M. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity in humans. J Infect Dis. 1994;169:927–931. doi: 10.1093/infdis/169.4.927. [DOI] [PubMed] [Google Scholar]
- 19.Guéry J C, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II restricted T cell after administration of protein in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess J, Gentschev I, Miko D, Welzel M, Ladel C, Goebel W, Kaufmann S H E. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection. Proc Natl Acad Sci USA. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 22.Hormaeche C E, Anjam Khan C M, Mastroeni P, Villarreal B, Dougan G, Chatfield S N. Salmonella vaccines: mechanisms of immunity and their use as carriers of recombinant antigens. In: Ala'Aldeen D A, Hormaeche C E, editors. Molecular and clinical aspects of vaccine development. Chichester, England: John Wiley & Sons; 1994. [Google Scholar]
- 23.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cells progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoo S H, St. Clair Roberts J, Mandal B K. Safety and efficacy of combined meningococcal and typhoid vaccine. Br Med J. 1995;310:908–909. doi: 10.1136/bmj.310.6984.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacsovics-Bankowski M, Rock K L. Presentation of exogenous antigens by macrophages: analysis of major histocompatibility complex I and II presentation and regulation by cytokines. Eur J Immunol. 1994;24:2421–2428. doi: 10.1002/eji.1830241024. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon γ by T cells. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leclerc C, Deriaud E, Rojas M, Whalen R G. The preferential induction of a Th1 immune response by DNA-based immunization is mediated by the immunostimulatory effect of plasmid DNA. Cell Immunol. 1997;179:97–106. doi: 10.1006/cimm.1997.1161. [DOI] [PubMed] [Google Scholar]
- 30.Lo-Man R, Martineau P, Dériaud E, Newton S M, Jehanno M, Clément J-M, Fayolle C, Hofnung M, Leclerc C D. Control by H-2 genes of the Th1 response induced against a foreign antigen expressed by attenuated Salmonella typhimurium. Infect Immun. 1996;64:4424–4432. doi: 10.1128/iai.64.11.4424-4432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martineau P, Guillet J G, Leclerc C, Hofnung M. Expression of heterologous peptides at two permissive sites of the MalE protein: antigenicity and immunogenicity of foreign B-cell and T-cell epitopes. Gene. 1992;113:35–46. doi: 10.1016/0378-1119(92)90667-e. [DOI] [PubMed] [Google Scholar]
- 32.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert P H, Siegrist C A. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 34.Murphy E E, Terres G, Macatonia S E, Hsieh C S, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon γ production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray J S, Ferrandis-Edwards D, Wolfe C J, Schountz T. Major histocompatibility complex regulation of T helper functions mapped to a peptide C terminus that controls ligand density. Eur J Immunol. 1994;24:2337–2344. doi: 10.1002/eji.1830241012. [DOI] [PubMed] [Google Scholar]
- 36.Murray J S, Madri J, Tite J, Carding S R, Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989;170:2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pamer E G, Sijts A J, Villanueva M S, Busch D H, Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pie S, Truffa-Bachi P, Pla M, Nauciel C. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect Immun. 1997;65:4509–4514. doi: 10.1128/iai.65.11.4509-4514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinet V, Malnati M S, Long E O. Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J Immunol. 1994;152:4852–4860. [PubMed] [Google Scholar]
- 42.Poirier T P, Kehoe M A, Beachey E H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988;168:25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Power C A, Wei G, Bretscher P A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1999;66:5743–5750. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu Y, Xu X, Wandinger N A, Dalke D P, Pierce S K. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994;125:595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathman M, Barker L P, Falkow S. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect Immun. 1997;65:1475–1485. doi: 10.1128/iai.65.4.1475-1485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roche P A. HLA-DM: an in vivo facilitator of MHC class II peptide loading. Immunity. 1995;3:259–262. doi: 10.1016/1074-7613(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 48.Rock K L, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996;156:3721–3726. [PubMed] [Google Scholar]
- 49.Sadegh-Nasseri S, Germain R N. A role for peptide in determining MHC class II structure. Nature. 1991;353:167–170. doi: 10.1038/353167a0. [DOI] [PubMed] [Google Scholar]
- 50.Sedlik C, Dériaud E, Leclerc C. Lack of Th1 or Th2 polarization of CD4+ T cell response induced by particulate antigen targeted to phagocytic cells. Int Immunol. 1997;9:91–103. doi: 10.1093/intimm/9.1.91. [DOI] [PubMed] [Google Scholar]
- 51.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 52.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 53.Svensson M, Stockinger B, Wick M J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 54.Tacket C O, Forrest B, Morona R, Attridge S R, LaBrooy J, Tall B D, Reymann M, Rowley D, Levine M M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990;58:1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tite J P, Russel S M, Dougan G, O'Callaghan D, Jones I, Brownlee G, Liew F Y. Antiviral immunity induced by recombinant nucleoprotein of influenza A virus. J Immunol. 1988;141:3980–3987. [PubMed] [Google Scholar]
- 56.Tulp A, Verwoerd D, Dobberstein B, Ploegh H L, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 57.Unanue E R. Mononuclear phagocytes. In: Paul W E, editor. Fundamental immunology. 3rd ed. Philadelphia, Pa: Lippincott-William & Wilkins; 1993. pp. 112–118. [Google Scholar]
- 58.van der Zee R, van Eden W, Meloen R H, Noordzij A, van Embden J D A. Efficient mapping and characterization of a T-cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989;19:43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez M A, Sicher S C, Proctor M L, Crowley J C, Lu C Y. Differential regulation of Ia expression and antigen presentation by listeriolysin-producing versus non-producing strains of Listeria monocytogenes. J Leukoc Biol. 1996;59:683–690. doi: 10.1002/jlb.59.5.683. [DOI] [PubMed] [Google Scholar]
- 60.West M A, Lucocq J M, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- 61.Wick M J, Harding C V, Normark S J, Pfeifer J D. Parameters that influence the efficiency of processing antigenic epitopes expressed in Salmonella typhimurium. Infect Immun. 1994;62:4542–4548. doi: 10.1128/iai.62.10.4542-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]
- 63.Zhong G M, Romagnoli P, Germain R N. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]