Abstract
Introduction
The onset of the COVID-19 pandemic and the first national lockdown implemented might have disrupted the epidemiology of deep venous thrombosis (DVT) and pulmonary embolism (PE). This study aimed to analyze time trends in patients hospitalized for DVT and PE in France and related in-patient and 90-day post-admission mortality rates.
Materials and methods
All patients hospitalized in France for DVT or PE between January and September (weeks 1–40) for each year from 2017 to 2020, were selected. Weekly incidence rate ratios (IRR) were computed to compare the rates of patients hospitalized in 2020 with those hospitalized in 2017–2019.
Results
Compared with the 2017–2019 study period, the rates of patients hospitalized with a primary diagnosis (PD) of DVT or PE in 2020 were significantly (50 and 40%, respectively) lower during weeks 12–13. The rate of patients hospitalized with an associated diagnosis (AD) of PE during weeks 12–19 of 2020 was twice as high as in the same period in 2017–2019. The prevalence of COVID-19 in patients hospitalized with a PD of DVT and PE, and in those hospitalized with an AD of DVT and PE reached respectively 4.0, 9.6, 17.2 and 44.6 during the country's first lockdown. Inpatients case-fatality rates in patients hospitalized with an AD of PE increased significantly during weeks 12–13.
Conclusions
Epidemiology of VT and PE was seriously impacted by the COVID-19 pandemic in 2020 in France, with a significant decrease in the rate of patients hospitalized for PE and a threefold increase in the related in-patient mortality rate. This highlight the need to inform the general population about the symptoms of PE and about the need to immediately seek medical care, particularly those infected with SARS-CoV-2.
Keywords: Deep venous thrombosis, Pulmonary embolism, COVID-19, Epidemiology, Mortality
1. Introduction
The epidemiology of deep venous thrombosis (DVT) and pulmonary embolism (PE) may have been strongly impacted not only by its direct association with SARS-CoV-2 infection [1], [2], [3], [4], but also by the obstacles to primary and hospital care brought about by the COVID-19 pandemic [5], [6], [7], [8]. More specifically, the global context of the pandemic has led to a change in the medical management for both inpatient and outpatient DVT and PE cases, leading to longer delays before access to healthcare structures [9], [10], [11], [12].
The first lockdown in France between 17 March and 11 May 2020 disrupted DVT and PE healthcare management systems [13]. This lockdown was very restrictive, as the entire population had to stay at home and could only move within 1 km of their residence. Furthermore, as in other countries, a global decrease in the number of all-cause hospitalizations was observed, the main hypothesis for this being that people were afraid of getting infected with SARS-CoV-2, and accordingly, delayed seeking medical care [14].
A higher risk of DVT and PE was found in patients infected with SARS-CoV-2 in 2020 [1], [2], [3], [4]. Several mechanisms might explain this, including severe viral pulmonary infection, inflammatory syndrome added to the usual risk factors of DVT, and increased levels of D-dimers, suggesting substantial coagulation activation and a hypercoagulable state [15]. Although many international studies have analyzed DVT and PE incidence and prevalence in COVID-19 patients [1], [2], [4], to date none has compared the time trends in the rates of hospitalized DVT and PE patients during the COVID-19 pandemic - and specifically during a lockdown period - with the rates from previous years.
This study aimed to analyze the impact of the COVID-19 pandemic on the epidemiology of hospitalizations for DVT and PE in France - with a special focus on the first lockdown period - in terms of the rates of patients hospitalized in 2020 versus 2017–19, their characteristics, care management, and related in-patient and 90-day (i.e., from hospital admission) case-fatality rates. A secondary objective aimed at describe the prevalence of COVID-19 among patients hospitalized with DVT and PE, respectively.
2. Methods
2.1. Data source
For this retrospective population-based cross-sectional study, all patients hospitalized with DVT or PE in France between January and October (i.e., weeks 1 to 40) each year from 2017 to 2020, were selected using the French national hospital discharge databases (Programme de médicalisation des systèmes d'information – PMSI). The PMSI exhaustively record all hospital stays in both the private and public healthcare sectors in France, and is part of the larger French national healthcare database (Systéme National des données de Santé-SNDS) which collects data on reimbursements for healthcare expenditures (drug treatments, medical procedures, specific care for long-term diseases, etc.) for the whole French population (approximatively 67 million people) [16]. The healthcare system in France guarantees partial or full reimbursement for all medical care.
The SNDS records disease codes for diagnosed cases using the international classification of diseases 10th revision (ICD-10). For the present study, DVT was defined as having any of the following codes: I80-I82 (except I80.0), O22.3, O87.1, whether reported as a primary (PD) or associated (AD) diagnosis. For PE, the codes were I26 or O88.2, again irrespective of PD or AD. For both illnesses, we analyzed patients with PD and AD separately. A PD of DVT or PE meant that the respective disease was the reason for the hospitalization or admission to a medical department. An AD meant that DVT or PE was a comorbidity associated with a PD of an illness considered more severe than DVT or PE - whether or not this illness was COVID-19 - and which required specific acute care.
For all the patients in the study, we also searched the SNDS database for a diagnosis of COVID-19, either during the index hospitalization for DVT/PE, or during a previous hospitalization for a non-DVT/PE condition occurring after 1 January 2020. Hospital diagnoses of COVID-19 cases were based on positive biological tests (polymerization chain reaction, antigen or serology tests) or on CT-scans showing pulmonary lesions specific to SARS-CoV-2 virus infection. COVID-19 hospitalization incidence started to increase exponentially in February 2020 in France [17]. The first wave of the epidemic peaked during week 13. Thereafter, incidence decreased and remained low until September 2020 (week 27) when it started to increase again, to later become the second wave [18].
2.2. Data collection
The following patient characteristics were collected from the databases: age, sex, medical history of venous thromboembolism (i.e., DVT or PE), stroke, coronary heart disease, heart failure, arrhythmia, valvulophathy, and any circulatory disease recorded in the five years before the index hospitalization for DVT/PE. Data were also collected on healthcare system reimbursements for hypertension, diabetes and hypercholesterolemia medication payments, as well as for other medications (antiplatelets, oral anticoagulants) with at least three deliveries in the 12 months preceding the DVT/PE index hospitalization. Using these data, cardiovascular and non-cardiovascular comorbidities were grouped according to the Charlson index score [19]. Three groups were defined according to the number of comorbidities as follows: 0–1 comorbidity group, 2–3 comorbidities group, and 4 or more comorbidities group. The inpatient case-fatality rate was recorded, as was the 90-day post-admission case-fatality rate, for DVT/PE patients whose mortality data could be correctly matched with hospital data (i.e. 99% of patients), and who were members of France's general health insurance scheme. Seventy-seven percent of our population's study were covered by this scheme, which is consistent with its overall percentage of the French population [16]. The evaluation of the 90-day case-fatality rate was restricted to these patients, as this rate is not recorded exhaustively by the other insurance schemes which cover the remaining 23% of France's population.
2.3. Statistical analysis
We described characteristics of patients hospitalized for DVT and PE for 2020 and the 2017–2019 period. Comparisons between both time periods for each of the two diseases were performed using the Chi2 test for categorical variables, and the Wilcoxon-Mann-Whitney test for quantitative variables. Means and standard deviations (SD) were calculated for each quantitative characteristic. Weekly incidence rate ratios (IRR) based on a Poisson distribution were computed to model the rates of patients hospitalized for DVT and PE in 2020 compared with 2017–2019. Weekly odds ratios (OR) were used to assess changes in patients' characteristics or case-fatality rates between 2017 and 2019 and 2020. Weekly OR for inpatient and 90-day post-admission case-fatality rates were only computed for patients hospitalized with a PD or AD of PE, as PE - unlike DVT - is directly involved in the process of death. All weekly indicators were based on the week of the index hospitalization for both diseases, and were adjusted for potential time trends between 2017 and 2020. Sensitivity analyses were conducted after excluding patients who were diagnosed with COVID-19 either during the index hospitalization for DVT/PE, or during a prior hospitalization after 1 January 2020 for a different condition (whether COVID-19 or not). Analyses were performed using the SAS Enterprise Guide 7.1 software tool.
2.4. Ethics approval
As per French governmental regulations and the National Ethics Committee's rules of practice, no patient consent was required for the present study. The databases used in the study contained pseudonymized patient information.
2.5. Data availability
Full access to the SNDS is granted to the National Agency for Public Health (Santé Publique France) by governmental decree (regulatory decision DE-2011-078). The data underlying this article cannot be shared publicly.
2.6. Permission information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
3. Results
Between week 1 (January) and week 40 (October) of 2020, a total of 121,225 individuals were hospitalized for DVT or PE. Of these, 9574 and 35,446 had a PD of DVT and PE, respectively, while 52,446 and 23,759 had an AD of DVT and PE, respectively (Table 1 ). The mean total number of patients hospitalized for both diseases during the same period in 2017–2019 was 113,106, demonstrating a combined overall increase of 7.2% in 2020 (+8119 cases).
Table 1.
Number of patients hospitalized for diagnosed VT and PE during the study time-period of each year from 2017 to 2020, and total proportion of hospital-based COVID-19 diagnoses in 2020.
| N |
% diagnosed with COVID-19 while in hospitala |
||||||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | During a prior hospitalization | During the index VT/PE hospitalization only | Overall | |
| Deep venous thrombosis | |||||||
| Primary diagnosis | |||||||
| W1-W40 (January–October) | 12,789 | 11,952 | 11,294 | 9574 | 0.9 | 0.6 | 1.5 |
| W1-W11 (pre-lockdown) | 3940 | 3614 | 3333 | 3167 | 0.1 | 0.2 | 0.3 |
| W12-W19 (lockdown) | 2553 | 2443 | 2421 | 1474 | 1.2 | 2.8 | 4.0 |
| W20-W40 (post-lockdown) | 6296 | 5895 | 5540 | 4933 | 1.2 | 0.3 | 1.5 |
| Associated diagnosis, % of all VT diagnoses | 78.6% | 80.3% | 82.2% | 84.6% | |||
| W1-W40 (January–October) | 46,963 | 48,975 | 52,311 | 52,446 | 1.0 | 3.6 | 4.6 |
| W1-W11 (pre-lockdown) | 14,879 | 14,949 | 15,818 | 16,694 | 0.1 | 0.7 | 0.8 |
| W12-W19 (lockdown) | 9216 | 9434 | 10,470 | 9501 | 1.9 | 15.3 | 17.2 |
| W20-W40 (post-lockdown) | 22,868 | 24,592 | 26,023 | 26,251 | 1.3 | 1.0 | 2.3 |
| Pulmonary embolism | |||||||
| Primary diagnosis | |||||||
| W1-W40 (January–October) | 32,439 | 33,016 | 33,158 | 35,446 | 1.0 | 2.7 | 3.7 |
| W1-W11 (pre-lockdown) | 10,285 | 10,618 | 10,197 | 9810 | 0.0 | 0.4 | 0.4 |
| W12-W19 (lockdown) | 6059 | 6520 | 6691 | 6855 | 1.8 | 7.8 | 9.6 |
| W20-W40 (post-lockdown) | 16,095 | 15,878 | 16,270 | 18,781 | 1.2 | 1.9 | 3.1 |
| Associated diagnosis, % of all PE diagnoses | 16.7% | 16.7% | 16.4% | 19.6% | |||
| W1-W40 (January–October) | 18,541 | 18,895 | 18,985 | 23,759 | 1.7 | 14.7 | 16.4 |
| W1-W11 (pre-lockdown) | 6194 | 6356 | 6131 | 6279 | 0.2 | 2.4 | 2.6 |
| W12-W19 (lockdown) | 3461 | 3654 | 3629 | 6453 | 3.4 | 41.2 | 44.6 |
| W20-W40 (post-lockdown) | 8886 | 8885 | 9225 | 11,027 | 1.6 | 5.9 | 7.5 |
DVT: Deep venous thrombosis; PE: Pulmonary embolism; W: week.
Diagnosis based on hospital biological test (PCR, antigen or serology tests) or CT scan.
3.1. Time-trends and profile of patients hospitalized with DVT and PE as primary diagnosis
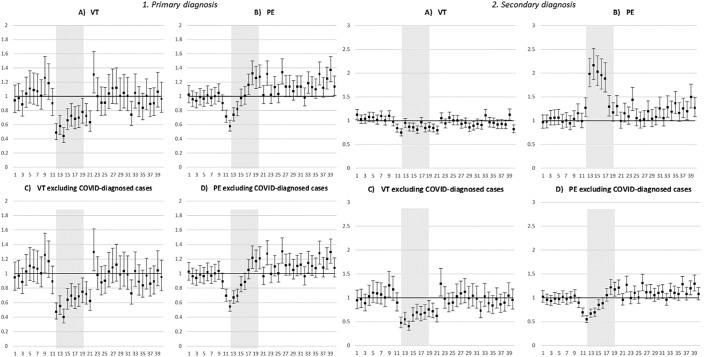
The number of patients hospitalized with a PD of DVT during France's first lockdown (weeks 12–19) in 2020 was almost half that for the same period in 2017–2019 (N2020 = 1474 versus N2017 = 2553, N2018 = 2443, N2019 = 2421). By contrast, the numbers for PE remained relatively stable (N2020 = 6855 versus N2017 = 6059, N2018 = 6520, N2019 = 6691) (Table 1). With regard to weekly IRR (Fig. 1 , A and B), very contrasting time-trends were observed, with decreases of 50% and almost 40% in DVT and PE, respectively, during the first two weeks of the lockdown (weeks 12–13). Lower hospitalization rates for DVT persisted until two weeks after the lockdown ended (week 20–21, Fig. 1, A). From week 22 until week 40 (i.e., the end of the study period), no significant IRR was observed meaning that hospitalization rates for DVT were similar to those for the same period (i.e., week 22–40) in 2017–2019.
Fig. 1.
Weekly incidence rate ratios* (IRR) of patients hospitalized with deep venous thrombosis (DVT) and pulmonary embolism (PE) in 2020 compared with the corresponding weeks in 2017–2019, according to type of diagnosis and excluding COVID-19 diagnosis during hospitalization (whether prior or index DVT/PE hospitalization)
Vertical axis: IRR; horizontal axis: week of hospitalization; grey zone: first national lockdown (weeks 12–19)
*adjusted for time-trends between 2017 and 2019 and 2020.
The pattern was different however for patients hospitalized with a PD of PE (Fig. 1, B). More specifically, although rates fell by almost 40% in weeks 12–13, they quickly increased in the following weeks of the lockdown (weeks 13–16), this increase becoming significant in weeks 17–20, where hospitalization was 30% higher than for the same period in 2017–2019 (Fig. 1, B). Furthermore, unlike the result for DVT, between weeks 20–40 of 2020, rates were regularly higher than in 2017–2019 (Fig. 1, B).
The percentage of patients hospitalized with a PD of DVT and PE in 2020 who were diagnosed in hospital with COVID-19 was 1.5 and 3.7%, respectively, reaching 4.0 and 9.6%, respectively, during the first lockdown (Table 1). For those diagnosed with COVID-19 during a prior hospitalization, the median delay before that hospitalization and the index hospitalization with a PD of DVT and PE was 46 (IQR: 14–130 days) and 20 (IQR: 8–65 days) days, respectively. Patients with a PD of DVT or PE who were diagnosed with COVID-19 were more frequently men and were younger (data not shown). In the sensitivity analysis, the exclusion of patients diagnosed with COVID-19 either during the index hospitalization for DVT/PE, or during prior hospitalization, did not modify these results (Fig. 1, C and D).
Compared with 2017–2019, patients hospitalized in 2020 with a PD of DVT/PE were slightly younger (64.7 and 68.5 years old, respectively, versus 65.6 and 69.0 years old, p < 0.01), and were more likely to be in the 0–1 comorbidity group (in terms of the Charlson index score), to have a history of PE/DVT, and a history of medication for hypertension and hypercholesterolemia (Table 2 ).
Table 2.
Characteristics of patients hospitalized for VT and PE, and of the related hospital stay between January (Week 1) and October (Week 40) 2020 compared with the corresponding time period in 2017–2019, according to the type of diagnosis. Bold: p-value < 0.05.
| Deep venous thrombosis as primary diagnosis |
Pulmonary embolism as primary diagnosis |
Deep venous thrombosis as associated diagnosis |
Pulmonary embolism as associated diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p |
| Patients' characteristics | ||||||||||||
| Women, % | 52.8 | 52.4 | 0.50 | 52.6 | 52.0 | 0.05 | 48.2 | 46.6 | <0.01 | 50.6 | 47.3 | <0.01 |
| Age, mean (std) | 65.6 (19.4) | 64.7 (19.9) | <0.01 | 69.0 (16.8) | 68.5 (17.2) | <0.01 | 67.0 (18.8) | 66.9 (18.6) | 0.18 | 70.3 (16.0) | 69.4 (16.1) | <0.01 |
| 0–65 years old, % | 41.6 | 42.9 | 0.03 | 33.9 | 35.1 | <0.01 | 38.0 | 37.9 | 0.79 | 31.1 | 33.6 | <0.01 |
| Charlson index score, % | 0.02 | <0.01 | <0.01 | <0.01 | ||||||||
| • 0–1 | 42.5 | 43.5 | 49.8 | 50.5 | 38.1 | 38.2 | 35.2 | 41.5 | ||||
| • 2–3 | 27.6 | 26.2 | 27.5 | 27.7 | 31.9 | 32.4 | 33.9 | 31.6 | ||||
| • 4+ | 29.9 | 30.3 | 22.7 | 21.8 | 30.0 | 29.4 | 30.8 | 26.9 | ||||
| Hospitalization or official ‘long-term disease’ status in the five years preceding the index hospitalizationa | ||||||||||||
| • Pulmonary embolism | 3.6 | 2.7 | <0.01 | 10.0 | 9.3 | <0.01 | 2.6 | 1.7 | <0.01 | 13.4 | 9.6 | <0.01 |
| • Venous thromboembolism | 16.9 | 17.8 | 0.04 | 12.2 | 10.9 | <0.01 | 11.4 | 9.8 | <0.01 | 16.0 | 11.4 | <0.01 |
| • Coagulation abnormalities | 9.8 | 10.1 | 0.42 | 6.2 | 6.3 | 0.52 | 7.8 | 7.6 | 0.07 | 6.4 | 5.3 | <0.01 |
| • Stroke | 5.8 | 5.9 | 0.52 | 5.6 | 5.7 | 0.36 | 8.1 | 8.4 | 0.03 | 9.7 | 9.4 | 0.36 |
| • Ischemic heart disease | 15.5 | 14.8 | 0.08 | 12.9 | 12.2 | <0.01 | 16.8 | 17.2 | 0.06 | 15.4 | 14.1 | <0.01 |
| • Heart failure | 13.7 | 13.2 | 0.21 | 14.0 | 13.4 | 0.01 | 15.4 | 15.6 | 0.13 | 16.6 | 14.2 | <0.01 |
| Treatment delivery in the year preceding the index hospitalizationa | ||||||||||||
| • Antihypertensives | 53.8 | 52.6 | 0.03 | 53.7 | 51.1 | <0.01 | 57.3 | 57.3 | 0.77 | 58.1 | 54.9 | <0.01 |
| • Antidiabetics | 15.7 | 16.2 | 0.27 | 12.0 | 11.9 | 0.69 | 17.6 | 18.7 | <0.01 | 15.6 | 16.0 | 0.20 |
| • Lipid-lowering medications | 26.7 | 25.2 | <0.01 | 27.0 | 24.4 | <0.01 | 27.7 | 27.9 | 0.20 | 28.4 | 26.6 | <0.01 |
| • Oral anticoagulants | 11.0 | 10.5 | 0.15 | 7.3 | 5.9 | <0.01 | 14.9 | 14.7 | 0.16 | 14.8 | 11.1 | <0.01 |
| • Heparin | 5.6 | 4.7 | <0.01 | 2.6 | 2.1 | <0.01 | 6.2 | 4.8 | <0.01 | 5.2 | 3.5 | <0.01 |
| • Antiplatelets | 24.5 | 23.2 | <0.01 | 22.3 | 20.9 | <0.01 | 25.1 | 25.1 | 0.96 | 24.2 | 23.0 | <0.01 |
| Disease management and hospital stay | ||||||||||||
| Length of stay, mean (std) | 7.1 (9.0) | 7.2 (9.9) | <0.01 | 9.3 (9.4) | 9.0 (9.0) | <0.01 | 16.1 (16.8) | 16.5 (17.3) | <0.01 | 17.7 (18.2) | 18.0 (17.9) | <0.01 |
| Admitted to intensive care unit, %b | 4.4 | 4.8 | 0.09 | 26.7 | 26.1 | 0.02 | 16.9 | 19.8 | <0.01 | 23.4 | 28.0 | <0.01 |
| In-patient mortality, % | 1.7 | 1.8 | 0.53 | 4.8 | 4.9 | 0.51 | 7.3 | 7.8 | <0.01 | 15.3 | 16.1 | <0.01 |
| 90-day post-admission mortality, %b | 7.6 | 8.3 | 0.06 | 7.2 | 8.0 | <0.01 | 12.0 | 11.9 | 0.82 | 15.4 | 15.0 | 0.27 |
std: standard deviation; p: 2020 vs. 2017–2019.
Available for patients without linkage error (i.e., 99% of patients).
Available for patients affiliated to France's general healthcare insurance scheme (i.e., 77% of included patients).
3.2. Time-trends and profile of patients hospitalized with DVT and PE as an associated diagnosis
Although the number of patients hospitalized with an AD of DVT in weeks 12–19 (i.e., the first lockdown) in 2020 remained stable with respect to 2017/2019, the number of patients hospitalized with an AD of PE doubled (weeks 12–19: N2020 = 6453) (N2017 = 3461; N2018 = 3654; N2019 = 3629) (Table 1).
Weekly IRR differed between patients hospitalized with an AD of DVT and PE (Fig. 1, E and F), and were also different from those found for patients hospitalized with a PD of DVT and PE (described in the previous section) (Fig. 1, A and B). In terms of hospitalization rates for an AD of PE, a large and significant increase was observed throughout all of the first lockdown (weeks 12–19) in 2020, with respect to the same period in 2017–2019. Specifically, the weekly hospitalization rate for weeks 13–17 was twice as high (Fig. 1, F). The rates of hospitalizations for an AD of DVT or PE were higher for patients under 75 years old (versus those over 75 years old) (See supplemental Figure e1). On the contrary, a slight but significant decrease in hospitalization rates with an AD of DVT was observed during the lockdown (Fig. 1, E). This decrease was only significant in people over 75 years old (See supplemental Fig. e2).
The percentage of patients with an index hospitalization with an AD of DVT and PE in 2020 who were diagnosed in hospital with COVID-19 was 4.6 and 16.4%, respectively, reaching 17.2 and 44.6% during the first lockdown (weeks 12–19). Specifically, for those diagnosed with COVID-19 during a prior hospitalization, the median delay before that hospitalization and the index hospitalization for an AD of DVT/PE was shorter than that for a PD of DVT/PE (see preceding section), at 26 (IQR: 7–86 days) and 10 (IQR: 4–31 days) days, respectively. In the sensitivity analysis, the exclusion of patients diagnosed with COVID-19 either during the index hospitalization for DVT/PE or during a prior hospitalization on or after 1 January 2020, did not modify these results (Fig. 1, G and H).
Compared with 2017–2019, the population of patients hospitalized with an AD of PE in 2020 were more likely to be men (52.7% vs 40.4%, p < 0.01), to be in the 0-1 comorbidity group (41.1% vs 34.7%, p < 0.01), and to be admitted to an intensive care unit (ICU) (28.0% vs 23.4%, p < 0.01) at some point during the hospitalization. They also had a higher inpatient case-fatality rate (16.1% vs 15.3%, p < 0.01). Similarly, the patient profile of persons hospitalized with an AD of DVT changed between 2017 and 2019 and 2020 (See supplemental Fig. e2), with a large increase in the proportion of men and in persons under 65 years old, and a substantial decrease in the proportion of patients with at least two comorbidities (i.e., the 2–3 and 4 or more comorbidity groups). These trends were greater for PE than DVT (See supplemental Fig. e2).
3.3. In-hospital and 90-day post-admission mortality in patients hospitalized with a primary or associated diagnosis of PE
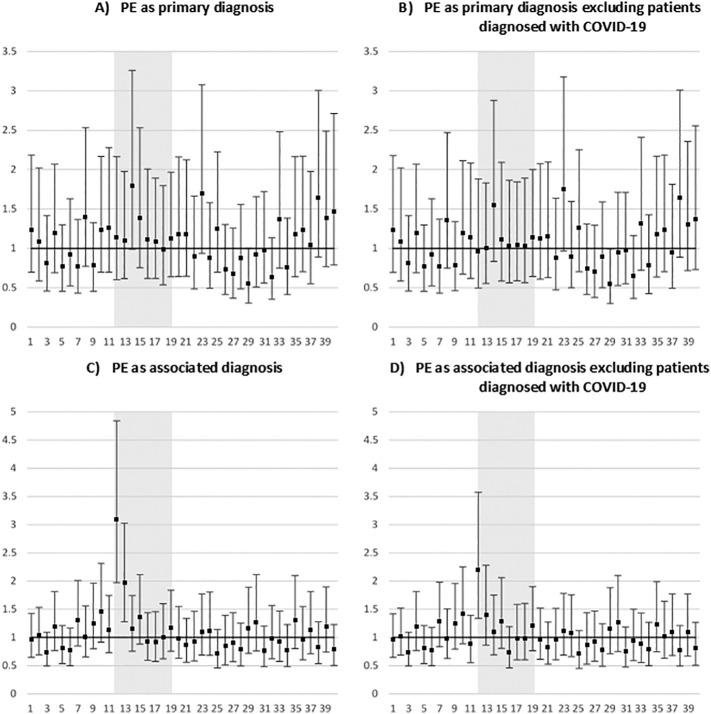
Compared with the corresponding time period in 2017–2019 (weeks 1–40), the OR for in-patient case-fatality rates did not change significantly for hospitalizations with a PD of PE in 2020 (Fig. 2 , A and B). On the contrary, inpatients hospitalized in 2020 with an AD of PE were, respectively, three times more likely to die than their counterparts hospitalized in 2017–2019 during the first week of lockdown (week 13, Fig. 2, C). After excluding cases diagnosed with COVID-19 in hospital, significantly higher case-fatality rates were still observed for week 12 of 2020 with respect to the same week in 2017–2019 patients hospitalized with an AD of PE (Fig. 2, D).
Fig. 2.
Weekly adjusted* odds ratios (OR) of in-patient hospital case-fatality rates among patients hospitalized with a pulmonary embolism (PE) in 2020 compared with the corresponding weeks in 2017–2019, according to the type of diagnosis and COVID-19 diagnosis during hospitalization
Vertical axis: IRR; horizontal axis: week of hospitalization; grey zone: first national lockdown (weeks 12–19)
*adjusted for time-trends between 2017 and 2019 and 2020, age and sex.
The 90-day post-admission case-fatality rates significantly increased between the two study periods, but in an irregular fashion; specifically, increases were seen for weeks 11 and 17 for persons with a PD of PE (See supplemental Fig. e3, A and B) and for weeks 5, 18 and 28 for those with an AD of PE (See supplemental Fig. e3, C). These observations remained after excluding patients diagnosed with COVID-19 in hospital (See supplemental Fig. e3, B and D).
4. Discussion
Our study showed that significant changes occurred in the epidemiology of hospitalized DVT and PE in France in 2020 compared with 2017–2019. This was particularly true during the country's first lockdown (weeks 12–19), with a significant decrease being observed in hospitalizations of people with a PD of DVT and PE by 50 and 40%, respectively, in the first two weeks (weeks 12–13), and a significant increase in hospitalizations for those with an AD of PE, reaching up to 200% during the first five weeks of the lockdown (weeks 12–17). During the first lockdown, 10 and 45% of persons hospitalized with a PD and an AD of PE, respectively, were diagnosed with COVID-19 during hospitalization (whether index DVT/PE hospitalization or prior hospitalization). The inpatient case mortality rate was three times higher in persons hospitalized with an AD of PE in the first week of the lockdown (week 12) in 2020 than in their hospitalized counterparts during the corresponding week in 2017–2019.
The relative decrease (i.e., compared with the same period in 2017/2019) in the rates of patients hospitalized with a PD of DVT and PE during the first two weeks of the lockdown (weeks 12–13) in 2020, was consistent with the decline observed in France and elsewhere in hospitalizations for other acute cardiovascular diseases - such as stroke [20], [21] and acute myocardial infarction [22] - and with the global decrease in the use of healthcare services [23]. The main hypothesis explaining these time-trends is that people were afraid of getting COVID-19 in hospital and therefore decided to delay contacting healthcare services. Nevertheless, the effect of this on PE was short-lived, as the numbers of hospitalizations started to increase again as early as the second week of the lockdown (week 13), and returned to normal (i.e., compared with the mean level for 2017/2019) during week 15.
The decrease in both DVT and PE hospitalizations during the first lockdown may also be partly linked to cancellations by health authorities of non-urgent surgery (mainly orthopedic and digestive surgery), and to the decrease in trauma surgery in that period [24], [25]. Between the end of the first lockdown (week 19) and the end of the study period (week 40), the weekly hospitalization rates for people with a PD of PE were often higher in 2020 than the mean for 2017–2019. This may be due to PE occurring as a consequence of an increase in COVID-19 cases. This point underlines the importance of extending thrombo-prophylactic measures to outpatient COVID-19 cases at high risk of PE [26]. Aktaa et al. showed a substantial increase in mortality from PE without COVID-19 diagnosis during the first wave of the pandemic in the UK. The authors linked this to the lack of testing outside hospitals during this period [27]. In terms of hospitalizations of people with a PD of DVT, the lower rates observed at the beginning of the lockdown persisted during and after the entire lockdown period until week 21. The difference in the patterns observed for DVT and PE might be explained by the fact that a majority of patients diagnosed with DVT were provided care outside the hospital setting when the peak of the first wave of the pandemic in France occurred in March 2020.
The increase in the numbers of patients hospitalized with an AD of PE in France during the whole first lockdown period was directly linked to the explosion in the numbers of SARS-CoV-2 infections in the general population, which resulted in mass hospitalizations for COVID-19 and related PE [1], [2], [4]. This finding is consistent with the increase in the rates of patients hospitalized with venous thromboembolism events in the abovementioned UK study [27]. Many studies have described the association between COVID-19 and PE. In ours, 45% of patients hospitalized with an AD of PE during France's first lockdown were diagnosed with COVID-19. Therefore, at that time, the epidemiology of PE and COVID-19 converged, which explained the substantial increase in the proportion of men, younger patients, and ICU admissions (data not shown for this last one) we found in patients hospitalized with an AD of PE. We cannot exclude the possibly - as hypothesized in the UK study above [27] - that the incidence of venous thromboembolism increased as a consequence of people staying at home too long without doing any physical activity.
The substantial increase in inpatient case-fatality rates for persons hospitalized with an AD of PE in the first two weeks of France's first lockdown (weeks 12–13) in 2020, might be related to the difficulty to manage patients who have both a severe form of COVID-19 and PE, and to the greater severity of COVID-19-related PE cases [28]. The rapid implementation of anticoagulation therapy and, when necessary, mechanical thromboprophylaxis (as per several national and international recommendations [26], [29], [30], [31]) in hospitalized COVID-19 cases with a severe form of the disease - especially those admitted to ICU - together with the greater awareness by healthcare professionals of the high risk of thromboembolic events in severe COVID-19 patients, might have reduced PE incidence in severe COVID-19 cases and therefore limited the inpatient case-fatality rate.
The increase (with respect to 2017–2019) in adjusted DVT and PE inpatient case-fatality rates which we saw in 2020, may also be related to longer delays before accessing hospital services during the first two weeks (weeks 12–13) of the first lockdown, whether because people waited longer before seeking medical attention, or because hospitals were too overwhelmed. This increase is consistent with the global excess in mortality observed in France in the initial weeks of the first lockdown [32]. A return to normality (with respect to 2017–2019) in adjusted DVT and PE inpatient case-fatality rates was observed from week 14 until the end of July 2020 in patients who were diagnosed with COVID-19. This may be related to better management of COVID-19 patients with thrombosis (thanks to widespread diffusion of effective recommendations [26], [29]), to the decline in the COVID-19 epidemic at that point in time, to the end of the first lockdown (and therefore possibly faster access to hospital), and to less fear about getting the disease in hospital (and therefore seeking care earlier) [18]. However, the reasons for the subsequent increase in inpatient case-fatality rates after July 2020 in this same population remain unclear.
4.1. Public health and clinical implications
Our results highlight that in the context of the ongoing COVID-19 pandemic and venous thromboembolism, the related lockdown measures to contain the pandemic, the general public's fear of getting COVID-19 if they go to hospital (resulting in a delay before seeking medical attention), and the structural delays in being able to access healthcare structures, all need to be tackled by increasing the general population's awareness of the severity of venous thromboembolism and its complications, especially if medical care is not sought immediately after symptoms appear. The association between DVT after prolonged sitting was first recognized during the blitzkrieg in World War II, when fatal PE were observed in Londoners obliged to stay for long periods of times in air shelters [33]. The restrictive effects of COVID-19-related lockdowns on people's movements, within and outside of their homes, highlights a parallel situation and similar risk.
Furthermore, the increased risk of venous thromboembolism in patients with COVID-19 which led to increased hospitalizations for DVT and PE in France in 2020, highlights the need to: (a) adjust the quantity and type of resources allocated to the management of venous thromboembolism in hospitals, (b) ensure adapted anticoagulation therapy in patients with COVID-19 at risk of DVT [26], [29], and (c) advocate for closer follow-up of these patients. Further research is also necessary to evaluate whether the spread of new SARS-CoV-2 variants in the ongoing pandemic has differentially impacted the incidence of DVT in France and elsewhere.
4.2. Strengths and limitations
To the best of our knowledge, this is the first nationwide study to exhaustively investigate the epidemiology of hospitalized DVT and PE cases, as well as associated case-fatality rates in France over a relatively long and recent time period, as cases until October 2020 were included. The main limitation of the study was the unavailability of data on outpatient DVT and PE cases, and associated case-fatality rates, in a context where related inpatient mortality in France increased by 9% with respect to 2017–2019 [32], [34]. A second limitation is that the time between DVT/PE symptom onset and hospitalization was not available in the database used, which limits the interpretation of our results. Finally, another limitation of our study was the general use of administrative medical databases which were not conceived for epidemiological but economic purposes.
5. Conclusion
The present study showed that the epidemiology of DVT and PE was seriously impacted by the COVID-19 pandemic in 2020. First, measures taken to limit population movements, especially lockdowns, were associated with a 40% decrease in the rate of patients hospitalized for PE with respect to 2017–2019, and a threefold increase in the related in-patient mortality rate, although the effect did not last for a long time. Second, COVID-19 itself directly led to an increase in the numbers of patients hospitalized with DVT and PE in France. This finding highlights the importance of promoting anticoagulation therapy in COVID-19 patients at risk of DVT and advocating for close follow-up of COVID-19 patients at risk of PE. More generally, efforts to increase the general population's awareness of DVT and PE need to be intensified, and the quantity and type of inpatient resources devoted to the management of these diseases need to be increased.
Funding sources
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2021.09.009.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Suh Y.J., Hong H., Ohana M., Bompard F., Revel M.P., Valle C., et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2):E70–e80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Minno A., Ambrosino P., Calcaterra I., Di Minno M.N.D. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin. Thromb. Hemost. 2020;46(7):763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georger F., Dos Santos E., Gazagne L., Berdagué P., Saib A., Nahon S., et al. COV IMPACT: stress exposure analysis among hospital staff in 2 hospitals in France during the COVID-19 pandemic. Ann. Cardiol. Angeiol. 2020;69(5):227–232. doi: 10.1016/j.ancard.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danguy des Déserts M., Mathais Q., Luft A., Escarment J., Pasquier P. Conception and deployment of a 30-bed field military intensive care hospital in Eastern France during the 2020 COVID-19 pandemic. Anaesth. Crit. Care Pain Med. 2020;39(3):361–362. doi: 10.1016/j.accpm.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuteifan K., Pasquier P., Meyer C., Escarment J., Theissen O. The outbreak of COVID-19 in Mulhouse : hospital crisis management and deployment of military hospital during the outbreak of COVID-19 in Mulhouse, France. Ann. Intensive Care. 2020;10(1):59. doi: 10.1186/s13613-020-00677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefrant J.Y., Fischer M.O., Potier H., Degryse C., Jaber S., Muller L., et al. A national healthcare response to intensive care bed requirements during the COVID-19 outbreak in France. Anaesth. Crit. Care Pain Med. 2020;39(6):709–715. doi: 10.1016/j.accpm.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosovsky R.P., Grodzin C., Channick R., Davis G.A., Giri J.S., Horowitz J., et al. Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: a position paper from the national PERT consortium. Chest. 2020;158(6):2590–2601. doi: 10.1016/j.chest.2020.08.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davin-Casalena B., Jardin M., Guerrera H., Mabille J., Tréhard H., Lapalus D., et al. The impact of the COVID-19 pandemic on first-line primary care in southeastern France: feedback on the implementation of a real-time monitoring system based on regional health insurance data. Rev. Epidemiol. Sante Publique. 2021;69(3):105–115. doi: 10.1016/j.respe.2021.04.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier J.P., Amélineau J.B., Hild S., Nguyen-Soenen J., Daviot A., Simonneau B. Patient-safety incidents during COVID-19 health crisis in France: an exploratory sequential multi-method study in primary care. Eur. J. Gen. Pract. 2021;27(1):142–151. doi: 10.1080/13814788.2021.1945029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesieur O., Quenot J.P., Cohen-Solal Z., David R., De Saint Blanquat L., Elbaz M. Admission criteria and management of critical care patients in a pandemic context: position of the Ethics Commission of the French Intensive Care Society, update of April 2021. Ann. Intensive Care. 2021;11(1):66. doi: 10.1186/s13613-021-00855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauchemez S., Kiem C.T., Paireau J., Rolland P., Fontanet A. Lockdown impact on COVID-19 epidemics in regions across metropolitan France. Lancet (London, England). 2020;396(10257):1068–1069. doi: 10.1016/s0140-6736(20)32034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morello F., Bima P., Ferreri E., Chiarlo M., Balzaretti P., Tirabassi G., et al. After the first wave and beyond lockdown: long-lasting changes in emergency department visit number, characteristics, diagnoses, and hospital admissions. Intern. Emerg. Med. 2021;1–8 doi: 10.1007/s11739-021-02667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page E.M., Ariëns R.A.S. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb. Res. 2021;200(1–8) doi: 10.1016/j.thromres.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuppin P., Rudant J., Constantinou P., Gastaldi-Menager C., Rachas A., de Roquefeuil L., et al. Value of a national administrative database to guide public decisions: from the systeme national d'information interregimes de l'Assurance maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev. Epidemiol. Sante Publique. 2017;65(Suppl 4):S149–s67. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Bernard Stoecklin S., Rolland P., Silue Y., Mailles A., Campese C., Simondon A. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro. Surveill. 2020;25(6) doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santé Publique France Point Epidémiologique du 8 Octobre 2020. 2021. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-8-octobre-2020
- 19.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 20.Gabet A., Grave C., Tuppin P., Chatignoux E., Béjot Y., Olié V. Impact of the COVID-19 pandemic and a national lockdown on hospitalizations for stroke and related 30-day mortality in France: a nationwide observational study. Eur. J. Neurol. 2021 doi: 10.1111/ene.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogueira R.G., Abdalkader M., Qureshi M.M., Frankel M.R., Mansour O.Y., Yamagami H., et al. Global impact of COVID-19 on stroke care. Int. J. Stroke. 2021;16(5):573–584. doi: 10.1177/1747493021991652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesnier J., Cottin Y., Coste P., Ferrari E., Schiele F., Lemesle G., et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5(10):e536–e542. doi: 10.1016/s2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moynihan R., Sanders S., Michaleff Z.A., Scott A.M., Clark J., To E.J., et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barro K., Malone A., Mokede A., Chevance C. Management of the COVID-19 epidemic by public health establishments-Analysis by the Fédération Hospitalière de France. J. Visc. Surg. 2020;157(3s1):S19–s23. doi: 10.1016/j.jviscsurg.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Druel T., Andeol Q., Rongieras F., Bertani A., Bordes M., Alvernhe A. Evaluation of containment measures’ effect on orthopaedic trauma surgery during the COVID-19 pandemic: a retrospective comparison between 2019 and 2020. Int. Orthop. 2020;44(11):2229–2234. doi: 10.1007/s00264-020-04712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khider L., Soudet S., Laneelle D., Boge G., Bura-Rivière A., Constans J., et al. Proposal of the French Society of Vascular Medicine for the prevention, diagnosis and treatment of venous thromboembolic disease in outpatients with COVID-19. J. Med. Vasc. 2020;45(4):210–213. doi: 10.1016/j.jdmv.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktaa S., Wu J., Nadarajah R., Rashid M., de Belder M., Deanfield J., et al. Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: multi-sourced population-based health records cohort study. Thromb. Res. 2021;202(17–23) doi: 10.1016/j.thromres.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planquette B., Le Berre A., Khider L., Yannoutsos A., Gendron N., de Torcy M., et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: a nested case-control study. Thromb. Res. 2021;197(94–9) doi: 10.1016/j.thromres.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huisman M.V., Klok F.A., Kaptein F.H.J., Stals M.A.M. Prophylaxis and treatment of COVID-19 related venous thromboembolism. Postgrad. Med. 2021:1–9. doi: 10.1080/00325481.2021.1891788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susen S., Tacquard C.A., Godon A., Mansour A., Garrigue D., Nguyen P., et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit. Care. 2020;24(1):364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontana P., Casini A., Robert-Ebadi H., Glauser F., Righini M., Blondon M. Venous thromboembolism in COVID-19: systematic review of reported risks and current guidelines. Swiss Med. Wkly. 2020;150 doi: 10.4414/smw.2020.20301. [DOI] [PubMed] [Google Scholar]
- 32.Fouillet A., Pontais I., Caserio-Schönemann C. Excess all-cause mortality during the first wave of the COVID-19 epidemic in France, March to May 2020. Eurosurveillance. 2020;25(34) doi: 10.2807/1560-7917.ES.2020.25.34.2001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson K. Shelter deaths from pulmonary embolism. Lancet. 1940;236(6120):744. doi: 10.1016/S0140-6736(00)92078-6. [DOI] [Google Scholar]
- 34.Institut national de la statistique et des études économiques . 2020 : une hausse des décès inédite depuis 70 ans. Insee Première. Mars 2021. p. 1847.https://www.insee.fr/fr/statistiques/5347349 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Data Availability Statement
Full access to the SNDS is granted to the National Agency for Public Health (Santé Publique France) by governmental decree (regulatory decision DE-2011-078). The data underlying this article cannot be shared publicly.