Abstract
Mycobacterium avium is an opportunistic pathogen that primarily infects immunocompromised individuals, although the frequency of M. avium infection is also increasing in the immunocompetent population. The antigen repertoire of M. avium varies from that of Mycobacterium tuberculosis, with the immunodominant 35-kDa protein being present in M. avium and Mycobacterium leprae but not in members of the M. tuberculosis complex. Here we show that a DNA vector encoding this M. avium 35-kDa antigen (DNA-35) induces protective immunity against virulent M. avium infection, and this protective effect persists over 14 weeks of infection. In C57BL/6 mice, DNA vaccines expressing the 35-kDa protein as a cytoplasmic or secreted protein, both induced strong T-cell gamma interferon (IFN-γ) and humoral immune responses. Furthermore, the antibody response was to conformational determinants, confirming that the vector-encoded protein had adopted the native conformation. DNA-35 immunization resulted in an increased activated/memory CD4+ T-cell response, with an accumulation of CD4+ CD44hi CD45RBlo T cells and an increase in antigen-specific IFN-γ production. The protective effect of the DNA-35 vectors against M. avium infection was comparable to that of vaccination with Mycobacterium bovis BCG and significantly greater than that for previous treated infection with M. avium. These results illustrate the importance of the 35-kDa protein in the protective response to M. avium infection and indicate that DNA vaccination successfully promotes a sustained level of protection during chronic M. avium infection.
Mycobacteria are widespread in nature and remain an important cause of infection in humans worldwide. Most often mycobacterial disease is associated with Mycobacterium tuberculosis and Mycobacterium leprae, the causative agents of tuberculosis and leprosy, respectively. There is, however, an increasing incidence of opportunistic infections caused by atypical mycobacterial species such as Mycobacterium avium, particularly in human immunodeficiency virus-infected patients (26). Until recently, M. avium complex (MAC) organisms were rarely reported to cause disease in individuals without predisposing lung disease or AIDS (5). Recent reports indicate that pulmonary MAC infections are becoming a more prevalent clinical problem in individuals without predisposing conditions (26), particularly in the older female population (6). Furthermore, studies have shown that non-AIDS-related pulmonary disease caused by MAC is as common as pulmonary tuberculosis in many areas of the United States (23).
M. avium is resistant to many antimycobacterial drugs, and the current treatment for M. avium infection requires multidrug therapy (MDT) with a combination of two to four agents (3). With the emergence of drug-resistant M. avium, alternative therapy is required in order to control infection (12). The vaccine Mycobacterium bovis Bacille Calmette-Guerin (BCG) reduces the incidence of M. avium infection in humans (27); however, BCG offers only moderate levels of protection in animal models (25). A more effective vaccine combined with MDT may contribute to the control of M. avium infections. One vaccine strategy is immunization with DNA plasmids encoding microbial genes. This approach has had successful application in respect to viral, bacterial, and protozoan infections in animal models (9, 15, 19, 32). Protection of mice against M. tuberculosis infection after DNA vaccination has been reported using the hsp65 (21, 29, 32), 85A (15), 85B (18), PstS-3 (31), and 38-kDa (39) antigens (Ags). The Ag repertoire of MAC includes some shared with the M. tuberculosis complex but also includes proteins not present in BCG. The 35-kDa protein, first identified in M. leprae (16, 38), has a homologue in M. avium with 95% amino acid identity but not in the M. tuberculosis complex (35). The 35-kDa protein is an immunodominant Ag in the human response to M. leprae (22, 30, 34) and is recognized during murine infection with M. avium (11, 35). Therefore, we have constructed DNA vectors expressing the 35-kDa protein with and without a eukaryotic leader sequence. Vaccination stimulated strong Ag-specific T-cell responses to 35-kDa protein from M. avium and antibody responses to conformational determinants of the antigen. These vaccines induced significant persistent protection against M. avium infection, which was of the same magnitude afforded by BCG vaccination.
MATERIALS AND METHODS
Bacteria.
The M. avium isolate used is a virulent strain of serotype 8 isolated from an AIDS patient and was kindly provided by C. Cheers (University of Melbourne, Victoria, Australia). It was grown in Middlebrook 7H9 broth with supplement (Difco Laboratories, Detroit, Mich.) and frozen in 1-ml ampoules at −70°C. Before use, the suspension was thawed at 37°C and sonicated for 10 s to disperse clumps. For manipulation of plasmids, Escherichia coli MC1061, grown in Luria-Bertani broth or agar (28) supplemented with ampicillin (100 μg/ml) as required, was used. For large-scale plasmid purification, the transformed bacteria were grown in Circlegrow broth (Bio 101, Vista, Calif.) supplemented with ampicillin.
Protein purification from recombinant Mycobacterium smegmatis and antibodies (Abs).
The recombinant M. avium 35-kDa protein (r35-kDa protein) was purified by monoclonal Ab (MAb) affinity chromatography as described previously (35). Murine anti-M. leprae 35-kDa protein MAbs CS-38 and ML03 were kind gifts of P. J. Brennan (Colorado State University, Fort Collins) and J. Ivanyi (Hammersmith Hospital, London, United Kingdom), respectively.
Production of DNA vaccines.
The vector, pJW4303, kindly provided by J. I. Mullins, University of Washington, Seattle, contains the cytomegalovirus early-immediate promoter with intron A upstream of the gene of interest and a bovine growth hormone polyadenylation sequence downstream. For prokaryotic manipulations, the selectable marker was the ampicillin resistance gene. The gene for the M. avium 35-kDa protein (for simplicity also referred to as 35 kDa) was amplified from plasmid pAJ9 (35). The 35-kDa-encoding gene was cloned into pJW4303 (DNA-Neg), using standard molecular biology techniques (28) and the 35-kDa-specific primers 5′ GCTAGAAGCTTATGACGTCGGCTC and 3′ CTACCGGACTCACTTGTACTCA to yield plasmid pJAM35 (DNA-35Cyt), containing the M. avium 35-kDa-encoding gene. The same gene was also cloned in frame with the tissue plasminogen activator (tPA) signal sequence of pJW4303, using the primers 5′ AATAGGCTAGCATGACGTCGGCTC and 3′ CTACCGGATCCTCACTTGTAC. This clone, pJAS35 (DNA-35Sec), permitted secretion of the mycobacterial protein from eukaryotic cells. The gene sequences were confirmed by double-stranded sequencing (Sequitherm; Epicentre Technologies, Madison, Wis.). DNA for immunization was purified by CsCl centrifugation, adjusted to 1 mg/ml in phosphate-buffered saline (PBS), and stored at −20°C until required.
COS cell transfection.
COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 2 mM glutamate (complete DMEM). The cells were transfected using DEAE-dextran as described previously (4) with DNA-35Sec, DNA-35Cyt, or DNA-Neg. The cells were harvested and lysed, and the presence of the 35-kDa protein was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with CS-38.
Immunization of animals.
C57BL/6 female mice were supplied as specific-pathogen-free mice by ARC (Perth, Australia) and were maintained in specific-pathogen-free conditions. Mice were immunized between 8 and 12 weeks of age with 50 μg of each plasmid by intramuscular injection into the tibialis anterior muscle of each hind leg. Control mice were immunized with PBS or DNA-Neg. Mice were immunized one to three times at biweekly intervals. For protein immunization, mice were injected subcutaneously at the base of the tail with 50 μg of the r35-kDa protein in incomplete Freund's adjuvant (IFA; Sigma, St. Louis, Mo.). Control groups received PBS in IFA.
Ab determination.
Mice were bled biweekly after the first immunization, and the presence of Ag-specific Abs was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (34, 35), using recombinant mycobacterial proteins (at 10 μg/ml) and alkaline phosphatase-conjugated goat anti-murine immunoglobulin G (IgG; Sigma). To determine the titer of the Ag-specific antibody, the mean absorbance plus 3 standard deviations of normal mouse sera, at a dilution of 1:100, was adopted as the cutoff absorbance. For ELISAs carried out with denatured Ag, the 35-kDa protein was heated to 95°C for 10 min.
Lymphocyte proliferation and cytokine assays.
The inguinal, axillary, and para-aortic lymph nodes and the spleen were collected from immunized mice, and single-cell suspensions were prepared in complete RPMI medium supplemented with 2 mM glutamate, 50 μM β-mercaptoethanol, and 10% FCS. Lymphocyte proliferation and cytokine assays for gamma interferon (IFN-γ) were carried out as described previously (18). Briefly, IFN-γ was detected with MAbs R46A2 and biotinylated XMG 1.2 (Endogen, Woburn, Mass.) and a recombinant murine IFN-γ standard (5.08 × 106 U/mg; Genzyme, Cambridge, Mass.). The limit of detection was 0.4 U/ml (1 U is equivalent to 197 pg/ml).
Mycobacterial challenge.
Six weeks after the last boost with either DNA-35Cyt or DNA-35Sec, mice were infected by an intravenous (i.v.) challenge with 106 CFU of M. avium. Mice were sacrificed at 2, 4, 8, and 14 weeks after the infection, and bacteria in the spleen and liver homogenates were enumerated on Middlebrook 7H11 Bacto Agar. Mice were vaccinated with 5 × 104 CFU of BCG (CSL) i.v. or 105 CFU of M. avium (MAC primed) i.v. and 6 weeks later were treated with isoniazid (25 mg/kg) for 12 weeks. The presence of mycobacteria in organs was examined at the time of challenge and presented as mean CFU ± standard error of the mean (SEM).
Flow cytometry.
To identify leukocyte populations, cell surface molecules were labeled with Abs and analyzed by flow cytometry as described previously (7). The following MAbs were used for flow cytometry: anti-CD44-fluorescein isothiocyanate (FITC), anti-IFN-γ-FITC, anti-CD45RB-phycoerythrin (PE), anti-B220-PE, and anti-MAC1-FITC (Pharmingen, San Diego, Calif.). Anti-CD4-Tricolor, anti-CD8-Tricolour, and isotype control Abs were purchased from Caltag (San Francisco, Calif.).
Intracellular cytokine staining.
Cells were cultured at 37°C and 5% CO2 for 6 h in the presence of phorbol myristate acetate-iodomycin (PMA/Io; 50 ng/ml). Brefeldin A (10 μg/ml) was then added for a further 16 h. Cells were washed and stained with rat anti-mouse CD4 MAb (Caltag). Intracellular staining was carried out as described previously (8).
ELISPOT assay for cytokine-producing cells.
IFN-γ-secreting cells were quantified as described previously (18). Splenic mononuclear cells from immunized and M. avium-infected mice were purified by centrifugation on Histopaque-1083 (ρ = 1.083; Sigma). The cells were added to 96-well plates (4 × 105/well) and incubated with 35 kDa (10 μg/ml), M. avium sonicate (10 μg/ml), PMA/Io (50 ng/ml), or medium alone. The plates were incubated for 48 h at 37°C in an atmosphere of 5% CO2. The cells were then collected, washed, and counted, and the enzyme-linked spot (ELISPOT) assay was conducted as described previously, using MAb R46A2 (Endogen) for capture and XMG 1.2 (Endogen) for recognition of IFN-γ-secreting cells (18).
RESULTS
DNA vaccines expressed the 35-kDa protein.
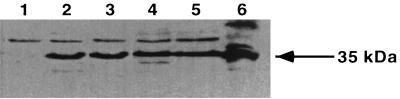
To ensure that the plasmid DNA vaccine constructs were functional, we sequenced the plasmids and analyzed expression in vitro by transient transfection of COS-7 cells and Western blotting (Fig. 1). Transfection with DNA-35Cyt and DNA-35Sec resulted in similar levels of expression of the 35-kDa protein. Ag-specific Abs were detected 2 weeks after the first immunization of C57BL/6 mice with DNA-35 vectors (data not shown). Increasing titers of specific IgG were generated over the course of immunization with either DNA-35Cyt or DNA-35Sec, resulting in log10 titers of 4.43 ± 0.1 and 4.27 ± 0.15, respectively, at 6 weeks. To determine whether the antibody responses recognised conformational determinants on the 35-kDa antigen, ELISAs were conducted with denatured and nondenatured 35-kDa antigen and MAbs recognizing both linear and conformational determinants on the native 35-kDa protein. MAb CS-38, generated to the purified native 35-kDa protein (14), recognizes a linear determinant whereas MAb ML04 binds only the native 35-kDa protein in its nondenatured state (17). As shown in Table 1, sera from DNA-35 immunized mice bound to the native protein but not to denatured 35-kDa antigen. ML04 also failed to bind to the denatured antigen, while MAb CS-38 reacted with both denatured and native protein.
FIG. 1.
Expression of the 35-kDa protein by DNA-35-transfected COS-7 cells. The COS-7 cells were transfected with DNA-35Sec or DNA-35Cyt expressing the M. avium 35-kDa protein with and without a secretory signal sequence. Whole cell lysates were analyzed by SDS-PAGE and immunoblotting with anti-35 kDa MAb CS-38. Lane 1, DNA-Neg; lanes 2 and 3, DNA-35Cyt; lanes 4 and 5, DNA-35Sec; lane 6, 1 μg of r35-kDa protein.
TABLE 1.
Immune responses to 35-kDa protein following immunization with DNA vaccines or recombinant protein
| Immunizationa | Mean ± SEMb
|
Ab level (mean A405 ± SD)e
|
||
|---|---|---|---|---|
| Δcpmc | IFN-γ (U/ml)d | Denatured 35 kDa | Nondenatured 35 kDa | |
| DNA-35Cyt | 29,528 ± 1,307 | 44.25 ± 5.760 | 0.0850 ± 0.001 | 0.732 ± 0.05 |
| DNA-35Sec | 25,883 ± 250 | 45.31 ± 4.127 | 0.0795 ± 0.005 | 0.963 ± 0.03 |
| DNA-Neg | 2,120 ± 219 | 9.544 ± 1.623 | 0.0750 ± 0.001 | 0.136 ± 0.02 |
| r35 kDa + IFA | 25,908 ± 122 | 32.69 ± 4.772 | 0.0624 ± 0.012 | 0.824 ± 0.11 |
| PBS + IFA | 2,476 ± 226 | 8.254 ± 1.247 | 0.0542 ± 0.004 | 0.112 ± 0.14 |
| CS-38f | NDg | ND | 0.7560 ± 0.010 | 0.875 ± 0.03 |
| ML04h | ND | ND | 0.0720 ± 0.004 | 0.963 ± 0.05 |
Mice were immunized by injection intramuscularly three times with 100 μg of DNA-35Cyt, DNA-35Sec, or DNA-Neg or subcutaneously once with 50 μg of r35 kDa or PBS in IFA.
T-cell proliferative and IFN-γ responses to the 35-kDa Ag (10 μg/ml), measured 2 weeks after the last immunization.
Mean specific [3H]thymidine incorporation for each group of five mice.
Measured by ELISA and represented as the mean for five mice.
Measured at a serum dilution of 1:100.
A MAb that recognizes a linear determinant on the 35-kDa Ag (14).
ND, not determined.
A MAb that recognizes a conformational determinant on the 35-kDa Ag (17).
DNA vaccines generated T-helper response and cytokine production.
Comparable levels of specific proliferation of splenocytes (Table 1) and lymph node cells (data not shown) were observed in mice immunized with DNA-35Cyt and DNA-35Sec or with 35-kDa protein, but not with PBS or the control vector, DNA-Neg. Mice immunized with either DNA-35Cyt, DNA-35Sec, or r35-kDa protein produced high levels of IFN-γ (Table 1). Therefore, the insertion of the tPA secretory signal sequence before the 35-kDa encoding gene did not increase antibody or T-cell responses. Mice immunized with suboptimal doses of the DNA-35 vaccines (one or two doses) also generated strong antigen-specific immune responses; however, at all times tested the responses did not exceed the immune response generated with three immunizations (data not shown).
Increase in the number of IFN-γ-producing CD4+ T cells following DNA-35 vaccination.
To examine the contribution of CD4+ T cells to IFN-γ production, spleen cells from DNA-35-immunized mice and DNA-Neg- or BCG-immunized mice were restimulated with PMA/Io for 16 h and stained for intracytoplasmic IFN-γ production. Following DNA vaccination, the proportions of CD4+ IFN-γ-producing cells were significantly greater in DNA-35 immunized mice (5.5% ± 0.86%) than in recipients of control DNA (2.25% ± 1.24%; P < 0.05). The response in BCG recipients was 4.3% ± 1.0%.
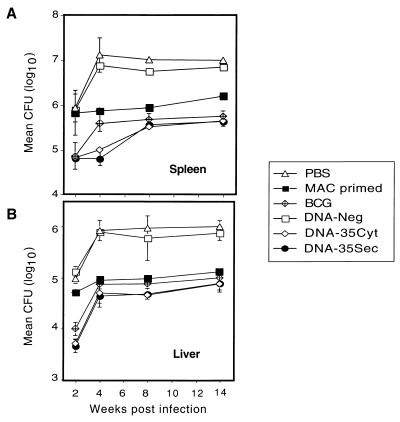
Protection against M. avium infection by DNA vaccination.
Immunization with DNA-35Cyt or DNA-35Sec achieved a persistent and highly significant level of protection against M. avium infection in both the spleen and liver (P < 0.01 at 4 and 14 weeks in the spleen) (Fig. 2). Interestingly at week 4, DNA-35 immunized mice had significantly higher protection in the spleen than BCG-immunized mice (P < 0.05; Fig. 2), but the effect was equivalent at 8 to 14 weeks. Mice infected with M. avium, treated, and rechallenged (MAC primed) also had significant protection (P < 0.01 at 4 and 14 weeks in the spleen) compared to DNA-Neg-immunized mice (Fig. 2). In the spleen, DNA-35-immunized mice demonstrated increased protection compared to MAC-primed mice at both weeks 4 and 14 (P < 0.01; Fig. 2A). Immunization schedules with only one or two doses of DNA-35 also resulted in significant protection against M. avium infection; however, three immunizations were always more effective (data not shown). The protective effect of DNA-35 was compared with that induced by r35-kDa Ag in IFA. Recombinant protein alone led to reductions in CFU by 0.5 log10 at 4 and 8 weeks, both of which were significantly less (P < 0.01) than the protection afforded by DNA-35.
FIG. 2.
DNA-35 protects against virulent M. avium infection. Mice (n = 5) were immunized with DNA-35Cyt, DNA-35Sec or DNA-Neg, three times at biweekly intervals. Six weeks after the last injection, mice were infected i.v. with 106 M. avium serovar 8. Other mice received BCG (5 × 104 CFU of live BCG i.v.) or M. avium (MAC primed; 105 CFU i.v.) followed by antibiotic treatment or were not immunized. Results represent the mean CFU ± SEM of five mice per group tested separately in the spleen (A) and liver (B). The differences between the following groups of mice were significant by Mann-Whitney test: DNA-35 and DNA-Neg in the spleen and liver at all time points tested (P < 0.01); DNA-35 and BCG in the spleen at week 4 (P < 0.05); MAC primed and DNA-Neg in the spleen and liver from week 4 onward (P < 0.01); and DNA-35 and MAC primed in the spleen (P < 0.01). These data are representative of two similar experiments.
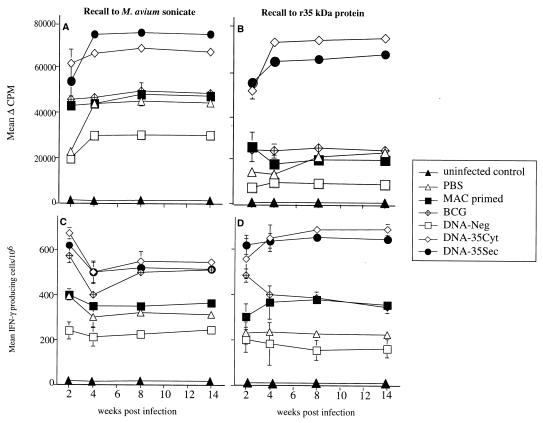
Enhanced Ag-specific T-cell activation by DNA-35 immunization during M. avium infection.
Immunization with either DNA-35Cyt or DNA-35Sec stimulated an Ag-specific proliferative response of T cells from the spleen throughout the course of M. avium infection (Fig. 3A). This proliferative response to M. avium was greater than that in mice given either BCG or M. avium (MAC primed). In vitro stimulation with r35-kDa protein resulted in the production of an Ag-specific proliferative response, with mice immunized with DNA-35 producing a response threefold higher than that of BCG or M. avium (MAC primed)-immunized mice (Fig. 3B). Therefore DNA-35 immunization primes for a greater and more rapid Ag-specific proliferative response, which is retained throughout the course of the infection.
FIG. 3.
Immunization with DNA vaccines results in significantly higher Ag-specific T-cell proliferative responses and higher frequency of Ag-specific IFN-γ-secreting cells. Splenocytes from mice immunized as for Fig. 2 were restimulated with M. avium sonicate (10 μg/ml) (A and C) or r35-kDa antigen (10 μg/ml) (B and D) for 48 h. The mean level of specific [3H]thymidine incorporation ± SEM was determined for five mice per group, tested separately. The differences between the following groups were significant by Mann-Whitney test: DNA-35 and either BCG, MAC primed, or DNA-Neg from 4 weeks onward, where P < 0.01 (B). The numbers of splenocytes secreting IFN-γ in response to either M. avium sonicate (10 μg/ml) (C) or 35-kDa Ag (10 μg/ml) (D) were analyzed by ELISPOT assay and represented as the mean ± SEM of five mice per group, tested separately. The differences between DNA-35- and BCG-immunized groups were significant at P < 0.01, determined by the Mann-Whitney test (D). The data are from one of two separate experiments.
IFN-γ-producing cells following DNA vaccination and M. avium infection.
Following in vitro stimulation of splenic lymphocytes with M. avium sonicate, IFN-γ-secreting cells were maximum at 2 weeks after infection in DNA-35- immunized mice (Fig. 3C). The combination of DNA immunization and M. avium infection resulted in over a threefold increase in cytokine-producing cells throughout the time course, compared to the number emerging in mice immunized with the control vector. In vitro stimulation of splenic lymphocytes with r35-kDa Ag showed a similar pattern (Fig. 3D). There were significantly higher levels of IFN-γ-secreting splenocytes in DNA-35-immunized mice than in BCG (P < 0.01)-, M. avium-, and control vector-immunized mice from 4 weeks onward. Therefore, DNA immunization primed for a higher frequency of Ag-specific IFN-γ-secreting cells throughout the course of M. avium infection.
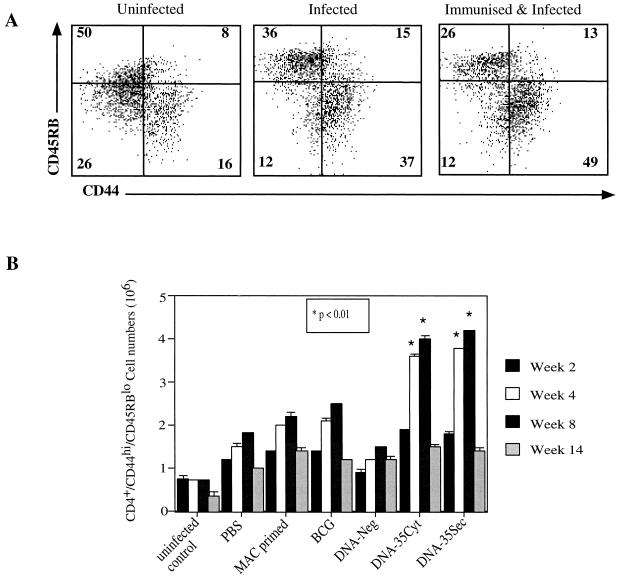
Protective cellular response elicited by DNA-35 vaccination.
Mice immunized with BCG, DNA-35Cyt, or DNA-35Sec showed significant increases in total spleen cell, CD4 and CD8 T-cell, and B-cell numbers compared to DNA-Neg-immunized or nonimmunized mice (data not shown). CD4+ T cells with dual expression of CD44hi and CD45RBlo markers were defined as activated cells and were observed in uninfected, M. avium-infected, and DNA-35-immunized, M. avium-infected mice (Fig. 4A). There was a significant difference in both percentage and absolute number of activated T cells in the spleen between DNA-35-vaccinated and BCG-immunized mice at 4 to 8 weeks postinfection (P < 0.01) (Fig. 4B). The maximum number of organisms (CFU) in the spleen was observed at week 4 (Fig. 2A), whereas the number of spleen activated T cells did not peak until week 8 and then decreased by week 12 (Fig. 4).
FIG. 4.
The expression of CD44 and CD45RB on CD4+ T cells from the spleens of uninfected, M. avium-infected, or DNA-35-immunized, and M. avium-infected mice were analyzed by flow cytometry. The profiles are representative of five mice from one of two experiments. Numbers in quadrants are percentages of CD4+ T cells (A). The number of CD44hi CD45RBlo CD4+ T cells in the spleens from mice in the seven groups shown were analyzed at 2, 4, 8, and 14 weeks. The data are the mean ± SEM from five mice per group from one of two experiments. The differences between DNA-35- and BCG-immunized groups were determined by the Mann-Whitney test where P < 0.01 at weeks 4 and 8, as indicated.
DISCUSSION
DNA vaccination has emerged as an effective strategy for expression of foreign antigens in vivo leading to immunization against viruses and protozoa (reviewed in reference 33). Since mycobacterial genes have a higher GC content than eukaryotic and certain other prokaryotic genes, and posttranslational modifications of genes may vary between mycobacterial and eukaryotic cells (36), expression of mycobacterial genes by DNA vaccination may not be optimal. Expression of the 35-kDa-encoding DNA constructs in COS-7 cells in vitro indicated that the constructs led to expression of the 35-kDa-encoding gene in eukaryotic cells (Fig. 1). The native 35-kDa protein forms multimers expressing conformational determinants which stimulate strong IgG antibody responses (35). Mice immunized with either of the two DNA constructs mounted a strong IgG antibody response to conformational determinants on the protein, confirming that the protein assembles into multimers in eukaryotic cells (Table 1).
Strong Ag-specific Th1 responses, characterized by IFN-γ secretion, are crucial for protection against mycobacterial infection (10). Immunization with the DNA-35 vaccines induced strong IFN-γ responses (Table 1), with levels of T-cell proliferation and IFN-γ release greater than those observed with DNA vaccines expressing secreted proteins of M. tuberculosis (18). Increased IFN-γ release was associated with an increased frequency of IFN-γ-secreting CD4+ T cells (data not shown). Interestingly, there was no difference observed between the construct designed to express the 35-kDa protein in the secreted or nonsecreted form. The presence of a secretory signal sequence did not influence the response to genes encoding either Ag85A or Ag85B of M. tuberculosis (18, 36). In a separate study, DNA vaccines expressing tuberculosis proteins fused to tPA signal sequences elicited similar IFN-γ responses, but slightly greater protective activity to those without tPA signal sequences. This difference was thought to be due to elevated concentrations of tPA fusion proteins relative to native Ags in transfected cells (20).
Both DNA-35Cyt and DNA-35Sec induced a highly significant and sustained reduction in mycobacterial load in the spleen and liver following challenge with virulent M. avium (Fig. 2). At 4 weeks there was a 2-log10 reduction in M. avium in the spleen compared to mice immunized with control vector (Fig. 2A), with sustained protection out to 14 weeks. These levels of protection were greater than the four- to eightfold reductions in M. avium infection induced with DNA vaccines expressing M. tuberculosis HSP65 or Ag85A or Ag85B (37) or the 0.4- to 0.8-log10 levels of protection conferred by DNA vaccines against M. tuberculosis (15, 18). This may relate to the nature of the 35-kDa protein or the virulence of the M. avium strain. Interestingly, the DNA-35 vectors were significantly more effective than BCG at 4 weeks and thereafter as effective as BCG up to 14 weeks (Fig. 2). The protective efficacy of BCG in established M. avium murine model is controversial, with levels of protection ranging from nil to modest (0.9 log10) protection (1, 13, 37). The level of protection afforded by BCG varies according to the strain of M. avium used, as well as the dose and route of infection. Interestingly, mice with specific memory responses to M. avium following antibiotic treatment for M. avium infection were less effectively protected at early stages of infection than mice immunized with DNA-35 or BCG (Fig. 2).
The specific protection conferred by the DNA-35 vectors was accompanied by enhanced T-cell proliferation and IFN-γ secretion which was sustained for at least 14 weeks of chronic mycobacterial infection. The recall responses to the 35-kDa protein were significantly higher in DNA-35-immunized mice than in mice immunized with BCG or primed with MAC (Fig. 3B and D). This enhanced effect was also observed when M. avium sonicate was used as the recall antigen (Fig. 3A and C), consistent with the 35-kDa protein being a dominant Ag among those present in crude M. avium sonicate. Despite the high Ag load during the infection in control infected mice which had received empty DNA vector or PBS, their Ag-specific IFN-γ responses never reached those in DNA-35-immunized mice, indicating that DNA immunization primes for sustained enhancement of Th1 responses.
CD4+ T cells are considered to be the major T-cell subset responsible for immunity against M. avium (2, 13), and in M. tuberculosis infection the CD4+ T cells associated with protection have further been delineated as CD44hi CD45RBlo T cells (8, 24). DNA-35 immunization resulted in a significant increase in this memory phenotype (Fig. 4). Interestingly, the maximum number of activated/memory T cells is at weeks 4 and 8 in DNA vaccinated mice, although protection is evident at week 2 and is still maintained at week 14 (Fig. 2) when the number of activated/memory T cells has fallen (Fig. 4B). There are several possible explanations for this observation. First, there may be an early release of IFN-γ from memory cells prior to expansion. It should be noted that at 2 weeks the number of activated/memory CD4+ T cells is already higher than in control DNA-immunized mice (Fig. 4B). Then later in the course of the infection, at 14 weeks, the pattern of the immune response established by vaccination may maintain the bacterial load at a lower level. This effect may operate through a more effective local granulomatous response. This prolonged effect of DNA vaccination may be important in clinical applications, as it may limit the immunopathology even though sterilizing immunity is not achieved.
In summary, DNA vaccination using the M. avium 35-kDa protein was successful at generating an activated/memory immune response which resulted in sustained protection during chronic M. avium infection. In a separate study, DNA vaccines expressing M. leprae 35-kDa protein induced protective immunity against M. leprae infection in the mouse footpad model comparable to BCG immunization (E. Martin, unpublished data). Therefore, DNA vaccination has the potential to improve on the current BCG vaccine against mycobacterial infections and, if used in conjunction with effective MDT, may contribute to the control of these diseases.
ACKNOWLEDGMENTS
This study was supported financially by the National Health and Medical Research Council of Australia (NHMRC) and the CRC for Vaccine Technology (CRC-VT). E. Martin and A. Kamath are recipients of Australian Postgraduate Research Awards, and E. Martin received a CRC-VT scholarship. James Triccas is a recipient of an NHMRC Peter Doherty Fellowship.
We thank A. Bean and Carl Feng for helpful discussions.
REFERENCES
- 1.Appelberg R. Protective role of interferon gamma, tumour necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis infection. J Immunobiol. 1994;191:520–526. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R, Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992;87:379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson C A. Treatment of disseminated disease due to the Mycobacterium avium complex in patients with AIDS. Clin Infect Dis. 1994;18:S273–S242. doi: 10.1093/clinids/18.supplement_3.s237. [DOI] [PubMed] [Google Scholar]
- 4.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. 1995. Coordinating ed., R. Coico. Wiley, London, England. [Google Scholar]
- 5.Collins F M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989;2:360–367. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, Appelberg R, Orme I M. Immunopathogenesis of Mycobacterium avium infection. Front Biosci. 1998;3:141–148. doi: 10.2741/a287. [DOI] [PubMed] [Google Scholar]
- 7.Demangel C, Bean A G D, Martin E, Feng C G, Kamath A T, Britton W J. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guerin-infected dendritic cells. Eur J Immunol. 1999;29:1972–1979. doi: 10.1002/(SICI)1521-4141(199906)29:06<1972::AID-IMMU1972>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Feng C G, Bean A G D, Hooi H, Briscoe H, Britton W J. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67:3242–3247. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine P E M, Rodrigues L C. Modern vaccines: mycobacterial diseases. Lancet. 1991;335:1016–1020. doi: 10.1016/0140-6736(90)91074-k. [DOI] [PubMed] [Google Scholar]
- 10.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelber R H, Brennan P J, Hunter S W, Munn M W, Monson J M, Murray L P, Siu P, Tsang M, Engleman E G, Mohagheghpour N. Effective vaccination of mice against leprosy bacilli subunit of Mycobacterium leprae. Infect Immun. 1990;58:711–718. doi: 10.1128/iai.58.3.711-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsburgh C R J, Havlik J A, Ellis D A, Kennedy E, Fann S A, DuBois R E, Thompson S E. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991;144:557–559. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard R D, Flory C M, Collins F M. T-cell immune responses in Mycobacterium avium-infected mice. Infect Immun. 1992;60:150–153. doi: 10.1128/iai.60.1.150-153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter S W, Rivoire B, Mehra V, Bloom B R, Brennan P J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990;265:14065–14068. [PubMed] [Google Scholar]
- 15.Huygen K, Content J, Olivier D, Montgomery D L, Yawman A M, Deck R R, De Witt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 16.Ivanyi J, Morris J A, Keen M. Studies with monoclonal antibodies to mycobacteria. In: Macario J L, de Macario E C, editors. Monoclonal antibodies to bacteria. London, England: Academic Press; 1985. p. 51. [Google Scholar]
- 17.Ivanyi J, Sinha S, Aston R, Cussel D, Kenn M, Sengupta U. Definition of species-specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983;52:528–536. [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai W C, Bennet M, Johnston S A, Barry M A, Pakes S P. Protection against Mycoplasma pulmonis infection by genetic vaccination. Cell Biol. 1995;14:643–648. doi: 10.1089/dna.1995.14.643. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowrie D B, Tascon R E, Bonato V L D, Lima V M F, Facciolo L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 22.Mohagheghpour N, Munn M W, Gelber R H, Engleman E G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect Immun. 1990;58:703–710. doi: 10.1128/iai.58.3.703-710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris S L, Bermudez L, Chaparas S D. Mycobacterium avium complex disease in patients with AIDS: seroreactivity to native and recombinant mycobacterial antigens. J Clin Microbiol. 1991;29:2715–2719. doi: 10.1128/jcm.29.12.2715-2719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 25.Orme I M, Collins F M. Prophylactic effect in mice of BCG vaccination against non-tuberculosis mycobacterial infections in mice. Tubercle. 1985;6:117–120. doi: 10.1016/0041-3879(85)90076-5. [DOI] [PubMed] [Google Scholar]
- 26.Prince D S, Peterson D D, Steiner R M, Gottlieb J E, Scott R, Israel H L, Figeroa W G, Fish J E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 27.Romanus V. Childhood tuberculosis in Sweden. Tubercle. 1983;64:101–110. doi: 10.1016/0041-3879(83)90034-x. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Silva C L, Pietro R L R, Januario A, Bonato V L D, Lima V M F, Silva M F, Lowrie D B. Protection against tuberculosis by bone marrow cells expressing mycobacterial hsp65. Immunology. 1995;86:519–524. [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Sengupta U, Ramu G, Ivanyi J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr. 1985;53:33–38. [PubMed] [Google Scholar]
- 31.Tanghe A, Lefevre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 32.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 33.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–96. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 34.Triccas J A, Roche P W, Winter N, Feng C G, Butlin C R, Britton W J. A 35-kDa protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–5177. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triccas J A, Winter N, Roche P W, Gilpin A, Kendrick K E, Britton W J. Molecular and immunological analysis of the Mycobacterium avium homologue of the immunodominant Mycobacterium leprae 35-kDa protein. Infect Immun. 1998;66:2684–2690. doi: 10.1128/iai.66.6.2684-2690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulmer J B, Liu M A, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Content J, Huygen K. Expression and immunogenicity of Mycobacterium tuberculosis antigen 85 by DNA vaccination. Vaccine. 1997;15:792–794. doi: 10.1016/s0264-410x(96)00255-1. [DOI] [PubMed] [Google Scholar]
- 37.Velaz-Faircloth M, Cobb A J, Horstman A L, Henry S C, Frothingham R. Protection against Mycobacterium avium by DNA vaccines expressing mycobacterial antigens as fusion proteins with green fluorescent protein. Infect Immun. 1999;67:4243–4250. doi: 10.1128/iai.67.8.4243-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter N, Triccas J A, Rivoire B, Pessolani M C, Eiglmeier K, Lim E M, Hunter S W, Brennan P J, Britton W J. Characterisation of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol Microbiol. 1995;16:865–876. doi: 10.1111/j.1365-2958.1995.tb02314.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]