Abstract

Carbide slag is a solid waste with a high content of reactive CaO, which can be used as an active material for the chemical absorption of CO2 and calcium looping. Calcium looping of CaO-based absorbents is one of the most promising methods of thermochemical energy storage. However, the sintering of pores and a reduction in the CO2 diffusion rates as the carbonization/calcination cyclic reaction progresses have posed challenges to the practical application of CaO-based absorbents. This study proposes a method for alleviating the sintering of the pore structure by improving the activity and cycling stability of such absorbents by doping carbide slag with MgO and ZnO powders. Results showed that the raw material ratio, reaction temperature, and reaction time have a considerable influence on the CO2 absorption rate. Furthermore, the specific surface area and pore volume of the absorbents increased with increasing ZnO and MgO doping levels in the carbide slag. Thus, the problems of sintering and clogging of pores in CaO-based absorbents were effectively alleviated, and the MgO and ZnO-doped absorbents CMZ85 and CMZ90 maintained 41–42% CO2 absorption after 10 cycles. These results confirmed that the cyclic stability and absorbent activity improved significantly with the MgO and ZnO doping of carbide slag for the calcium looping process.
1. Introduction
A concentrated solar power (CSP) system for capturing solar radiation and converting this radiation into electricity has received extensive attention in recent years. Owing to the abundant and inexhaustible nature of solar energy resources, solar energy has been rapidly employed in industrial applications.1 Solar energy has also been used globally to reduce and control CO2 emissions.2 CSP generation is considered a promising renewable energy technology with the attributes of high efficiency and low costs.3 However, the intermittent nature of solar energy remains the main drawback of CSP systems.4 The intermittent nature of solar energy is as follows: this energy is influenced by changes in the weather, as well as changes in the time of day and seasonal variations, thereby preventing the widespread use of solar energy. This problem can be solved by installing thermal energy storage (TES) systems in CSP plants. Specifically, the intermittent heat collected by a CSP plant is stored as thermal energy. When energy is required, the heat stored from chemical reactions can be converted into a steady output of heat.3 TES can be classified into three main types, namely, sensible energy storage (SES), latent energy storage (LES), and thermochemical energy storage (TCES).5 SES is achieved by exploiting the high heat capacity of materials such as water, molten salts, and ceramics.6 LES uses phase change materials that can undergo phase changes to store latent heat.7 However, SES and LES are characterized by an energy loss problem. Compared with SES and LES, TCES can achieve a higher operating temperature and energy storage density through reversible chemical reactions.8
Calcium looping technology is mainly based on cyclic carbonization and calcination reactions of CaO. In addition, CaO-based materials are widely available from a range of sources, have low operating costs, and have a high energy storage density. Therefore, calcium looping technology is considered a promising technology for achieving thermochemical energy storage. The carbonization reaction of CaO-based materials can be used to absorb CO2 gas, and the heat released by the reaction can be used for thermochemical energy storage.9 This process is also an economical and efficient thermochemical energy storage method. Two main CaO-based materials in the thermochemical energy storage system are CaO/Ca(OH)210,11 and CaO/CaCO3.12,13 However, for high-temperature thermochemical energy storage, the CaO/CaCO3 system is the most reliable system, which relies primarily on the reversible carbonization and calcination reaction that proceeds as follows:
| 1 |
| 2 |
eq 1 describes a carbonization reaction process which is an exothermic reaction where the released energy can be converted and used. eq 2 describes an endothermic reaction process where the calcination of CaCO3 at high temperatures yields CaO and CO2. In this process, an external heat source (such as solar energy) can drive the heat absorption reaction, and the heat absorbed by the reaction is converted into chemical energy stored in the CaO particles. The results show that the reactivity of both CaO and CO2 decreases with increasing number of carbonization/calcination reaction cycles when high-temperature thermochemical energy storage is achieved via calcium looping.14,15
The deactivation mechanism of the CaO-based absorbents subjected to the cyclic carbonization/calcination reaction is shown in Figure 1a. The sintering of CaO-based absorbents usually occurs at temperatures far below the melting point of CaO because the contact between adjacent CaO particles increases when the solid reaches the Tamman temperature. Consequently, the particles combine, agglomerate, and grow, leading to the collapse or disappearance of the pore structures associated with the CaO particles, and the porosity and specific surface area of the CaO absorbents decrease.16 The ability of the CaO-based absorbents to absorb CO2 is reduced in the subsequent calcium looping. This leads to two results: (1) The CaO grains can be easily sintered during high-temperature calcination, and CO2 absorbed in the carbonization reaction will be reduced. As a result, the energy released in the carbonization reaction decreases. (2) The CaCO3 layer generated via the carbonization reaction can easily clog the pores of CaO particles (this clogging plays an important role in thermochemical energy storage). Therefore, the sintering and clogging of pores in CaO absorbents are the main problems associated with these absorbents in thermochemical energy storage. To reduce the sintering of CaO-based absorbents, researchers have proposed a method for preparing highly active CaO-based absorbents in the process of realizing calcium cycling thermochemical energy storage. This method involves doping modification, where dopants, such as metal oxides MgO,17 Al2O3,18 TiO2,19 and SiO2,20 added to CaO-based absorbents with a high Tamman temperature can be used as skeleton support materials. This doping can effectively delay the melting and growth of CaO particles and inhibit the sintering of CaO-based absorbents. Doping-modified CaO-based absorbents can improve the activity and cyclic stability of CaO, thus improving the thermochemical energy storage performance.21−25Figure 1b shows the mechanism governing the sintering resistance of CaO-based absorbents doped with inert oxides. The doped inert oxides act mainly as an internal skeleton to support the CaCO3 structure during the calcium cycle. This allowed more CO2 gas molecules (than those occurring for the nondoped material) to enter the interior of the CaCO3 layer for a reaction with CaO, thus improving the thermochemical energy storage performance. The CaO-based absorbent is modified with an inert material, which is dispersed in the absorbent. After the carbonization/calcination reaction cycle, the modified absorbent maintains a better pore structure than pure CaO, and the modification performance improves with increasing dispersion uniformity of the inert material. The modification process of CaO-based raw materials doped with inert substances is simple and yields good performance and is therefore considered a promising modification method. This process improves the conversion rate and stability of cyclic energy storage to a certain extent, but the deactivation of long-cycle energy storage materials persists despite the improvements achieved. Therefore, studies focused on the preparation of high-activity cyclic stable energy storage materials are ongoing.26
Figure 1.
Sintering mechanisms of CaO-based absorbents and antisintering mechanisms of CaO-based absorbents doped with inert oxides.
Al2O3 is the most commonly used carrier owing to its high Tamman temperature, excellent mechanical strength, and low operating cost. Benitez-Guerrero et al.25 evenly mixed nanometer A12O3 powder and limestone powder to prepare composite materials with different A12O3 content (5, 10, and 20%). The results revealed that the energy storage conversion rate of CaO is higher than that of the original material. This results from the fact that, as the activity increases, the rate of the reaction involving CaO carbonation with high concentrations of CO2 at high temperatures increases. The addition of 5% Al2O3 to the limestone powder yields significant improvement in the CaO conversion rate and stability of composite materials. If the addition of Al2O3 is further increased, the energy storage conversion rate will decrease. This is mainly due to the formation of calcium aluminates in the calcium cycle, which helps to stabilize the microstructure of CaO and reduce the clogging of pores, but CaO is consumed.27 MgO is considered an excellent inert carrier for reducing the activity loss of CaCO3 resulting from high-temperature sintering.28,29 André et al.30 investigated the energy storage stability of CaO/MgO composites after mechanical mixing of different proportions of MgO (0, 20, 30, and 45%) with limestone. The results revealed that the energy storage conversion rate decreased with the number of cycles after the addition of 30% MgO. In addition, the deactivation rate decreased, and the energy storage cycle stability was enhanced. Increasing the MgO content (45%) yielded no further enhancement in the energy storage stability, and the energy storage conversion rate decreased with increasing number of cycles.
Aihara et al.31 used TiO2 to modify limestone, and the resulting CaTiO3 improved the energy storage properties of the material. The calcination reaction occurred in a N2 atmosphere, and the carbonization reaction occurred in a N2/CO2 atmosphere with 20% CO2. After 10 cycles, CaO-based energy storage materials maintain a 0.65 energy storage conversion rate and exhibit good stability and sintering resistance.
Chen et al.22 prepared composite materials by mechanically mixing nano-SiO2 and limestone powder. The results showed that the calcination conversion rate of limestone improved with the addition of SiO2 to the limestone, and the optimal SiO2 mixing ratio was 5%. Moreover, the specific heat capacity of the composites increased by nearly 20%, and the apparent activation energy decreased by ∼40 kJ/mol. This phenomenon can be explained by the theory of heat and mass transfer. That is, the calcination of CaCO3 at high temperatures is a surface reaction, and the high thermal conductivity of SiO2 increases the rate of heat transfer from the environment to the surface of the particles. Therefore, the reaction temperature is rapidly reached. Another reason is that nano-SiO2 is dispersed in the CaCO3 crystals, affecting the internal structure and increasing the volume of pores, thereby increasing the specific surface area and improving the energy storage rate.
Khosa et al.32 investigated the effect of ZnO addition to the material on the kinetic and thermodynamic parameters of thermochemical energy storage. The cyclic stability of CaO-based materials was also analyzed, and the minimum temperature at which energy can be stored during the thermochemical energy storage process was determined. The rapidity of the calcination reaction shows that the addition of ZnO to CaCO3 is practical and allows the CaCO3/CaO system to store energy in a shorter time than pure CaCO3. The effect of this addition changed only slightly with the ZnO content (2, 5, 10, and 15%). However, doping with 5% ZnO yielded the best heat storage and exothermic capacity of the samples. Therefore, in the CaCO3/CaO system, the optimum content of ZnO was 5% and the heat storage capacity and heat release capacity were 1478.8 and 613.39 J/g, respectively. Thermodynamic analysis of the pure sample and 5% doped sample revealed that the specific heat capacity of the doped samples was 68% higher than that of the undoped samples. This difference was attributed to the high thermal conductivity of ZnO.
Carbide slag is a chemical waste generated in acetylene production by calcium carbide hydrolysis, where the main component is calcium hydroxide. Typically, carbide slag is used to produce cement, thereby reducing the cost of production. However, for industrial purposes, carbide slag is produced in large quantities, thereby providing a solution to the problem of waste treatment, but the economic benefit is minimal. As a result, a large amount of slag can only be accumulated and buried. The total amount of carbide slag, which has reached hundreds of millions of tonnes, takes up valuable land resources, pollutes the air and water, and even affects human health. As a means of solving this problem, the use of solid waste carbide slag has received widespread attention. The conceptual scheme of using solid waste carbide slag to prepare a CaO-based solar energy storage material for the CSP model is illustrated in Figure 2. Carbide slag contains a large amount of Ca(OH)2, which can be converted into active CaO, and a small amount of Al2O3, SiO2, and MgO. However, CaO can be used as the active substance for chemical absorption of CO2.26 Therefore, carbide slag can be used to prepare highly active CaO-based absorbents. CaO in the slag is essential for CO2 absorption and thermochemical energy storage. The principle of thermochemical heat storage via the calcium looping process is based upon the decomposition of CaCO3 and the carbonation of CaO. In the calciner, the decomposition of CaCO3 is an endothermic reaction that converts solar energy into solid particles for storage. In the carbonator, the reaction of CaO with CO2 is an exothermic reaction. The heat released during the carbonization process can be used for heating, power generation, and other applications. Such a CaL-looping process uses CaO as an intermediate medium and is, therefore, very important for developing cheap CaO materials.
Figure 2.
Schematic showing the calcium looping (CaL) process.
The present work focuses mainly on improving the stability of CaO-based absorbents using carbide slag subjected to cyclic reactions. MgO and ZnO doping of the slag was proposed. This doping allowed reasonable use of solid waste carbide slag and improved the cyclic stability of the CaO/CaCO3 system and energy storage performance. MgO is an excellent inert additive with high stability and a high Tamman temperature and therefore can improve the sintering resistance of the material and improve the cycling stability of the CaO-based absorbent. As a cheap and abundant material, ZnO as a dopant in the CaO/CaCO3 system enhanced the reactivity and cyclic stability.32 The energy storage characteristics of the MgO and ZnO-doped carbide slag were evaluated via material preparation and CO2 absorbability tests.
2. Experimental Section
2.1. Materials and Absorbent Preparation
Carbide slag was obtained from Inner Mongolia Zhonggu Mining Co., Ltd. The chemical composition of the slag (see Table 1) was determined by means of X-ray fluorescence spectrometry. The main component of the slag is CaO. ZnO (≥99.0%) was obtained from Shanghai Guangnuo Chemical Technology Co., Ltd. MgO (≥98.0%) was obtained from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. In addition, CO2 and N2 gases were provided by Jinghua Gas Co., Ltd.
Table 1. Chemical Composition of Carbide Slag.
| composition | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | others | LOI |
|---|---|---|---|---|---|---|---|
| content (wt %) | 70.35 | 0.51 | 2.00 | 1.18 | 0.24 | 3.01 | 22.71 |
The preparation process of the carbide slag absorbent is shown in Figure 3. First, original samples of the slag were dried for 12 h in a convection oven at 80 °C to remove the water contained in the slag. The dried slag was then calcined in a muffle furnace at 800 °C for 1 h and ground into powder in a mortar. Afterward, the carbide slag powder was ground for 40 min in a mortar with MgO powder (5, 10, 15, and 20 wt %) and ZnO powder (5 wt %) in different preparation ratios (90, 85, 80, and 75 wt %) and mixed evenly. The ground and mixed samples were then calcined at a high temperature for 20 min in a tubular heating furnace. The sorbent type, composition, and notation of each carbide slag absorbent are listed in Table 2.
Figure 3.
Preparation process of the doped carbide slag.
Table 2. Preparation of the Doped Carbide Slag.
| amount
of additive (wt %) |
|||||
|---|---|---|---|---|---|
| sorbent type | method | carbide slag | ZnO | MgO | notation |
| carbide slag | 100 | CS | |||
| carbide slag with MgO and ZnO | dry mixing | 90 | 5 | 5 | CMZ90 |
| dry mixing | 85 | 5 | 10 | CMZ85 | |
| dry mixing | 80 | 5 | 15 | CMZ80 | |
| dry mixing | 75 | 5 | 20 | CMZ75 | |
2.2. Cyclic Experiments
The cycling performance of the carbide slag absorbent as a thermochemical energy storage material was investigated in a tubular heating furnace. In the cyclic experiments, the effect of carbide slag absorbents on the sinterability with increasing number of calcination/carbonization cycle reactions was investigated. Good carbonation performance and cyclic stability of the carbide slag absorbents are essential for achieving efficient thermochemical energy storage of the CaO/CaCO3 system. Cycle experiments include carbonization and calcination cycles. In this study, the carbonization reaction in the calcium cycle is divided into three processes: heating process, reaction process, and cooling process. The operating conditions of calcination/carbonization reactions have an important effect on thermochemical energy storage when carbide slag is used as the absorbent. Operating conditions such as the reaction temperature, reaction time, reaction atmosphere, and other parameters have an important influence on the performance and cycle stability of thermochemical energy storage. Taking into account the cyclical nature of industrial applications and investment costs, 10 times are set for each cycle. Ten cycles of calcination/carbonization reactions of carbide slag absorbents were performed. Sintering and CO2 absorption of carbide slag absorbents in multiple cycles were explored by determining the changes in the absorption rate during the cycles.
Carbide slag absorbents were placed in a tubular heating furnace for the calcination/carbonization reaction. As shown in Table 3, the carbonization reaction occurs at 600 °C/650 °C, and the calcination reaction occurs at 800/850 °C. For each absorbent, 0.5 g of the absorbent was placed in a tube furnace for the cyclic calcination/carbonization reaction. Pure N2 and CO2 gases at a flow rate of 100 mL/min were employed for the calcination and carbonation reactions, respectively. In the carbonation reaction, the N2 and CO2 atmospheres were varied during the temperature-increase period, and the effect of gas termination temperatures (100, 300, and 500 °C) on CO2 absorption was explored. The samples were cooled to room temperature after each carbonization and calcination cycle and then weighed on a precision electronic balance.
Table 3. Reaction Conditions.
| associated illustration | calcination | carbonation | heating (°C/min) | atmosphere | cooling | gas termination temperatures (°C) |
|---|---|---|---|---|---|---|
| Figure 4 | 800 °C, 20 min | 600 °C, 20 min | 20 | N2–CO2–CO2 | nature | 100 |
| 800 °C, 20 min | 600 °C, 20 min | 20 | CO2–CO2–CO2 | nature | 100 | |
| Figure 5 | 800 °C, 20 min | 600 °C, 20 min | 20 | CO2–CO2–CO2 | nature | 500, 300 and 100 |
| Figure 6a | 800 °C, 20 min | 600 °C, 20 min | 20 | CO2–CO2–CO2 | nature | 500 |
| 850 °C, 20 min | 650 °C, 20 min | 20 | CO2–CO2–CO2 | nature | 500 | |
| Figure 6b | 850 °C, 20 min | 650 °C, 20, 30, 40, 50, and 60 min | 20 | CO2–CO2–CO2 | nature | 500 |
| Figure 7 | 850 °C, 20 min | 650 °C, 40 min | 20 | CO2–CO2–CO2 | nature | 500 |
In this paper, the ability of thermochemical energy storage materials to release and store thermal energy during cyclic carbonization/calcination reactions is described by the amount of CO2 absorbed by CaO in the carbide slag absorbent. The amount of CO2 absorbed by CaO in the reaction is expressed in terms of the CO2 absorption rate obtained from the sample weight change after calcination and carbonization in each cycle. The absorption rate is calculated as follows:
| 3 |
Here, N, CN, mcalc,N, and mcarb,N represent the number of cyclic reactions, absorption rate of CO2 in the N cycle, mass of absorbents after the N cycle calcination, and mass of absorbents after the N cycle carbonization, respectively.
2.3. Characterization of Absorbents
To elucidate the structure–performance relationship of the material, the surface area was determined using the Brunauer–Emmett–Teller (BET) method, and the pores were characterized using a porosimetry analyzer (ASAP 2460, Micromeritics). The samples were degassed entirely at 200 °C prior to the N2 adsorption and desorption process. Moreover, the phase composition of carbide slag absorbents was determined by means of X-ray diffraction (XRD; SmartlabSE, Japan). The crystal structure and phase structure of the material were observed over an angle range of 20–80°. To further understand the morphology and structure of the absorbents, each absorbent and the corresponding aggregation state were characterized via scanning electron microscopy (FE-SEM; SYGMA500, Germany). X-ray photoelectron spectroscopy (XPS; ESCALAB Xi+) is an important characterization method for analyzing materials. This evaluation method was used to qualitatively analyze the elemental composition, chemical state, and molecular structure of the carbide slag absorbents after 10 calcination/carbonization reactions.
3. Results and Discussion
3.1. Effect of the Carbonation Reaction Atmosphere on Absorbent Performance
Figure 4 shows the effect of different reaction atmospheres on the CO2 absorption rate during the carbonization process of the calcination/carbonization reaction at 800/600 °C. As shown in the figure, the CO2 absorption rate for the carbonation reaction with a CO2–CO2–CO2 atmosphere is higher than the rate associated with a N2–CO2–CO2 atmosphere. For increasing the MgO content of the carbide slag absorbent, the absorption rate increases initially and then decreases for the CO2–CO2–CO2 atmosphere but decreases continuously for the N2–CO2–CO2 atmosphere. The CO2 absorption rate of the CMZ85 absorbent in the CO2–CO2–CO2 atmosphere was 40.99%, which is 1.5 times higher than that obtained for the N2–CO2–CO2 atmosphere (27.37%). Thus, compared with N2–CO2–CO2, CO2–CO2–CO2 is considered a more suitable atmosphere for the carbonization process.
Figure 4.
CO2 absorption rate of absorbents associated with different reaction atmospheres.
3.2. Effect of Gas Termination Temperature on Absorbent Performance
The effect of different gas termination temperatures on the CO2 absorption rate associated with a carbonization reaction in a CO2–CO2–CO2 atmosphere is shown in Figure 5. For this reaction atmosphere, the value of the absorption rate at different gas termination temperatures shows good characteristics at 500 °C. The CO2 absorption rate at a gas termination temperature of 500 °C is 41% for the CMZ85 absorbent. This value is 1.04 and 1.08 times higher than the absorption rates associated with 300 and 100 °C, respectively. Therefore, 500 °C is considered the most suitable gas termination temperature for the carbonization reaction.
Figure 5.

CO2 absorption rate of absorbents at different gas termination temperatures associated with the CO2–CO2–CO2 atmosphere.
3.3. Effect of Different Reaction Temperatures and Reaction Times on Absorbent Performance
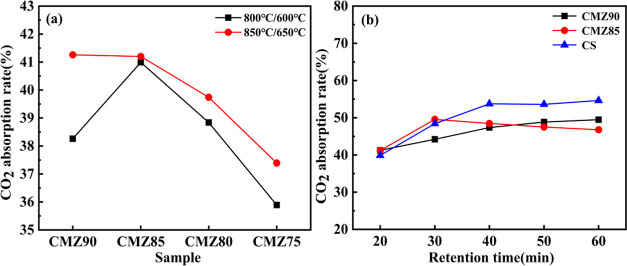
The effect of different reaction temperatures on the CO2 absorption rate of absorbents is shown in Figure 6a. Analysis of the operating conditions associated with the calcination/carbonization reaction revealed that the optimum reaction temperature for the reaction is 850/650 °C. The corresponding CO2 absorption rates of the CMZ90, CMZ85, CMZ80, and CMZ75 absorbents are 41.3, 41.2, 39.7, and 37.4%, respectively. These values are significantly higher than the CO2 absorption rates of each absorbent involved in the calcination/carbonization reaction at 800/600 °C. The influence of retention time (20, 30, 40, 50, and 60 min) on the absorption rate during the carbonization reaction process was investigated. The investigation was performed on the CMZ90 and CMZ85 absorbents with the highest CO2 absorption rate in this study and CS absorbents prepared with pure carbide slag. The effect of the retention time on the CO2 absorption rate at 850/650 °C is shown in Figure 6b. As shown in the figure, the absorption rate of each absorbent changed only slightly for carbonization reaction times exceeding 40 min, and therefore, 40 min was selected to reduce the time required for experiments.
Figure 6.
CO2 absorption rate associated with different (a) reaction temperatures and (b) reaction times.
3.4. Cyclic CO2 Absorption Capacity of Modified Carbide Slag Absorbents
In this study, the optimal reaction conditions for the carbide slag absorbent calcium cycle are as follows: The calcination reaction occurs at 850 °C with a pure N2 atmosphere and a reaction time of 20 min. The carbonization reaction occurs at 650 °C with a pure CO2 atmosphere and a reaction time of 40 min. The gas termination temperature associated with the CO2 absorption rate in the calcium cycle is 500 °C for the CO2 atmosphere.
The CO2 absorption rate of the carbide slag absorbents during 10 cycles is shown in Figure 7. The absorption rate of each absorbent decreases gradually with increasing cycling time, and the lowest rate occurs in the second cycle. This results from the self-activation phenomenon associated with the Mg content of an absorbent.33 The self-activation of carbide slag absorbents occurred after the first cycle, and hence the CO2 absorption rate was low in the second cycle and then increased after the third cycle. However, the CO2 absorption decreased slightly with increasing cycle number. CMZ80-90 exhibited more stable absorption than CMZ75, owing to the fact that excessive MgO is added to the absorbent, leading to a reduction in the content of active component CaO. Furthermore, compared with CS, the doped absorbents exhibited superior CO2 absorption characteristics, indicating that ZnO and MgO doping of CS can enhance CO2 absorption.
Figure 7.
CO2 absorption rate of carbide slag absorbents during 10 cycles.
The CO2 absorption rate of CS absorbent prepared with pure carbide slag is 55.06% in the first cycle and is 1.22 times higher than that of the CMZ90 absorbent. After 10 cycles, the absorption rates of the CS absorbent and CMZ85 absorbent are 31.21% and (1.35 times higher) 42.12%, respectively. However, the CO2 absorption rate of the CaO-based absorbent prepared with pure CaCO3 as a calcium source was 57.8% in the first cycle and 25.4% after 10 cycles.34 The highest absorption rates (41–42%) are obtained for CMZ85 and CMZ90 after 10 cycles. These data revealed that improved solid waste carbide slag used to prepare CO2 absorption energy storage materials exhibits good absorption performance.
3.5. Effect of MgO and ZnO on the Carbide Slag Absorbent Structures
The adsorption–desorption N2 isotherms are shown in Figure 8a. All four curves can be classified as type IV isotherms, and the hysteresis loop is of type H3,35 which indicates that absorbents are formed via the agglomeration of particles.36Figure 8b shows the cumulative pore volume distribution of the four absorbents after 10 cycles. For pore widths ranging from 0 to 250 nm, the pore volume of each absorbent increased initially and then leveled off as the pore width increased. After 10 cycles, the CMZ absorbent has a high cumulative pore volume, confirming the abundance of the adsorbent in the pore structure.37 The cumulative pore volume of the CS-10 absorbent was considerably lower than that of the CMZ absorbent.
Figure 8.
Quantity absorbed of N2 (a) and pore volume (b) of the four absorbents after 10 cycles were analyzed in BET.
Nitrogen adsorption analyzers are mainly used to determine the pore microstructure of absorbents. In particular, the specific surface area is determined according to the Brunauer–Emmett–Teller (BET) method, while the pore volume and pore diameter are determined according to the Barrett–Joyner–Halenda (BJH) method. Table 4 shows the specific surface area, pore volume, and pore diameter of the fresh absorbents and absorbents after 10 cycles. For fresh CS absorbents, the specific surface area, pore volume, and pore size were 9.5344 m2/g, 0.0266 cm3/g, and 14.2956 nm, respectively, higher than those of the CMZ absorbents. After 10 cycles of calcination/carbonization reactions, the specific surface area, pore volume, and pore size of the CS absorbent decreased significantly to 5.7706 m2/g, 0.0160 cm3/g, and 8.0521 nm, respectively. This indicates that CM undergoes considerable sintering during the cycles. In contrast, the specific surface area, pore volume, and pore diameter of CMZ80-90 remained almost unchanged after 10 cycles. This indicates that the specific surface area, pore volume, and cyclic stability of the absorbent subjected to calcination/carbonation reaction cycles can be improved through MgO and ZnO doping of the carbide slag. The specific surface area is an important factor affecting cycle performance. Compared with a smaller specific surface area, a larger area leads to a faster reaction rate and higher conversion in a limited reaction time.38 Therefore, compared with the nondoped absorbents, the absorbents doped with MgO and ZnO in multiple cycles have a larger surface area and pore volume and are, therefore, more conducive to energy storage.
Table 4. Particle Properties (Determined Using the BET Method) of Fresh Absorbents and after 10 Cycles of Carbonation–Calcination Reactions.
| surface
area (m2/g) |
pore volume (cm3/g) |
pore diameter (nm) |
||||
|---|---|---|---|---|---|---|
| sorbent | fresh | 10th | fresh | 10th | fresh | 10th |
| CS | 9.5344 | 5.7706 | 0.0266 | 0.0160 | 14.2956 | 8.0521 |
| CMZ90 | 7.9315 | 9.1233 | 0.0228 | 0.0240 | 10.6464 | 13.8744 |
| CMZ85 | 8.7484 | 8.7074 | 0.0176 | 0.0227 | 8.5544 | 8.1499 |
| CMZ80 | 7.1952 | 7.6652 | 0.0180 | 0.0208 | 7.6069 | 7.7009 |
| CMZ75 | 9.2667 | 7.5895 | 0.0233 | 0.0195 | 8.4346 | 8.0032 |
Figure 9 shows the FE-SEM results of the fresh absorbent and the absorbent after 10 cycles. Figure 9a–c shows the SEM images of fresh absorbents CS, CMZ85, and CMZ90. The pore structure of the CS absorbent, where many small particles are observed on the surface, is inhomogeneous compared with those of the other absorbents. After ZnO and MgO doping of the carbide slag, the structure and shape of the slag absorbents change significantly. Furthermore, only a few small particles are observed on the surface, and hence the structure of the doped absorbents undergoes little or no sintering. Figure 9d–f shows the SEM images of CS, CMZ85, and CMZ90 absorbents after 10 cycles. After 10 cycles, the carbide slag absorbents are characterized mainly by an amorphous structure, with many small particles attached to the surface of each absorbent. The structure of the CS absorbent undergoes considerable sintering where the particle size increases and the surface area decreases. However, with ZnO and MgO doping of the slag, the surface of the absorbent becomes smooth, and the structure shape of the absorbent differs only slightly between the fresh and cycled states. Many voids occur on the surface of the CMZ absorbents, and a few small particles appear on the surface. Some of these particles become clustered with increasing MgO content of the absorbent. More particles are dispersed on the surface of the absorbent (than in other regions), suggesting that small cluster particles enhance the skeleton structure. Therefore, after 10 cycles of calcination/carbonization reactions, the CMZ absorbents undergo less sintering than the CS absorbents. The CO2 absorption in the carbonization reaction occurs mainly in two stages. The first step is a short and fast process, where a CO2 injection reaction occurs directly on the surface of the absorbents. The second step is a mass transfer process involving CO2 and/or ion diffusion through the crust of the absorbent.33 The pore size of the CMZ absorbent remained almost the same after 10 cycles and was conducive to the diffusion reaction of CO2 but had some impact on the absorption rate. Therefore, many holes were present in the absorbent, thereby weakening the sintering inactivation phenomenon of CaO to a certain extent. As shown in Figure 9, the structure of the CMZ absorbent is superior to that of the CS absorbent after 10 cycles; this superior structure is more conducive to the diffusion of CO2 into CaCO3, and the reaction with internal CaO releases more energy. A comparison of the absorbents revealed that the MgO content of the best absorbent ranges from 5 to 10%.
Figure 9.
SEM images of fresh absorbents (a–c) and absorbents after 10 cycles (d–f).
The EDS elemental mapping diagram and surface scanning diagram of the CMZ85-10 absorbent are shown in Figure 10. The elements C, O, Ca, Mg, Zn, and Si are present in both diagrams. The Si element results from the fact that the carbide slag contains a small amount of SiO2, which is an inert component. The EDS mapping presented in Figure 10 shows the distribution of different elements (Ca, Mg, and Zn) in the CMZ85 absorbent. The uniform distribution of each element indicates that the skeleton structure of the absorbent is maintained after 10 cycles. That is, ZnO and MgO mixed in the slag are well distributed in the absorbents, and hence the doped slag absorbents have no effect on pore formation in the repeat process of the cycle process. The homogeneous distribution of ZnO and MgO in the CMZ absorbent can support the CaO structure maintained after high-temperature sintering. Doping of the slag can improve the structural stability of CaO in cyclic carbonization/calcination reactions and inhibit the high-temperature sintering of CaO grains. Therefore, compared with nonmodified CMZ, modified CMZ exhibits superior cyclic stability during the carbonization/calcination reactions.
Figure 10.
EDS elemental mapping diagram and elemental surface scanning diagram (CMZ85-10).
3.6. Role of Promoters and Their Loadings
XRD spectra of fresh absorbents are shown in Figure 11a. The diffraction peaks of CaO crystals were detected in CS absorbents and CMZ absorbents, and the peaks of MgO and ZnO were detected for CMZ90 and CMZ85 absorbents. However, the diffraction peaks corresponding to other components of the carbide slag were absent from the XRD spectra. Figure 11b shows the spectra of the slag absorbents after 10 cycles. Among these, the diffraction peaks of the CaO crystal were still detected for CS and CMZ absorbents after the 10th cycle (CS-10 and CMZ-10). Moreover, sharp characteristic peaks of MgO were observed for the absorbent CMZ85, but the peaks of ZnO were quite weak. The intensity of the characteristic peaks corresponding to ZnO decreased gradually with increasing number of cycles. This may have resulted from the smaller size of ZnO after cycling (compared with the size before cycling), which is below the lower limit of XRD detection or the size of the amorphous state. It is noteworthy that a broad halo can be found between 15 and 25 in Figure 11a,b, which is caused by the presence of a small amount of SiO2 particles in carbide slag. Thus, the main crystalline phase component of the carbide slag absorbent is CaO.
Figure 11.
XRD patterns of the (a) fresh absorbents and (b) absorbents after 10 cycles.
XPS, an important characterization method for analyzing materials, was used to qualitatively evaluate the elemental composition, chemical state, and molecular structure of the CMZ85-10 absorbent after 10 cycles. Figure 12a shows the full spectrum XPS scanning results of the CMZ85-10 absorbent. Sharp peaks of Ca 2p, O 1s, C 1s, Mg 1s, and Zn 2p consistent with the results of EDS scanning are observed. Figure 12b–d shows the scanning results of fine XPS spectra obtained for the CMZ absorbent. The high-resolution Ca 2p, Mg 1s, and Zn 2p spectra revealed two characteristic peaks of the 2P orbital corresponding to Ca. The electron binding energies associated with the peaks are 347.1 and 350.6 eV. These values are consistent with the binding energy corresponding to the 2p orbital of the Ca element in CaO, indicating that the phase form of the Ca element is CaO. For the element Mg, a characteristic peak attributed to the 1s orbital occurs at a binding energy of 1303.9 eV. This is consistent with the electron binding energy associated with the 1s orbital of the Mg in MgO, indicating that Mg occurs as the MgO phase in the CMZ absorbent. Regarding the element Zn, two characteristic peaks associated with the 2p orbital occur at binding energies of 1022.1 and 1045.1 eV. This is consistent with the electron binding energy of Zn in ZnO in the 2p orbital, indicating that Zn occurs as the ZnO phase in the CMZ absorbent. Therefore, MgO and ZnO doped in the carbide slag are mainly used to support the structure of CaO and ease the sintering of the absorbent.
Figure 12.
(a) XPS full spectrum scan and (b) Ca 2p, (c) Mg 1s, and (d) Zn 2p fine spectra of the CMZ85 absorbent.
To summarize, MgO and ZnO doping of carbide slag has a significant impact on the structure of the carbide slag absorbent. N2 adsorption–desorption experiments (Table 3) and FE-SEM analysis (Figure 9) were performed on the carbide slag absorbents. The results revealed that, after 10 cycles, the pore structure of the CMZ absorbent with MgO and ZnO doping of the slag was better than that of the CS absorbent without doping. The structures of CMZ90-10 and CMZ85-10 were relatively stable. This is related to the XRD-determined phase composition (Figure 11), XPS-evaluated elemental composition, chemical state, and molecular structure of the adsorbent (Figure 12), and the EDS-determined uniformity of elemental distribution (Figure 10). The results obtained in this study indicate that MgO and ZnO doped in the carbide slag are effective in alleviating the sintering of the absorbent and improving the diffusion rate of CO2.
4. Conclusions
The effect of ZnO and MgO doping on the cyclic carbonization/calcination reaction of carbide slag was studied. Cycle performance experiments revealed that the raw material ratio, reaction temperature, and reaction time considerably influence the CO2 absorption rate. After 10 cycles of reactions, the CO2 absorption rate of CMZ90-10 and CMZ85-10 reached 41–42%, significantly higher than those obtained by other carbide slag absorbents. Based on the structural characterization of the absorbents, ZnO and MgO doping of the carbide slag increases the specific surface area and pore volume of the slag absorbents. This doping also effectively alleviates the problems of sintering and clogging of pores in the absorbents and improves the activity and cycling stability of the absorbents. The improvement in the pore structure of the absorbents enables more CO2 to diffuse into the interior of the CaCO3 layer and react with CaO to release more energy. Therefore, for a given amount of ZnO doping, the addition of 5–10% MgO to the slag can significantly improve the cyclic stability and absorption activity of thermochemical energy storage. The results indicate that the modification of carbide slag presents a promising method for the preparation of energy storage materials.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Ningxia Province (Grant Nos. 2022AAC03218 and 2021AAC03170).
Author Contributions
C.G.: funding acquisition and writing—review and editing; Y.Z.: formal analysis and writing—original draft; D.L.: writing—review and editing; and M.L.: data curation.
The authors declare no competing financial interest.
References
- Benitez-Guerrero M.; Valverde J. M.; Sanchez-Jimenez P. E.; Perejon A.; Perez-Maqueda L. A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. 10.1016/j.solener.2017.05.068. [DOI] [Google Scholar]
- Fernández A. G.; Gomez-Vidal J.; Oró E.; Kruizenga A.; Solé A.; Cabeza L. F. Mainstreaming commercial CSP systems: A technology review. Renewable Energy 2019, 140, 152–176. 10.1016/j.renene.2019.03.049. [DOI] [Google Scholar]
- Wu S.; Zhou C.; Tremain P.; Doroodchi E.; Moghtaderi B. A phase change calcium looping thermochemical energy storage system based on CaCO3/CaO-CaCl2. Energy Convers. Manage. 2021, 227, 113503 10.1016/j.enconman.2020.113503. [DOI] [Google Scholar]
- Tian H.; Wang W.; Ding J.; Wei X. Thermal performance and economic evaluation of NaCl–CaCl2 eutectic salt for high-temperature thermal energy storage. Energy 2021, 227, 120412 10.1016/j.energy.2021.120412. [DOI] [Google Scholar]
- Kuravi S.; Trahan J.; Goswami D. Y.; Rahman M. M.; Stefanakos E. K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. 10.1016/j.pecs.2013.02.001. [DOI] [Google Scholar]
- Khare S.; Dell’amico M.; Knight C.; McGarry S. Selection of materials for high temperature sensible energy storage. Sol. Energy Mater. Sol. Cells 2013, 115, 114–122. 10.1016/j.solmat.2013.03.009. [DOI] [Google Scholar]
- Agyenim F.; Hewitt N.; Eames P.; Smyth M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renewable Sustainable Energy Rev. 2010, 14, 615–628. 10.1016/j.rser.2009.10.015. [DOI] [Google Scholar]
- Mahlia T. M. I.; Saktisahdan T. J.; Jannifar A.; Hasan M. H.; Matseelar H. S. C. A review of available methods and development on energy storage; technology update. Renewable Sustainable Energy Rev. 2014, 33, 532–545. 10.1016/j.rser.2014.01.068. [DOI] [Google Scholar]
- Zhang S.; Wang X.; Mao Z.; Li Y.; Jin B.; Xiao R. Effect of calcination condition on the performance of iron ore in chemical-looping combustion. Fuel Process. Technol. 2020, 203, 106395 10.1016/j.fuproc.2020.106395. [DOI] [Google Scholar]
- Criado Y. A.; Alonso M.; Abanades J. C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. 10.1016/j.solener.2016.06.056. [DOI] [Google Scholar]
- Sakellariou K. G.; Criado Y. A.; Tsongidis N. I.; Karagiannakis G.; Konstandopoulos A. G. Corrigendum to “Multi-cyclic evaluation of composite CaO-based structured bodies for thermochemical heat storage via the CaO/Ca(OH)2 reaction scheme” [Solar Energy 146 (2017) 65–78]. Sol. Energy 2017, 150, 619–620. 10.1016/j.solener.2017.05.049. [DOI] [Google Scholar]
- Ortega-Fernández I.; Calvet N.; Gil A.; Rodríguez-Aseguinolaza J.; Faik A.; D’Aguanno B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. 10.1016/j.energy.2015.05.153. [DOI] [Google Scholar]
- Shimizu T.; Hirama T.; Hosoda H.; Kitano K.; Inagaki M.; Tejima K. A Twin Fluid-Bed Reactor for Removal of CO2 from Combustion Processes. Chem. Eng. Res. Des. 1999, 77, 62–68. 10.1205/026387699525882. [DOI] [Google Scholar]
- Chen Z.; Song H. S.; Portillo M.; Lim C. J.; Grace J. R.; Anthony E. J. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuels 2009, 23, 1437–1444. 10.1021/ef800779k. [DOI] [Google Scholar]
- Manovic V.; Anthony E. J. Long-term behavior of CaO-based pellets supported by calcium aluminate cements in a long series of CO2 capture cycles. Ind. Eng. Chem. Res. 2009, 48, 8906–8912. 10.1021/ie9011529. [DOI] [Google Scholar]
- Lysikov A. I.; Salanov A. N.; Okunev A. G. Change of CO2 carrying capacity of CaO in isothermal recarbonation-decomposition cycles. Ind. Eng. Chem. Res. 2007, 46, 4633–4638. 10.1021/ie0702328. [DOI] [Google Scholar]
- Naeem M. A.; Armutlulu A.; Imtiaz Q.; Donat F.; Schublin R.; Kierzkowska A.; Müller C. U. Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents. Nat. Commun. 2018, 9, 2408 10.1038/s41467-018-04794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Li Y.; Zhang W.; Wang Z.; Zhao J. DFT study of CO2 adsorption across a CaO/Ca12Al14O33 sorbent in the presence of H2O under calcium looping conditions. Chem. Eng. J. 2019, 370, 10–18. 10.1016/j.cej.2019.03.176. [DOI] [Google Scholar]
- Sun J.; Guo Y.; Yang Y.; Li W.; Zhou Y.; Zhang J.; Liu W.; Zhao C. Mode investigation of CO2 sorption enhancement for titanium dioxide-decorated CaO-based pellets. Fuel 2019, 256, 116009 10.1016/j.fuel.2019.116009. [DOI] [Google Scholar]
- Valverde J. M.; Perejon A.; Perez-Maqueda L. A. Enhancement of fast CO2 capture by a nano-SiO2/CaO composite at Ca-looping conditions. Environ. Sci. Technol. 2012, 46, 6401–6408. 10.1021/es3002426. [DOI] [PubMed] [Google Scholar]
- Benitez-Guerrero M.; Valverde J. M.; Perejon A.; Sanchez-Jimenez P. E.; Perez-Maqueda L. A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. 10.1016/j.apenergy.2017.10.109. [DOI] [Google Scholar]
- Chen X.; Jin X.; Liu Z.; Ling X.; Wang Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. 10.1016/j.energy.2018.05.016. [DOI] [Google Scholar]
- Sakellariou K. G.; Tsongidis N. I.; Karagiannakis G.; Konstandopoulos A. G.; Baciu D.; Charalambopoulou G.; Steriotis T.; Stubos A.; Arlt W. Development and evaluation of materials for thermochemical heat storage based on the CaO/CaCO3 reaction couple. AIP Conf. Proc. 2016, 1734, 050040 10.1063/1.4949138. [DOI] [Google Scholar]
- Obermeier J.; Sakellariou K. G.; Tsongidis N. I.; Baciu D.; Charalambopoulou G.; Steriotis T.; Müller K.; Karagiannakis G.; Konstandopoulos A. G.; Stubos A.; Arlt W. Material development and assessment of an energy storage concept based on the CaO-looping process. Sol. Energy 2017, 150, 298–309. 10.1016/j.solener.2017.04.058. [DOI] [Google Scholar]
- Benitez-Guerrero M.; Valverde J. M.; Sanchez-Jimenez P. E.; Perejon A.; Perez-Maqueda L. A. Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. 10.1016/j.cej.2017.11.183. [DOI] [Google Scholar]
- Geng Y.-q.; Guo Y.; Fan B.; Cheng F.; Cheng H. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification. J. Fuel Chem. Technol. 2021, 49, 998–1013. 10.1016/S1872-5813(21)60040-3. [DOI] [Google Scholar]
- Kierzkowska A. M.; Pacciani R.; Muller C. R. CaO-based CO2 sorbents: from fundamentals to the development of new, highly effective materials. ChemSusChem 2013, 6, 1130–1148. 10.1002/cssc.201300178. [DOI] [PubMed] [Google Scholar]
- Sun J.; Liu W.; Li M.; Yang X.; Wang W.; Hu Y.; Chen H.; Li X.; Xu M. Mechanical Modification of Naturally Occurring Limestone for High-Temperature CO2 Capture. Energy Fuels 2016, 30, 6597–6605. 10.1021/acs.energyfuels.6b01131. [DOI] [Google Scholar]
- Daud F. D. M.; Vignesh K.; Sreekantan S.; Mohamed A. R. Improved CO2 adsorption capacity and cyclic stability of CaO sorbents incorporated with MgO. New J. Chem. 2016, 40, 231–237. 10.1039/C5NJ02081F. [DOI] [Google Scholar]
- André L.; Abanades S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. 10.1016/j.est.2017.07.014. [DOI] [Google Scholar]
- Aihara M.; Nagai T.; Matsushita J.; Negishi Y.; Ohya H. Development of porous solid reactant for thermal-energy storage and temperature upgrade using carbonation/decarbonation reaction. Appl. Energy 2001, 69, 225–238. 10.1016/S0306-2619(00)00072-6. [DOI] [Google Scholar]
- Khosa A. A.; Yan J.; Zhao C. Y. Investigating the effects of ZnO dopant on the thermodynamic and kinetic properties of CaCO3/CaO TCES system. Energy 2021, 215, 119132 10.1016/j.energy.2020.119132. [DOI] [Google Scholar]
- Choi D.; Shin J.; Park Y. Effects of CaCl2 on cyclic carbonation-calcination kinetics of CaO-based composite for potential application to solar thermochemical energy storage. Chem. Eng. Sci. 2021, 230, 116207 10.1016/j.ces.2020.116207. [DOI] [Google Scholar]
- Luo C.; Zheng Y.; Xu Y.; Ding N.; Shen Q.; Zhen C. Wet mixing combustion synthesis of CaO-based sorbents for high temperature cyclic CO2 capture. Chem. Eng. J. 2015, 267, 111–116. 10.1016/j.cej.2015.01.005. [DOI] [Google Scholar]
- Li Y.-j.; Zhao C.; Duan L.; Liang C.; Li Q.; et al. Cyclic calcination/carbonation looping of dolomite modified with acetic acid for CO2 capture. Fuel Process. Technol. 2008, 89, 1461–1469. 10.1016/j.fuproc.2008.07.008. [DOI] [Google Scholar]
- Radfarnia H. R.; Iliuta M. C. Limestone Acidification Using Citric Acid Coupled with Two-Step Calcination for Improving the CO2 Sorbent Activity. Ind. Eng. Chem. Res. 2013, 52, 7002–7013. 10.1021/ie400277q. [DOI] [Google Scholar]
- Luo T.; Luo C.; Shi Z.; Li X.; Wu F.; Zhang L. Optimization of sol-gel combustion synthesis for calcium looping CO2 sorbents, part I: Effects of sol-gel preparation and combustion conditions. Sep. Purif. Technol. 2022, 292, 121081 10.1016/j.seppur.2022.121081. [DOI] [Google Scholar]
- Teng L.; Xuan Y.; Da Y.; Liu X.; Ding Y. Modified Ca-Looping materials for directly capturing solar energy and high-temperature storage. Energy Storage Mater. 2020, 25, 836–845. 10.1016/j.ensm.2019.09.006. [DOI] [Google Scholar]