Abstract
Objective
This study evaluated the effect of microbiome-targeted therapies (pre-, pro-, and synbiotics) on weight loss and other anthropometric outcomes when delivered as an adjunct to traditional weight loss interventions in overweight and obese adults.
Methods
A systematic review of three databases (Medline [PubMed], Embase, and the Cochrane Central Register of Controlled Trials) was performed to identify randomized controlled trials published between January 1, 2010 and December 31, 2020, that evaluated anthropometric outcomes following microbiome-targeted supplements in combination with dietary or dietary and exercise interventions. The pooled mean difference (MD) between treatment and control groups was calculated using a random effects model.
Results
Twenty-one trials with 1233 adult participants (76.4% female) with overweight or obesity were included. Separate meta-analyses were conducted for probiotics (n=11 trials) and synbiotics (n=10 trials) on each anthropometric outcome; prebiotics were excluded as only a single study was found. Patient characteristics and methodologies varied widely between studies. All studies incorporated some degree of caloric restriction, while only six studies included recommendations for adjunct exercise. Compared with dietary or dietary and exercise interventions only, probiotics resulted in reductions in body weight (MD: −0.73 kg; 95% confidence interval [CI]: −1.02 to −0.44, p < 0.001), fat mass (MD: −0.61 kg; 95% CI: −0.77 to −0.45; p<0.001) and waist circumference (MD: −0.53 cm; 95% CI: −0.99 to −0.07, p=0.024) while synbiotics resulted in reductions in fat mass (MD: −1.53 kg; 95% CI: −2.95 to −0.12, p=0.034) and waist circumference (MD: −1.31 cm; 95% CI: −2.05 to −0.57, p<0.001).
Conclusion
This analysis indicates that microbiome-targeted supplements may enhance weight loss and other obesity outcomes in adults when delivered as an adjunct to dietary or dietary and exercise interventions. Personalized therapy to include microbiome-targeted supplements may help to optimize weight loss in overweight and obese individuals.
Keywords: microbiome, obesity, adjunctive therapy, weight loss
Introduction
Obesity is one of the most widespread chronic diseases worldwide. According to the World Health Organization (WHO), global rates of obesity have nearly tripled since 1975, with over 650 million adults estimated to have obesity in 2016.1 Obesity was associated with 4.7 million deaths in 2017 worldwide, and by 2025, more than 1 billion adults are predicted to be obese, with 177 million developing severe conditions.2,3 Excessive adiposity is associated with impaired quality of life and a myriad of comorbidities that collectively heighten the risk of preventable mortality.4,5 Identifying therapeutic options that accelerate weight loss or reduce the consequences of obesity is therefore of great importance.
A multitude of treatment approaches have been trialed to facilitate weight loss. Obesity management often begins with lifestyle interventions. Diet and exercise alone have been found to produce mixed results depending on the intervention, and are linked to regaining up to half of the lost weight within the first year.6 Pharmacological therapies have shown various degrees of success in achieving weight loss but are hampered by side effects and non-compliance with therapy.7 In more severe cases, and for non-responders, escalation to bariatric surgery promotes significant weight loss and reduction in mortality but is associated with wide-ranging adverse effects, in addition to elevated financial costs.8 Therefore, additional strategies are needed to overcome the constraints of existing therapies and yield more consistent outcomes.
Recent human and animal studies have shown that the commensal micro-organisms that make up the gut microbiome play a significant role in the etiology of obesity by modulating hunger, satiety, nutritional absorption, metabolism, and inflammation, among other mechanisms.9–11 Disturbances to the homeostasis of the microbiota, such as through dieting, can cause imbalances among the bacterial communities residing in the intestine.12 These imbalances, termed gut dysbiosis, can contribute to the development of metabolic and intestinal disease by triggering chronic inflammation, among other mechanisms.13 Conversely, a balanced intestinal microbiome can have protective health effects, including a role in preventing or alleviating obesity and metabolic diseases.14 Thus, implementing therapies that support microbiota homeostasis concurrent with weight loss therapies may be beneficial to the overall health of the patient.
Further, research indicates that the gut microbiome may differ between obese and lean individuals in both composition and function. One of the most cited factors differentiating the obese and lean microbiome is the ratio of bacterial microbiota belonging to the Firmicutes and Bacteroidetes phyla, which collectively account for around 90% of the adult intestinal microbiota.15 In general, obese individuals have been found to have a greater proportion of Firmicutes than Bacteroidetes phyla than lean individuals, although recent studies have questioned the validity of this ratio given the high variability in the abundance of both phyla.16 Individuals with obesity also have a lower richness and diversity of microbial species,17 which has been associated with low-grade inflammation and dysregulated metabolism.18 These differences between obese and lean individuals suggest that microbiome-targeted therapies (MTTs) could be evaluated as novel adjunct strategies in obesity management.
A broader understanding of the reciprocal relationship between the microbiome and obesity has heightened interest in MTTs, including probiotics, prebiotics, and synbiotics. Probiotics are defined as live microorganisms that confer health benefits to the host when administered in adequate amounts, while prebiotics are non-metabolized substances that are selectively utilized by gut microbes and confer a health benefit.19 Although fibers such as fructans (fructooligosaccharides and inulin) and galactans (galactooligosaccharides) dominate the literature, recent expert consensus indicates that other substances such as polyphenols and fatty acids could be considered prebiotics if demonstrated to exert beneficial effects in the host.19 The synergistic combination of both probiotics and prebiotics has been termed synbiotics.
Diet is the primary medium for microbial metabolism and accordingly plays a significant role in shaping an individual’s microbiome. Dietary changes can have a considerable and sustained effect on the composition of the microbiome, and alterations in the microbiome, in turn, may influence the absorption, breakdown, and storage of nutrients.11,18,20 Additionally, compositional differences in gut microbiota contribute to individual differences in the metabolic responses to specific foods.21,22 These differences have been associated with differential weight loss in response to certain diets.23 For example, two recent studies of overweight adults found that individuals with high Prevotella/Bacteroides ratios at baseline lost more weight and body fat after 6 months on a high-fiber dietary intervention compared to individuals with low Prevotella/Bacteroides ratio.23,24 Likewise, a metagenomic study that stratified obese individuals by the genomic profiles of their gut microbiota observed a more favorable response in inflammation variables following dietary intervention among individuals with greater baseline microbial diversity.18 As insights into the role of the intestinal microbiome in obesity grow, the possibility of managing weight through modulation of the gut microbiome becomes an increasingly appealing strategy.
The use of MTTs as a preventative and treatment strategy for a range of chronic diseases, including obesity, has been steadily increasing.25 Clinical studies have shown an association between probiotics and reductions in body weight and other anthropometric measures,26 between prebiotics and appetite suppression, reduced food intake, and altered composition and function of the gut,27 and between synbiotics and reductions in body mass index (BMI), waist circumference and hip circumference.28 Thus far, the positive outcomes observed in these studies suggest that continued exploration of MTTs is worthwhile for obesity management programs.
Given the complexity of obesity and the interconnected physiological mechanisms controlling weight and appetite, a combination of therapies may conceivably be more effective than a single strategy. A recent systematic review and meta-analysis demonstrated that the combination of exercise and dietary interventions was more effective for weight loss over the long term than either strategy alone.29 Likewise, combination pharmacotherapy that uses medications with complementary modes of action may outperform the same treatments administered individually.30 Recent studies demonstrate that MTTs can promote weight loss in individuals with overweight and obesity. Still, as far as we are aware, no review has been conducted on the potential benefit of delivering these therapies as an adjunct to traditional weight loss interventions (exercise and diet) in humans. Therefore, this systematic review and meta-analysis aimed to evaluate the extent to which MTTs amplify the effects of traditional weight loss interventions for the treatment of overweight and obesity in adults.
Methods
Search Strategy
This study was undertaken in accordance with the PRISMA guidelines for reporting systematic reviews and meta-analyses.31 A comprehensive search of Medline (PubMed), Embase, and the Cochrane Central Register of Controlled Trials was conducted for randomized controlled trials (RCTs) published between January 1, 2010 and December 31, 2020. The search strategy combined the controlled vocabulary terms for each concept alongside key word synonyms using Boolean operators. Reference lists of included studies and relevant reviews were also searched by hand to identify additional studies meeting eligibility criteria not captured by the database search. The complete search strategy can be found in the Supplementary Box S1.
Inclusion Criteria
We included studies of adults with overweight or obesity (BMI ≥ 25) aged 18 or older that were published in English, administered an MTT (prebiotics, probiotics, or synbiotics) as an intervention for obesity in conjunction with a dietary and/or exercise weight loss intervention, and included at least one of the following indices or measures of obesity: BMI, body weight, fat mass, or waist circumference, collected at baseline and end-of-treatment. We excluded studies that included pregnant females, the use of anti-obesity medications, or participants that had undergone bariatric surgery. Only original studies in which the full-text could be retrieved were included.
Data Extraction and Study Selection
The initial screening by title and abstract was performed by one reviewer (TP) and added to a database prepopulated with inclusion and exclusion criteria. A second reviewer (KW) independently assessed all entries marked for inclusion by the first reviewer, as well as a random subset of 50 entries marked for exclusion. Full-text review and data extraction were performed by two reviewers (TP and KW). In all stages, disagreements were resolved by discussion and consensus.
A structured data extraction form (Supplementary Table S1) was used for collecting relevant information from the selected studies. Data were extracted on participant characteristics, study design, study subgroups, type and dose of MTT, type and details of the secondary (exercise and/or diet) intervention, duration of treatment, and outcomes of interest.
Risk of Bias Assessment
To assess the internal quality of RCTs, the Cochrane Collaboration’s tool for assessing the risk of bias (RoB-2) was applied to each included study.32 The RoB-2 tool assessed the areas of sequence generation, allocation concealment, the blinding of participants and personnel, the blinding of outcomes, incomplete outcome data, selective reporting, and other potential biases. The potential risk of bias for each domain was graded as some, low, or high risk.
Authors of all trials selected for inclusion were contacted to request a copy of their statistical analysis plan and additional information on other unclear domains in the RoB-2. Only two authors responded to our request in the time given (six weeks), and our analysis for these studies was updated accordingly.
Statistical Analysis
Effect Size
All analyses were conducted in R version 4.0.4 using the “meta” package.33,34 Separate analyses were conducted for probiotics and synbiotics for each anthropometric measure (BMI, body weight, fat mass, and waist circumference) with sufficient data (n≥ 5 studies) for pooling. Prebiotics administered alone as a treatment were included in a single study and were therefore omitted from further analysis.
The effect size was used to determine the change between the baseline and post-intervention measures for treatment and control groups. Each anthropometric outcome was assessed using the mean difference (MD; ie the difference of the means) ± standard deviation (SD). Where provided, these values were taken directly from studies; otherwise, MD was calculated by subtracting the post-treatment mean from the baseline mean for each group and the SD was calculated using SDchange = (SDbase2 + SDfinal2 – (2 x Corr x SDbase x SDfinal)1/2 (where base = baseline and final = post-treatment). In instances where within-group SD (ie SDbase and SDfinal) was not provided, these values were estimated from the reported confidence intervals (CIs) or standard errors using the following formulas: SD = (N)1/2 x (upper CI – lower CI) / t-statistic (where the t-statistic was determined from t distribution tables for the appropriate degrees of freedom); and SD = SE x (N)1/2.
The Corr (ie correlation coefficient) value used in the SDchange estimation was set to 0.5, as there were very few studies included in our meta-analysis that reported SDchange from which to reliably estimate the correlation coefficient.35 To assess the effect of the correlation coefficient on the final pooled effect size, we also estimated SDchange for studies that did report it using a correlation coefficient of 0.2 and then 0.8, and re-ran the pooled effect size analysis using both scenarios. A visual inspection of the resulting forest plots showed that increasing or decreasing the correlation coefficient had a minimal effect on the pooled effect sizes.
The effect sizes were pooled between studies to assess the overall difference between treatment and control groups for each anthropometric outcome. Pooled MD was calculated using a random effects model using the Paule and Mandel estimator, as recommended for continuous data.36 Pooled effect sizes were reported as pooled MD (lower 95% CI, upper 95% CI). We calculated the percent change for each anthropometric measure using the pooled mean and SD values for both probiotics and synbiotics.
Sensitivity Analysis
The SD of change used to calculate the effect size tended to be larger than the SDs directly reported in the included studies. This resulted in the studies that reported SDs being assigned a greater weight in the pooled effect sizes. Therefore, a leave-one-out sensitivity analysis was also conducted for each anthropometric outcome to assess the influence of individual studies on the overall effect size.
Heterogeneity and Publication Bias
Between-study heterogeneity was evaluated using the I- square (I2) test and through visual inspection of the forest plots. We considered heterogeneity to be low, moderate, or high where I2 was greater than 25%, 50%, and 75%, respectively.37
Small study publication bias was evaluated through visual inspection of funnel plots and Egger’s test of regression for analyses that included ten or more studies. Analyses including fewer than ten studies are underpowered to distinguish chance from real asymmetry.38
Results
Study Selection
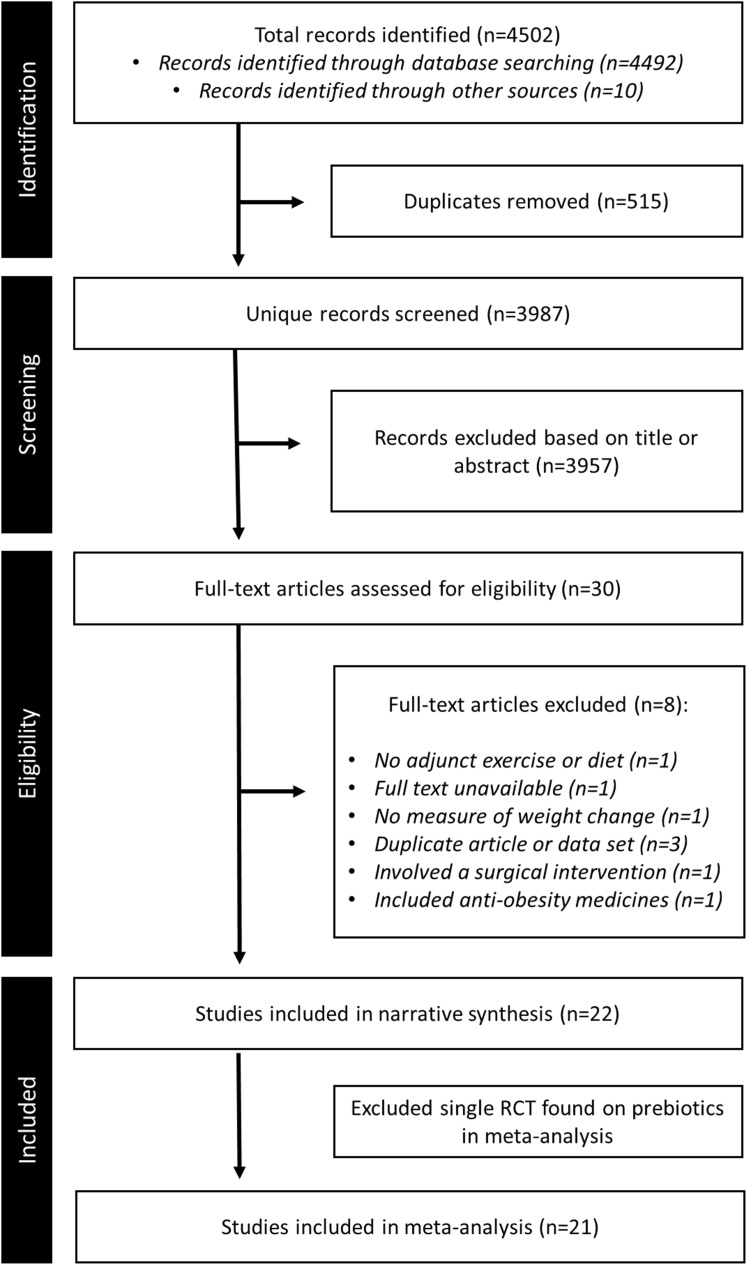
In total, 4502 records were identified in a combined search of electronic databases and reference lists. After removing duplicates and initial title and abstract screening, 30 records remained and were assessed for eligibility by full-text review. Of these, 22 RCTs met the criteria for inclusion in this report, and 21 were included in meta-analyses (Figure 1). The remaining study not included in the meta-analysis was the single record for prebiotics.
Figure 1.
Flowchart of study selection. Preferred Reporting in Systematic Reviews and Meta-analyses (PRISMA) flowchart showing the study selection process.
Fifteen of the included studies were double-blinded RCTs, four were single-blinded RCTs, one was triple-blind, and two were unblinded. Probiotics and synbiotics were evaluated alongside traditional weight loss therapy in 11 and 10 studies, respectively. Selected studies were conducted in Iran (n=10), Brazil (n=2), Canada (n=2), Poland (n=2), Italy (n=2), Korea (n=1), Estonia (n=1), Spain (n=1), and the USA (n=1).
General Characteristics of Studies
The primary characteristics of the included studies are described in Table 1. A total of 1233 participants were included across all studies, with a sample size ranging from 28 to 105 participants (mean=56.1 ± 22.4). All participants were ≥18 years old (mean age: 41.7 ± 8.9 years) with BMI ≥ 25 in all cases (weighted mean BMI: 32.6 ± 3.5 kg/m2). Three-quarters of all participants were female (76.4%). The majority of studies (n=13) included healthy participants without any major underlying health conditions. However, a subset of studies included patients with non-alcoholic fatty liver disease (n=3), metabolic syndrome (n=4), food addiction (n=1), and major depressive disorder (n=1).
Table 1.
Characteristics of Included Studies
| First Author, Year, Reference, Country | Study Design | Study Duration and Stages | Participant Characteristics (mean ± SD) | Microbiome-Targeted Therapy and Details | Dietary/Exercise Intervention | Groups | Significant Anthropometric Outcomes (at the End of Treatment) |
|---|---|---|---|---|---|---|---|
| Prebiotics | |||||||
| Vaghef-Mehrabany 201961 Iran | Randomized, double-blind, placebo-controlled study | 8 weeks | 45 obese women with the major depressive disorder; Age: 38.7 ± 7.8 years; BMI: 34.02 ± 3.9 kg/m2; 100% female; Attrition: 27.4% |
Prebiotic: Inulin (10 g/day sachet dissolved in water) | 25% calorie-restricted diet with meal plan, composed of 55%/ 15%/ 30% carbohydrate/ protein/ fat | A) Prebiotic + hypocaloric diet (n=22) B) Placebo + hypocaloric diet (n=23) |
Reduced ***BW, ***BMI, ***WC, and ***HC in both groups, but NS differences between groups. Reduced ***FM in group A only, but NS difference between groups. |
| Probiotics | |||||||
| Banach 202034 Poland | Randomized, single-blind, placebo-controlled study | 12 weeks | 54 healthy overweight and obese adults; Age: 34.5 ± 10.0 years; BMI: 34.9 ± 3.9 kg/m2; 65% female; Attrition: 27.4% |
Probiotic: 250 g/day yogurt containing Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12 | Individualized weight loss program with hypocaloric diet (500–800 Kcal/day reduction; 45–60%/ 15–25%/ 25–35% carbohydrate/ protein/ fat) + 150 min of moderate or 75 min of high-intensity exercise per week + behavioural aspects, such as goal setting |
A) Probiotic + weight loss program (n=27) B) Weight loss program only (n=27) |
Reduced *BW and *FM in both groups, but NS difference between groups. |
| Doria 201344 Italy | Randomized, double-blind, placebo-controlled study | 90 days (measurements taken at 30, 60, and 90 days) | 40 healthy slightly overweight women; Age: 41.4 ± 7.8 years; BMI: not given; 100% female; Attrition: 0% |
Probiotic: 25 mL of dietary supplement containing Lactobacillus casei (7.5 x 108), Lactobacillus acidophilus (7.5 x 108) and other nutrients (phloridzin, isoflavones (puerarin 67%, daidzin 0.5%, daidzein 1.6%, genistein 0.4%) | Hypocaloric diet (300 Kcal/day reduction) | A) Probiotic + diet (n=20) B) Placebo + diet (n=20) |
Greater reductions in **BW, **FM, ***WC, ***TC, and **BC in group A compared with group B. |
| Gomes 201542 Brazil | Randomized, double-blind, placebo- controlled study | 8 weeks | 43 healthy overweight or obese adults; Age: 20–59 years; BMI: 32.5 ± 4.4 kg/m2; 100% female; Attrition: 28.3% |
Probiotic: 4 sachets daily, each containing Lactobacillus acidophilus LA-14, Lactobacillus casei LC-11, Lactococcus lactis LL-23, Bifidobacterium bifidum BB-06, and Bifidobacterium lactis BL-4 (2 x 1010 CFU/day total) | Normocaloric diet (25–30 kcal/kg; energy intake according to expected BW for height) + maintenance of pre-trial physical activity | A) Probiotic sachets + diet B) Placebo sachets + diet |
Greater reduction in *WC in group A compared with B. Reduced *FM in group A only, but NS difference between groups. |
| Kim et al 201835 Korea | Randomized, double-blind, placebo- controlled study | 12 weeks | 90 healthy overweight and obese adults; Age: 38.4 ± 9.8 years; BMI: 28.4 ± 2.5 kg/m2; 70% female; Attrition: 14.4% |
Probiotic: 1600 mg/day of low dose (109 CFU) or high dose (1010 CFU) Lactobacillus gasseri BNR17 (BNR-H) | Hypocaloric diet (200 kcal/day reduction) + increased energy expenditure (100 kcal/day) | A) Low-dose probiotic (109 CFU) + diet/exercise plan (n=30) B) High dose probiotic (1010 CFU) + diet/exercise plan (n=30) C) Placebo + diet/exercise plan (n=30) |
Greater reduction in *FM in groups A and B compared with C. Reduced *WC in groups A & B, but NS difference between groups. |
| Madjd 201637 Iran | Randomized, double-blind, controlled study | 12 weeks | 89 healthy overweight and obese women; Age: 32.0 ± 6.8 years; BMI: 32.1 ± 3.6 kg/m2; 100% female; Attrition: 9% |
Probiotic: 400 g/day of probiotic yogurt containing Lactobacillus acidophilus LA5 and Bifidobacterium lactis BB12 (1 x 107 CFU) | Hypocaloric diet (500–1000 kcal/day reduction) + physical activity (gradual increase to 60 min of moderate exercise 5 days/ week) |
A) Probiotic yogurt + hypocaloric diet + exercise (n=44) B) Yogurt (no probiotic) + hypocaloric diet + exercise (n=45) |
Reduced **BM, **BMI, and **WC in both groups, but NS difference between groups. |
| Narmaki62 Iran | Randomized, double-blind, placebo-controlled study | 2 phases of 6 weeks: Phase 1: reduced calorie diet only Phase 2: reduced calorie diet + capsules |
62 obese women with food addiction; Age: 33.2 ± 6.5 years; BMI: 34.4 ± 2.9 kg/m2; 100% female; Attrition: 8% |
Probiotic: Capsules containing 6 species: Lactobacillus acidophilus (1.8 x 109 CFU/capsule), Bifidobacterium bifidum (1.8 x 109 CFU/capsule), Bifidobacterium lactis (1.8 x 109 CFU/capsule) Bifidobacterium longum (1.8 x 109 CFU/capsule), Lactobacillus rhamnosus (1 x 109 CFU/capsule), and Lactobacillus reuteri (1 x 109 CFU/capsule) | Personalized hypocaloric diet (300–500 Kcal/day reduction; approx. 55%/ 15%/30% carbohydrate/ protein/ fat) | A) Probiotic + hypocaloric diet (n=31) B) Placebo + hypocaloric diet (n=31) |
Reduced ***BW, ***BMI, ***WC, ***WHR, ***BF and ***TF in group A compared with group B. |
| Omar 201363 Canada | Randomized, double-blind, placebo- controlled, cross-over study | 3 Phase of 43 days, separated by a washout period of 6 weeks | 28 healthy overweight and obese adults; Age: 46.3 ± 2.4 years; BMI: 31.6 ± 0.7 kg/m2; 64% female; Attrition: not given |
Probiotic: 100 g/day yogurt containing 10 g of Lactobacillus amylovorus (1.39 x 109 CFU) and 10 g of Lactobacillus fermentum (1.08 x 109 CFU) | Hypocaloric diet containing 50%/ 15%/ 35% carbohydrate/ protein/ fat | A) Yogurt containing Lactobacillus fermentum + hypocaloric diet (n=14) B) Yogurt containing Lactobacillus amylovorus + hypocaloric diet (n=12) C) Regular yogurt + hypocaloric diet (n=12) |
Reduced *FM in all groups, but NS difference between groups. |
| Razmpoosh 201945 Iran | Randomized controlled study | 8 weeks | 65 overweight and obese women; Age: 36.5 ± 8.0 years; BMI: 31.5 ± 4.6 kg/m2; 100% female; Attrition: 7% |
Probiotic: 50g/day pasteurized liquid probiotic Kashk containing Lactobacillus acidophilus La5 (1.85 x 106 CFU/g) and Bifidobacterium lactis Bb12 (1.79 x 106 CFU/g) | Hypocaloric diet (500 kcal/day reduction; approx. 58%/ 15%/ 27% carbohydrate/ protein/ fat) | A) Probiotic + hypocaloric diet (n=32) B) Hypocaloric diet only (n=33) |
Reduced **BW, **BMI, **BFM, **BFP, and *WC in group A compared with group B. NS between-group differences in FFM. |
| Sharafedtinov 201364 Estonia | Randomized, parallel, double-blind, placebo- controlled pilot study | 3 weeks | 36 obese adults, with metabolic syndrome; Age: 51.9 ± 11.2 years; BMI: 37.2 ± 4.2 kg/m2; 68% female; Attrition: 10% |
Probiotic: 50 g/day of probiotic cheese containing Lactobacillus plantarum TENSIA | Hypocaloric diet (1512 kcal/day) | A) Probiotic cheese + hypocaloric diet (n=25) B) Regular cheese (no probiotic) + hypocaloric diet (n=11) |
Reduced *BMI and *WHR in group A compared with group B. NS between-group differences in BW and FM. |
| Zarrati 201836 Iran | Randomized, double-blind, placebo-controlled study | 8 weeks | 56 obese or overweight individuals; Age: 36.1 ± 9.0 years; BMI: 32.1 ± 4.4 kg/m2; 70% female; Attrition: 6.7% |
Probiotic: 200 g/day yogurt containing Lactobacillus acidophilus LA5, Lactobacillus casei DN001, and Bifidobacterium lactis Bb12 (1 x 108 CFU/g each) | Hypocaloric diet (500 kcal/day reduction; 55–60%/ 12–15%/ 30–35% carbohydrate/ protein/ fat) + 30–45 minutes walking 3–5 times/week | A) Probiotic + hypocaloric diet + exercise (n=26) B) Regular yogurt + hypocaloric diet + exercise (n=30) |
Reduced **BFP in group A compared with group B. NS between-group differences in BW, BMI, or WC. |
| Zarrati 201465 Iran | Randomized, double-blind, controlled study | 8 weeks | 75 healthy overweight and obese adults; Age: 35.7 ± 8.9 years; BMI: 33.2 ± 5.7 kg/m2; 68% female; Attrition: 0% |
Probiotic: 200 g/day probiotic yogurt containing Lactobacillus acidophilus La5 (1 x 107 CFU/mL), Bifidobacterium BB12, and Lactobacillus casei DN001 (1 x 107 CFU/mL) | Hypocaloric diet (details not provided) | A) Probiotic yogurt + hypocaloric diet (n=25) B) Probiotic yogurt only (n=25) C) Regular yogurt + hypocaloric diet (n=25) |
Reduced ***BW, ***BMI, and ***WC in group A compared with group B and group C compared with group B. |
| Synbiotics | |||||||
| Eslamparast 201438 Iran | Randomized, double-blind, placebo-controlled pilot study | 28 weeks | 38 adults with metabolic syndrome; Age: 46.8 ± 9.5 years; BMI: 31.8 ± 2.5 kg/m2; 60.5% female; Attrition: 0% |
Probiotic: Capsule containing 2×108 CFU of seven strains (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus) Prebiotic: 250mg FOS |
Hypocaloric diet (500–1000 kcal reduction) + 20–30 min of high-intensity exercise 3–4 day/week (or more) | A) Synbiotic capsule + diet + exercise (n=19) B) Placebo capsule + diet + exercise (n=19) |
NS within- or between-group differences in BMI and WC. |
| Eslamparast 2014b39 Iran | Randomized, double-blind, placebo-controlled pilot study | 28 weeks | 52 adults with non-alcoholic fatty liver disease; Age: 46.0 ± 9.2 years; BMI: 31.7 ± 2.4 kg/m2; 92.6% female; Attrition: 7.7% |
Probiotic: Capsule containing 2×108 CFU of seven strains (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus) Prebiotic: 250mg FOS |
Hypocaloric diet (500–1000 kcal reduction) + 20–30 min of high-intensity exercise 3–4 day/week (or more) | A) Synbiotic capsule + diet + exercise (n=26) B) Placebo capsule + diet + exercise (n=26) |
Reduced *BMI and *WHR in both groups, but NS differences between groups. |
| Ferolla 201666 Brazil | Randomized, controlled, single-blind study | 3 months | 50 adults with non-alcoholic fatty liver disease; Age: median=57.3 years BMI: 32.5 ± 4.0 kg/m2; 76% female; Attrition: 0% |
Probiotic: 2 capsules of Lactobacillus reuteri (1 x 108 CFU) Prebiotic: 4g partially hydrolyzed guar gum and inulin |
Hypocaloric diet (1500/1800 kcal for women/men – a 500–1000 kcal reduction) | A) Synbiotic capsule + diet (n=27) B) Placebo capsule + diet (n=23) |
Reduced *BW, *BMI, and **WC in Group A but not group B. |
| Gutiérrez-Repiso 201967 Spain | Randomized, single-blind, parallel study | 2 x 2-month stages: Phase 1: 2 months very-low-calorie diet Phase 2: 2 months of a low-calorie diet |
33 obese patients; Age: 45.4 ± 10.4 years; BMI: 32.9 ± 1.6 kg/m2; 61% female; Attrition: not given |
Phase 1 Probiotic: Bifidobacterium lactis, Lactobacillus rhamnosus, Bifidobacterium longum ES1 Prebiotic: Prebiotic fibre (unspecified) Phase 2 Probiotic: Bifidobacterium lactis Prebiotic: prebiotic fibre (unspecified) |
Phase 1: Very-low-calorie Ketogenic Diet (PnK method, 600–800 kcal/day) Phase 2: low-calorie diet (800–1500 kcal/day) |
A) Synbiotics during both phases (n=15) B) Placebo during very-low-calorie diet phase and synbiotic during low-calorie-diet phase (n=9) C) Placebo during both phases (n=9) |
Reduced *BW in group B compared with group A. |
| Janczy 202041 Poland | Randomized, single-blinded study | 12 weeks | 56 obese patients; Age: 37.2 ± 15.6 years; BMI: 33.8 ± 7.0 kg/m2; 75% female; Attrition: 5.1% |
Probiotic: Probiotic capsules (Bifidobacterium lactis ≥2.8 x 108, Lactobacillus acidophilus ≥1.2 x 108, Lactobacillus paracasei ≥0.9 x 108, Lactobacillus plantarum ≥1.1 x 108
Lactobacillus salivarius ≥0.9 x 108, Lactobacillus lactis ≥1.1 x 108) Prebiotic: 9.6 g FOS, 110.4 g inulin |
Hypocaloric diet (500 kcal reduction; 45–55%/ 20–25%/ 25–30% carbohydrate/ protein/ fat + maintenance of pre-trial physical activity | A) Synbiotic + hypocaloric diet (n=36) B) Placebo + hypocaloric diet (n=20) |
Reduced ***BW, ***BMI, and ***FM in both groups, but NS difference between groups. |
| Malaguarnera 201268 Italy | Randomized, parallel, double-blind, placebo-controlled study | 6 months | 66 males and females with excess weight and non-alcoholic fatty liver disease; Age: 46.8 ± 5.5 years; BMI: 27.3 ± 1.3 kg/m2; 50% female; Attrition: 0% |
Probiotic: Bifidobacterium longum W11 (5 x 109 CFU) Prebiotic: 2.5 g FOS |
Lifestyle modification program, including mild physical training + 1600 kcal/day diet | A) Synbiotic + lifestyle modification (n=34) B) Lifestyle modification only (n=32) |
Reduced *BMI in both groups but NS difference between groups. |
| Mohammadi-Sartang 201940 Iran | Randomized, parallel, double-blinded study | 10 weeks | 90 overweight or obese adults with metabolic syndrome; Age: 45.5 ± 8.8 years; BMI: 30.4 ± 2.4 kg/m2; 59% female; Attrition: 3.3% |
Probiotic: Fortified yogurt containing Bifidobacterium lactis Bb-12 (107 CFU/g) Prebiotic: 3 g inulin + 5g whey + 5 mg calcium + 500 IU vitamin D3 per serve |
Hypocaloric diet (500 kcal/day reduction) + maintenance of pre-trial physical activity | A) 2 daily servings of fortified yogurt + hypocaloric diet (n=44) B) 2 daily servings low-fat yogurt + hypocaloric diet (n=45) |
Reduced *FM in group A compared with group B. |
| Rabiei 201543 Iran | Randomized, triple-blind, controlled study | 12 weeks | 40 adults with metabolic syndrome; Age: 59.0 ± 7.6 years; BMI: 32.4 ± 4.7 kg/m2; 69.6% female; Attrition: 13% |
Probiotic: 2 capsules daily containing 2×108 CFU Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum, Lactobacillus bulgaricus Prebiotic: FOS |
Diet based on ideal weight + maintenance of pre-trial physical activity | A) 2 synbiotic capsules daily + personalized diet plan (n=20) B) 2 placebo capsules daily + personalized diet plan (n=20) |
Reduced ***BMI, ***WC, and ***HC in both groups but NS differences between groups. |
| Sanchez 201469 Canada | Randomized, double-blind, placebo-controlled study | 6 months: Phase 1: 12-weeks of weight loss Phase 2: 12-weeks of weight maintenance |
105 healthy obese adults; Age: 36.0 ± 10 years; BMI: 36.0 ± 2.2 kg/m2; 57% female; Attrition: not given |
Probiotic: Lactobacillus rhamnosus CGMCC1.3724 (1.62 x 108 CFU) Prebiotic: Oligofructose and inulin |
Phase 1: Personalized diet plan with a 500 kcal/day reduction Phase 2: Personalized diet plan without energy reduction |
A) 2 synbiotic capsules daily + personalized diet plan (n=62) B) 2 placebo capsules daily + personalized diet plan (n=63) |
Reduced *BW and *FM in group A females compared with group B females in both stages (NS difference when considering males and females together). NS differences in BW and FM in men in either group at either stage. |
| Sergeev 2020 70 USA | Randomized, placebo-controlled study | 12 weeks | 20 overweight or obese adults; Age: 47.4 ± 12.3 years; BMI: 33.5 ± 5 kg/m2; 75% female; Attrition: 0% |
Probiotic: Probiotic capsules containing 15×109 CFU (Lactobacillus acidophilus DDS-1, Bifidobacterium lactis UAB1a-12, Bifidobacterium longum UAB1-14, and Bifidobacterium bifidum UABb-10) Prebiotic: Trans-galactooligosaccharide |
Energy restricted diet recommended (low-carbohydrate, high-protein) – did not track adherence | A) Synbiotic + hypocaloric diet recommendations (n = 10) B) Placebo + hypocaloric diet recommendations (n = 10) |
NS difference in BW, BMI, FM, and BFP within or between groups. |
Notes: Significance is denoted by *p<0.05, **p<0.01, or ***p<0.001. Sample size refers to the number of participants included in the final analysis.
Abbreviations: BC, buttock circumference; BF, body fat; BFM, body fat mass; BFP, body fat percentage; BMI, body mass index; BW, body weight; FFM, fat-free mass; FM, fat mass; HC, hip circumference; NS, non-significant; TC, thigh circumference; TF, trunk fat; WC, waist circumference; WHR, weight-to-height ratio.
Studies ranged in duration from three weeks to seven months, with a mean duration of 13.8 ± 7.1 weeks. Of the 21 studies that included a probiotic (either on its own or as part of a synbiotic), 19 included Lactobacillus species, 14 included Bifidobacterium species, one included Lactococcus species, and three included Streptococcus species. By frequency for each genus, these were Lactobacillus acidophilus (n=13), casei (n=7), rhamnosus (n=6), bulgaricus (n=3), plantarum (n=2), reuteri (n=1), gasseri (n=1), fermentum (n=1), paracasei (n=1), and salivarius (n=1); Bifidobacterium lactis (n=10), longum (n=7), bifidum (n=4), and breve (n=3); and Streptococcus thermophilus (n=3), and Lactococcus lactis (n=1).
Of the eleven studies that included a prebiotic (either on its own or as part of a synbiotic), six used fructooligosaccharides, four used inulin, one used guar gum, and one used trans-galactooligosaccharide. MTT was most often administered in the form of capsules (n=10), followed by yogurt (n=6), liquid preparations (n=5), and cheese (n=1).
All studies incorporated some degree of caloric restriction into their intervention. Where the amount was specified, reductions ranged from 200–1500 kcal/day. Seven studies reported specific macronutrient distributions, with carbohydrate-protein-fat ratios ranging from 45–60% carbohydrate, 12–25% protein, and 25–35% fat. Six studies included recommendations for adjunct exercise,39–44 while four studies explicitly requested participants to avoid altering their normal pattern of physical activity.45–48
The Effect of Probiotic Supplementation on Obesity Outcomes
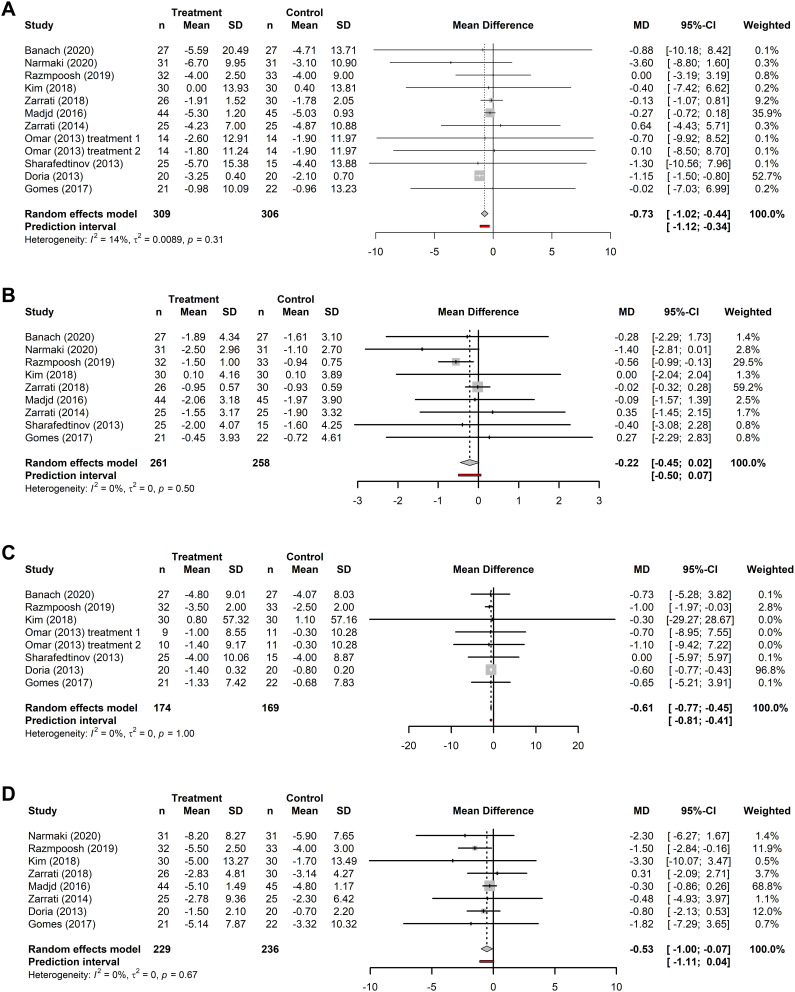
The effects of probiotic supplementation as an adjunct to diet and/or exercise programs for weight loss were examined in 11 RCTs. Meta-analyses were performed for 11 trials (n=309) for body weight, 9 trials for BMI (n=261), 7 trials for fat mass (n=174), and 8 trials for waist circumference (n=229). Forest plots for all analyses are presented in Figure 2. Meta-analyses indicated a reduction in body weight (MD: −0.73 kg; 95% CI: −1.02 to −0.44, p < 0.001), fat mass (MD: −0.61 kg; 95% CI: −0.77 to −0.45; p<0.001), and waist circumference (MD: −0.53 cm; 95% CI: −0.99 to −0.07, p=0.024) after probiotic supplementation and exercise/diet compared to control participants receiving exercise/diet only. The pooled effect size for BMI was not statistically significant. The I2 values were all below our low-heterogeneity criteria (body weight: I2=14%, p=0.31; BMI: I2=0%, p=0.5; fat mass: I2=0%, p=1; waist circumference: I2=0%, p=0.67).
Figure 2.
Forest plots of the effects of probiotics. Forest plots of the effects of probiotics on (A) body weight; (B) BMI; (C) fat mass; and (D) waist circumference. Analyses consider the pooled mean difference (MD) between baseline and end-of-treatment in patients receiving microbiome-targeted therapies as an adjunct to exercise/diet compared with patients receiving exercise/diet only. MDs are presented with the 95% CI for each study and for the combined results.
Compared to controls, the percentage change in the treatment group was 0.29% greater for body weight (treatment: 4.16%; control: 3.86%), 0.42% greater for BMI (treatment: 4.28%; control: 3.86%), 1.03% greater for waist circumference (treatment: 4.81%; control: 3.78%), and 1.67% greater for fat mass (treatment: 6.47%; control: 4.80%).
The leave-one-out sensitivity analysis suggested that studies with high weightings in the pooled MD did influence the pooled results; however, the pooled effect sizes when the highest weighted study was removed from the analysis suggested that body weight, fat mass, and waist circumference were still reduced after supplementation compared to the control group (Supplementary Figure S1).
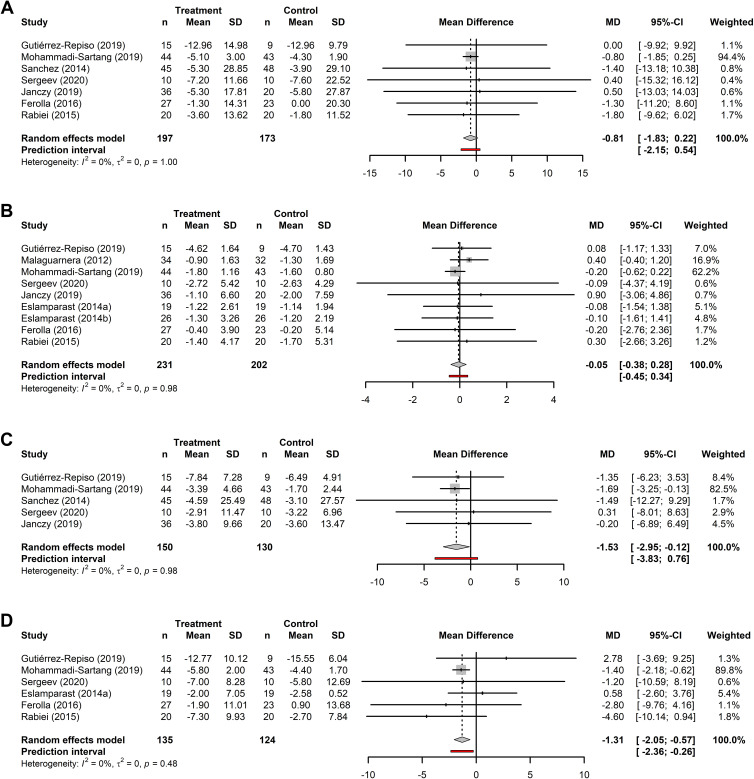
The Effect of Synbiotic Supplementation on Anthropometric Outcomes
The effects of synbiotic supplementation as an adjunct to diet or exercise programs for weight loss was examined in 10 RCTs. Meta-analyses were performed for 7 trials for body weight (n=197); 9 trials for BMI (n=231); 5 trials for fat mass (n=150) and 6 trials for waist circumference (n=135). Forest plots for all analyses are presented in Figure 3. Meta-analyses indicated a reduction in fat mass (MD: −1.53 kg; 95% CI: −2.95 to −0.12, p=0.034) and waist circumference (MD: −1.31 cm; 95% CI: −2.05 to −0.57, p<0.001) after synbiotic supplementation with exercise/diet compared to control participants receiving exercise/diet only. However, the pooled effect sizes for the reduction in body weight and BMI had non-statistically significant p-values. The I2 values were all below our low-heterogeneity criteria (body weight: I2=0%, p=1.0; BMI: I2=0%, p=1.0; fat mass: I2=0%, p=1; waist circumference: I2=0%, p=0.5).
Figure 3.
Forest plots of the effects of synbiotics. Forest plots of the effects of synbiotics on (A) body weight (kg); (B) BMI (kg/m2); (C) fat mass (kg); and (D) waist circumference (cm). Analyses consider the pooled mean difference (MD) between baseline and end-of-treatment in patients receiving microbiome-targeted therapies as an adjunct to exercise/diet compared with patients receiving exercise/diet only. MDs are presented with the 95% CI for each study and for the combined results.
Compared to controls, the percentage change in the treatment group was higher by 1.13% for body weight (treatment: 5.96%; control: 4.82%), 1.63% for waist circumference (treatment: 5.29%; control: 3.66%), and 4.32% for fat mass (treatment: 13.95%; control: 9.63%). The percentage change for BMI was 0.11% greater in the control group (treatment: 4.31%; control: 4.42%).
Like the probiotic analysis, the leave-one-out sensitivity analysis suggested that the most heavily weighted studies did have an influence on the pooled effect size; however, the pooled effect sizes when the highest weighted study was removed from the analysis suggested that fat mass and waist circumference were still reduced after supplementation compared to the control group (Supplementary Figure S2).
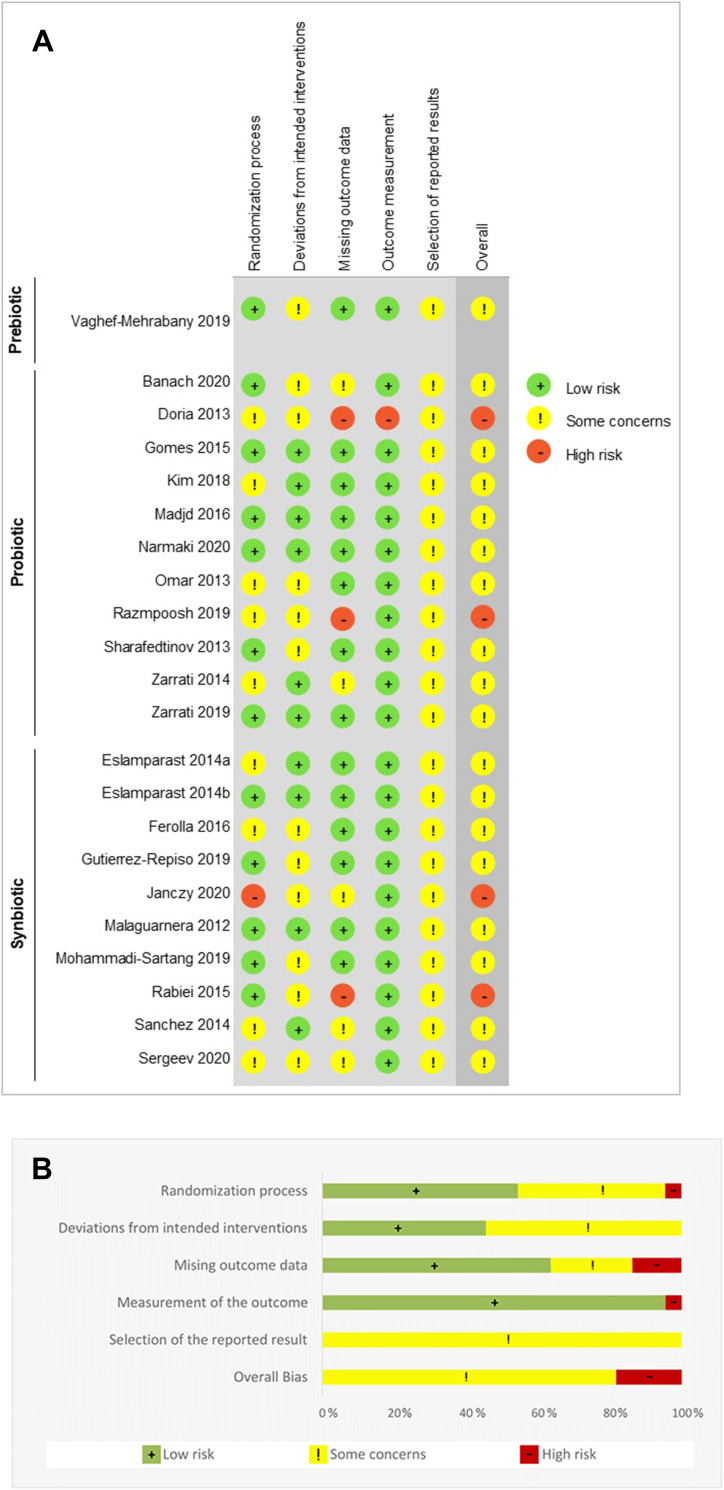
Risk of Bias
The risk of bias for each included RCT is shown in Figure 4A; Figure 4B summarizes the risk of bias across all RCTs. The overall risk of bias reflected some concerns for the majority (n=18) of studies, while four studies were scored as having a high risk of bias.46,48–50 A high risk of bias was present in three of the five domains: randomization, missing outcome data, and measurement of the outcome. Although numerous studies in both domains had some concerns, there were no substantial (high risk) deviations from the intended intervention or selection bias. The most common causes of concern included not providing sufficient detail on the randomization or concealment of the allocation sequence, not including an appropriate analysis to estimate the effect of assignment to the intervention, and lack of clarity regarding the extent to which published data were in accordance with a pre-specified analysis plan.
Figure 4.
Risk of bias assessment using the RoB-2 tool. The risk was assessed using the Cochrane Collaboration’s tool for qualitatively assessing the risk of bias.32 (A) Details of all included studies; (B) overall summary.
Publication Bias
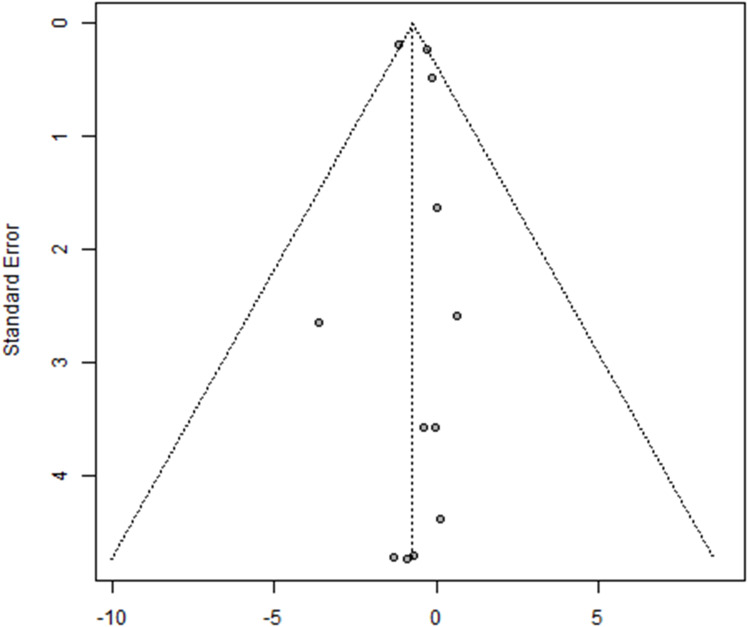
There was no evidence of publication bias in studies examining the effect of probiotics and exercise/diet on body weight (Egger’s test p=0.64; Figure 5), although the number of studies was small; a greater number of studies would give us more confidence in the results. Funnel plots for all other anthropometric outcome groups were not generated due to the low number of trials included.
Figure 5.
Funnel plot. Funnel plot for the meta-analysis of body weight in the probiotic group. The standard error and effect size are shown on the y- and x-axis, respectively. The circles represent the individual studies in the analysis.
Discussion
Obesity has reached epidemic proportions worldwide and continues to raise public health concerns.1 A multitude of treatment options have been investigated for obesity, including behavioral, dietary, pharmacological, and surgical options. However, no singularly effective, feasible, and sustainable intervention currently exists.
The results of this systematic review suggest that gut MTT, delivered in conjunction with dietary or dietary and exercise interventions, can have beneficial effects on anthropometric outcomes in adults with overweight and obesity. Specifically, meta-analyses of pooled MDs identified a positive impact of probiotic supplementation on body weight, fat mass, and waist circumference when delivered with dietary or dietary and exercise interventions. Likewise, synbiotics improved fat mass and waist circumference compared to control participants receiving the dietary or dietary and exercise intervention only.
However, the clinical significance of these changes is less clear. A 5% reduction in body weight is a commonly used metric for assessing the clinical relevance of obesity interventions and is associated with clinically significant improvements in cardiometabolic risk factors, while a ≥3% to <5% change from baseline reflects modest weight loss.51,52 By this definition, modest weight loss was experienced by both treatment and control groups across all outcomes. Considering outcomes that had a statistically significant change only, the addition of probiotics or synbiotics pushed the percentage change near or over the threshold for clinical significance. Specifically, probiotics increased the percentage change by 0.29% to 4.16% in body weight; by 1.03% to 3.78% in fat mass; and by 1.67% to 6.47% in waist circumference; and synbiotics increased the percentage change in waist circumference by 1.63% to 5.29%.
While the margin of difference between treatment and control groups is narrow, the overall percentage change for individuals in the treatment groups occupies the threshold between modest weight loss and clinically significant change. Further studies are required to clarify whether these differences are sufficient to warrant the inclusion of MTTs into traditional therapeutic modalities.
Our review compared individuals receiving dietary or dietary and exercise interventions plus an MTT to those receiving dietary/dietary and exercise interventions only; however, an additional control group receiving the MTT alone would have been informative. Several recent systematic reviews and meta-analyses have investigated the effects of MTTs alone for the management of overweight or obesity, and these have reported largely positive changes in anthropometric measures from probiotics9,53–55 and synbiotics.56 The positive changes observed in our study when probiotics or synbiotics are added to dietary or dietary and exercise interventions are promising as they highlight the potential to enhance the effects of traditional interventions by modulating the gut microbiota.
Strengths and Limitations
Our study had several strengths. We systematically reviewed a decade’s worth of literature to report the first comprehensive analysis of the beneficial role of adjunctive microbiome-targeted therapies on obesity outcomes. Three electronic databases and key reference lists were searched, and all authors from selected studies were contacted for additional information to ensure accuracy. Moreover, we performed separate analyses for probiotics and synbiotics and limited the population of interest to adults with a BMI ≥ 25, improving the specificity of our findings.
There were also limitations in this study. While we observed significant improvements in multiple obesity outcomes in the probiotic and synbiotic analyses, the overall effect sizes were small. Several outcomes (BMI with probiotics and BMI and body weight with synbiotics) had non-significant p-values with CIs including zero, which may in part be due to the relatively small number of studies included in these analyses. In addition, only a single study of prebiotics in combination with exercise and/or diet could be identified, precluding any further comment or analyses on this intervention.
Another potential concern is that most analyses were weighted in favor of a subset of the included studies. We ran a leave-one-out sensitivity analysis to address this concern and found that the pooled effect sizes shifted (increased in some cases and decreased in others) but remained negative after the consecutive removal of individual studies from the analysis, reflecting an improvement in each measure in comparison to the controls.
Study duration ranged from 3 to 28 weeks, and the extent to which the observed changes are sustained beyond this point is unclear. Previous studies indicate that a range of therapeutic modalities can be used to attain short-term clinically relevant weight loss; however, long-term maintenance of weight loss is more challenging.57 In a meta-analysis of 29 studies of structured weight loss programs, more than half of the weight lost was regained within two years, and 80% of weight lost was regained within five years.58 Studies investigating the role of MTTs on long-term clinical endpoints are therefore necessary to establish the clinical relevance of these therapies for sustained changes in obesity outcomes. Since the maximum length of study in our meta-analysis was 28 weeks, we were not able to compare the short-term and long-term influence of probiotics or synbiotics as an adjunct therapy for weight loss.
In addition, our meta-analyses included a relatively small number of studies and patients which precluded sub-group analyses. Although the outcomes measured were homogenous (as measured by the I2 statistic), there was notable heterogeneity in study methods, including the method of administration, study duration, MTT strain(s), dosage, patient characteristics, and the parameters of the exercise and/or dietary intervention. All these variables could have feasibly confounded the results and are worthy of investigation in their own right. For example, probiotics are known to have strain-specific effects on weight.59 A 2012 meta-analysis of human and animal studies reported that L. acidophilus, L. ingluviei, and L. fermentum were linked to weight gain, whereas L. gasseri and L. plantarum were linked to weight loss.60 As the breadth of research in this field increases, a future meta-analysis of MTTs plus exercise/dietary interventions that are sufficiently powered to include subgroups would clarify the therapeutic strains and doses that are most likely to be beneficial in a particular circumstance.
Conclusion
In summary, our work suggests that microbiome-targeted supplements may enhance weight loss and other obesity outcomes in adults when delivered as an adjunct to dietary or dietary and exercise interventions. Given the impact of the gut microbiota on obesity outcomes, modulation of the microbiome should continue to be explored in the pursuit of more effective and sustainable weight management strategies.
Funding Statement
No funding to declare.
Data Sharing Statement
The data supporting this systematic review and meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author by request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed submitting to Diabetes Metabolic Syndrome and Obesity: Targets and Therapy; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in relation to this work and that there are no conflicts of interest regarding the publication of this article.
References
- 1.World Health Organization. Obesity and overweight. Fact sheets; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed April 9, 2021.
- 2.Ritchie HRM. Obesity: share of deaths attributed to obesity and death rate from obesity, 1990 to 2017. Our world in data. 2008.
- 3.The World Obesity Federation. Prevalence of obesity. World obesity. Available from: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity. Accessed November 24, 2022.
- 4.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):1–12. doi: 10.21037/atm.2017.03.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7(5):273–289. doi: 10.1111/cob.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerveld D, Yang D. Through thick and thin: identifying barriers to bariatric surgery, weight loss maintenance, and tailoring obesity treatment for the future. Surg Res Pract. 2016;2016:1–7. doi: 10.1155/2016/8616581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events a systematic review and meta-analysis. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peckmezian T, Hay P. A systematic review and narrative synthesis of interventions for uncomplicated obesity: weight loss, well-being and impact on eating disorders. J Eat Disord. 2017;5(1). doi: 10.1186/s40337-017-0143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John GK, Wang L, Nanavati J, Twose C, Singh R, Mullin G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes. 2018;9(3):167. doi: 10.3390/genes9030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adalsteinsdottir SA, Magnusdottir OK, Halldorsson TI, Birgisdottir BE. Towards an individualized nutrition treatment: role of the gastrointestinal microbiome in the interplay between diet and obesity. Curr Obes Rep. 2018;7(4):289–293. doi: 10.1007/s13679-018-0321-z [DOI] [PubMed] [Google Scholar]
- 11.Duca I, Rusu F, Chira A, Dumitrascu DL. Gut microbiota and body weight – a review. Psihol Teme. 2018;27(1):33–53. doi: 10.31820/pt.27.1.3 [DOI] [Google Scholar]
- 12.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1–4. doi: 10.1136/gut.53.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8(9):523–531. doi: 10.1038/nrgastro.2011.133 [DOI] [PubMed] [Google Scholar]
- 14.John GK, Mullin GE. The gut microbiome and obesity. Curr Oncol Rep. 2016;18(7):2–8. doi: 10.1007/s11912-016-0528-7 [DOI] [PubMed] [Google Scholar]
- 15.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. doi: 10.3390/nu12051474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi: 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 19.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Cai W, Yu J, et al. Dietary advanced glycation end products shift the gut microbiota composition and induce insulin resistance in mice. Diabetes, Metab Syndr Obes Targets Ther. 2022;15:427–437. doi: 10.2147/DMSO.S346411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korem T, Zeevi D, Zmora N, et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017;25(6):1243–1253.e5. doi: 10.1016/j.cmet.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe. 2016;19(1):12–20. doi: 10.1016/j.chom.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 23.Hjorth MF, Roager HM, Larsen TM, et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes. 2018;42(3):580–583. doi: 10.1038/ijo.2017.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjorth MF, Blædel T, Bendtsen LQ, et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int J Obes. 2019;43(1):149–157. doi: 10.1038/s41366-018-0093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerdó T, García-Santos JA, Bermúdez MG, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11:635. doi: 10.3390/nu11030635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bin Wang Z, Xin SS, Ding LN, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and meta-analysis. Evid Based Complement Altern Med. 2019. doi: 10.1155/2019/3862971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoun A, Darwish F, Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev Nutr Food Sci. 2020;25(2):113–123. doi: 10.3746/pnf.2020.25.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrarese R, Ceresola ER, Preti A, Canducci F. Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. Eur Rev Med Pharmacol Sci. 2018;22(21):7588–7605. doi: 10.26355/eurrev-201811-16301 [DOI] [PubMed] [Google Scholar]
- 29.Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557–1568. doi: 10.1016/j.jand.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilleri M, Acosta A. Combination therapies for obesity. Metab Syndr Relat Disord. 2018;16(8):390–394. doi: 10.1089/met.2018.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 32.Higgins J, Savović J, Page MJ, Sterne JAC. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Br Med J. 2019;1–24. Available from https://methods.cochrane.org/. [Google Scholar]
- 33.R Core Team. R: a language and environment for statistical computing; 2020. Available from: https://www.r-project.org/. Accessed November 24, 2022.
- 34.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Heal. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 36.Veroniki AA, Jackson D, Bender R, et al. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res Synth Methods. 2019;10(1):23–43. doi: 10.1002/jrsm.1319 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT, Churchill R, Chandler J, Cumpston MS. Chapter 10: addressing reporting biases. Sterne JAC, Matthias Egger DM, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. ScienceDirect; 2017. Available from http://www.sciencedirect.com/science/article/pii/B9780122210907500129. [Google Scholar]
- 39.Banach K, Glibowski P, Jedut P. The effect of probiotic Yogurt containing lactobacillus acidophilus LA-5 and bifidobacterium lactis BB-12 on selected anthropometric parameters in obese individuals on an energy-restricted diet: a randomized, controlled trial. Appl Sci. 2020;10:17. doi: 10.3390/app10175830 [DOI] [Google Scholar]
- 40.Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: a randomized, double-blind, placebo-controlled trial. J Med Food. 2018;21(5):454–461. doi: 10.1089/jmf.2017.3937 [DOI] [PubMed] [Google Scholar]
- 41.Zarrati M, Raji Lahiji M, Salehi E, et al. Effects of probiotic yogurt on serum omentin-1, adropin, and nesfatin-1 concentrations in overweight and obese participants under low-calorie diet. Probiotics Antimicrob Proteins. 2018;11(4):1202–1209. doi: 10.1007/s12602-018-9470-3 [DOI] [PubMed] [Google Scholar]
- 42.Madjd A, Taylor MA, Mousavi N, et al. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: a randomized controlled trial. Am J Clin Nutr. 2016;103(2):323–329. doi: 10.3945/ajcn.115.120170 [DOI] [PubMed] [Google Scholar]
- 43.Eslamparast T, Zamani F, Hekmatdoost A, et al. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br J Nutr. 2014;112(3):438–445. doi: 10.1017/S0007114514000919 [DOI] [PubMed] [Google Scholar]
- 44.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99(3):535–542. doi: 10.3945/ajcn.113.068890 [DOI] [PubMed] [Google Scholar]
- 45.Mohammadi-Sartang M, Mazloomi SM, Fararouie M, Bedeltavana A, Famouri M, Mazloom Z. Daily fortified-synbiotic yogurt consumption facilitates appetite control in overweight and obese adults with metabolic syndrome during a weight-loss program: a 10-week randomized controlled trial. Prog Nutr. 2019;21(1):135–144. doi: 10.23751/pn.v21i2-S.6906 [DOI] [Google Scholar]
- 46.Janczy A, Aleksandrowicz-Wrona E, Kochan Z, Malgorzewicz S. Impact of diet and synbiotics on selected gut bacteria and intestinal permeability in individuals with excess body weight – a prospective, randomized study. Acta Biochim Pol. 2020;67(4):571–578. doi: 10.18388/ABP.2020_5443 [DOI] [PubMed] [Google Scholar]
- 47.Gomes AC, de Sousa RGM, Botelho PB, Gomes TLN, Prada PO, Mota JF. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: a double-blind, randomized trial. Obesity. 2016;25(1):30–38. doi: 10.1002/oby.21671 [DOI] [PubMed] [Google Scholar]
- 48.Rabiei S, Shakerhosseini R, Saadat N. The effects of symbiotic therapy on anthropometric measures, body composition and blood pressure in patient with metabolic syndrome: a triple blind RCT. Med J Islam Repub Iran. 2015;29(1):115. [PMC free article] [PubMed] [Google Scholar]
- 49.Doria E, Buonocore D, Michelotti A, Nobile V, Marzatico F. Evaluation of a phyto-supplement efficacy as adjuvant in reducing body weight and fat mass in overweight women. Curr Top Nutraceutical Res. 2013;11(1–2):21–27. [Google Scholar]
- 50.Razmpoosh E, Zare S, Fallahzadeh H, Safi S, Nadjarzadeh A. Effect of a low energy diet, containing a high protein, probiotic condensed yogurt, on biochemical and anthropometric measurements among women with overweight/obesity: a randomised controlled trial. Clin Nutr ESPEN. 2020;35(xxxx):194–200. doi: 10.1016/j.clnesp.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 51.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129(25suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 53.Perna S, Ilyas Z, Giacosa A, et al. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients. 2021;13(2):1–16. doi: 10.3390/nu13020666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin HY, Lo YT, Wang TJ, et al. Normalization of glycosaminoglycan-derived disaccharides detected by tandem mass spectrometry assay for the diagnosis of mucopolysaccharidosis. Sci Rep. 2019;9(1):10755. doi: 10.1038/s41598-019-46829-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35(7):566–575. doi: 10.1016/j.nutres.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 56.Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci Nutr. 2020;60(4):584–596. doi: 10.1080/10408398.2018.1545218 [DOI] [PubMed] [Google Scholar]
- 57.Loveman E, Frampton G, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15(2). doi: 10.3310/hta15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579 [DOI] [PubMed] [Google Scholar]
- 59.Kekkonen RA, Lummela N, Karjalainen H, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008;14(13):2029. doi: 10.3748/wjg.14.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53(2):100–108. doi: 10.1016/j.micpath.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 61.Vaghef-Mehrabany E, Ranjbar F, Asghari-Jafarabadi M, Hosseinpour-Arjmand S, Ebrahimi-Mameghani M. Calorie restriction in combination with prebiotic supplementation in obese women with depression: effects on metabolic and clinical response. Nutr Neurosci. 2019;0(0):1-15. doi: 10.1080/1028415X.2019.1630985 [DOI] [PubMed] [Google Scholar]
- 62.Narmaki E, Borazjani M, Ataie-Jafari A, et al. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: a randomized clinical trial. Nutr Neurosci. 2020;0(0):1-13. doi: 10.1080/1028415X.2020.1826763 [DOI] [PubMed] [Google Scholar]
- 63.Omar JM, Chan YM, Jones ML, Prakash S, Jones PJH. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J Funct Foods. 2013;5(1):116-123. doi: 10.1016/j.jff.2012.09.001 [DOI] [Google Scholar]
- 64.Sharafedtinov KK, Plotnikova OA, Alexeeva RI, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients - A randomized double-blind placebo-controlled pilot study. Nutr J. 2013;12(1):1-11. doi: 10.1186/1475-2891-12-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zarrati M, Salehi E, Nourijelyani K, et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J Am Coll Nutr. 2014;33(6):417-425. doi: 10.1080/07315724.2013.874937 [DOI] [PubMed] [Google Scholar]
- 66.Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8(7):1-20. doi: 10.3390/nu8070397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutiérrez-Repiso C, Hernández-García C, García-Almeida JM, et al. Effect of synbiotic supplementation in a very-low-calorie ketogenic diet on weight loss achievement and gut microbiota: a randomized controlled pilot study. Mol Nutr Food Res. 2019;63(19):1-10. doi: 10.1002/mnfr.201900167 [DOI] [PubMed] [Google Scholar]
- 68.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545-553. doi: 10.1007/s10620-011-1887-4 [DOI] [PubMed] [Google Scholar]
- 69.Sanchez M, Darimont C, Drapeau V, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111(8):1507-1519. doi: 10.1017/S0007114513003875 [DOI] [PubMed] [Google Scholar]
- 70.Sergeev IN, Aljutaily T, Walton G, Huarte E. Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients. 2020;12(1). doi: 10.3390/nu12010222 [DOI] [PMC free article] [PubMed] [Google Scholar]