Abstract
Alveolar bone resorption can be induced in specific-pathogen-free mice by oral infection with Porphyromonas gingivalis (P. J. Baker, R. T. Evans, and D. C. Roopenian, Arch. Oral Biol. 39:1035–1040, 1994). Here we used a mouse strain, C57BL/6J, which is relatively resistant to P. gingivalis-induced bone loss to examine whether partial or complete deletion of various adhesion molecules would increase susceptibility. Complete deletion of P-selectin or nearly complete lack of expression of intercellular adhesion molecule 1 (ICAM-1) led to increased susceptibility to bone resorption after oral infection, while a hypomorphic defect in β2-integrins did not. Both the total amount of bone lost and the number of sites at which there was significant loss were increased in mice deficient in either ICAM-1 or P-selectin. Each of the three adhesion molecule deficiencies was sufficient to decrease P. gingivalis-specific serum immunoglobulin G responses, but lower antibody titers did not lead to increased bone loss in partially β2-integrin-deficient mice. In conclusion, P-selectin and ICAM-1 deficiencies increase susceptibility to and severity of alveolar bone loss after P. gingivalis infection. This finding underscores the importance of innate immunity in protection against P. gingivalis-induced alveolar bone resorption.
Periodontal diseases are chronic inflammatory diseases which destroy the supporting tissues around the teeth and can lead to tooth loss. One aspect of periodontal disease is resorption of the alveolar bone, which forms the bony sockets to which the teeth are anchored (19, 23, 27). In the initiation of periodontal disease, cells of the innate immune system are recruited to the gingiva; neutrophils are thought to be protective against the disease (10). Periodontal disease in humans is associated with the black-pigmented, gram-negative anaerobic bacterium Porphyromonas gingivalis (18, 22, 25). Humans develop a variety of adaptive immune responses to P. gingivalis during the course of the disease (8, 9).
Using a mouse model in which oral infection with P. gingivalis results in loss of alveolar bone, we have previously shown that one aspect of the adaptive immune response, CD4+ T cells and their cytokines, rather than being protective, contribute to destructive bone remodeling (4). Here, using the same model, we examine the effects of adhesion molecule deficiencies on bone loss. Such deficiencies lead to defects in both innate and adaptive immunity, due in part to the role of adhesion molecules in extravasation of leukocytes from the circulation into the tissues.
Several families of adhesion molecules are utilized at different steps of the extravasation process. Neutrophil and macrophage rolling, the initial step in their leaving the blood vessel, is mediated by selectins upregulated by endothelial cells, such as those in blood vessels, in response to activation signals such as C5a, interleukin-1β, and tumor necrosis factor alpha. The integrin family of adhesion molecules on leukocytes then bind to the intercellular adhesion molecules (ICAM-1 and ICAM-2) on endothelial cells, attaching the leukocytes to the endothelial walls and aiding in transendothelial migration (28). The β2-integrins consist of a common β2 subunit (CD18) coexpressed with one of several α subunits (CD11a in lymphocyte function antigen LFA-1, CD11b in complement receptor type 3 [CR3 or Mac-1], and CD11c in complement receptor type 4 [CR4 or p150,95]) (1, 28). All three are present on neutrophils and macrophages. In addition to their role in granulocyte extravasation, the β2-integrins function in neutrophil phagocytosis and respiratory burst (1). ICAM-1 and ICAM-2 are expressed on leukocytes, epithelium, and fibroblasts in addition to endothelium and are upregulated by stimulation with bacterial lipopolysaccharide or inflammatory cytokines (14).
In addition to their roles in innate immunity, two of these adhesion molecules are also important in adaptive immunity. Integrin binding to ICAM-1 is a costimulatory signal for T- and B-lymphocyte activation, in addition to their extravasation (12).
In this study, we investigated the consequences of adhesion defects by comparing three strains of mice with a range of severity of adhesion deficiencies. One strain has a hypomorphic allele at the CD18 locus, resulting in decreased CD18 expression and thus in reduced cell surface expression of all of the β2-integrins. These mice are a model for the moderate form of human CD18 deficiency (28). The second mouse strain is severely but not entirely deficient in ICAM-1; these mice do express low levels of cell surface ICAM-1, but the expression is more severely inhibited than is integrin expression in the CD18 mutant mice (21, 28). However, even when mice are completely deficient in cell surface ICAM-1, neutrophil transendothelial migration is not completely eliminated, indicating that there are ICAM-1-independent mechanisms for extravasation (21). Therefore, we also tested a third mouse strain, in which there is a complete lack of expression of P-selectin. Both ICAM-dependent and ICAM-independent mechanisms of leukocyte emigration are blocked in P-selectin-deficient mice (17). The three mutant mouse strains thus provide a graded series of adhesion defects. We examined the effects of different degrees of adhesion molecule deficiency on alveolar bone loss in response to P. gingivalis and on specific antibody responses after infection.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free mice were bred and raised at The Jackson Laboratory (Bar Harbor, Maine). ICAM-1-deficient C57BL/6J-Icam1tm1Bay (21), CD18-deficient C57BL/6J-Itgb2tm1Bay mice (28), and P-selectin (Selp)-deficient C57BL/6J-Selptm1Bay mice were produced by gene targeting of strain 129-derived embryonal stem cells followed by at least 10 generations of backcrossing onto the C57BL/6J background mice. These mice and their wild-type C57BL/6J controls were kept on a 12-h light-dark cycle and received distilled water and food ad libitum. The animals within an experiment were age-matched females, 10 to 15 weeks old at the start of experiments. All experiments were approved by the Animal Care and Use Committee, Bates College.
Bacteria.
P. gingivalis ATCC 53977 (A7A1-28) was maintained frozen in defibrinated sheep blood at −70°C and by weekly transfer on supplemented blood agar (Trypticase soy agar base with 0.1% yeast extract, 5.0 μg of hemin per ml, 0.5 μg of menadione per ml, and 5% defibrinated sheep blood). For experiments, the bacteria were anaerobically grown under 5% CO2–10% H2–85% N2 on supplemented blood agar at 37°C for 4 to 7 days.
Oral infection.
As described previously (4), mice were given sulfamethoxazole-trimethoprim (Sulfatrim; Goldline Laboratories, Fort Lauderdale, Fla.), 10 ml per pint in deionized water, ad libitum for 10 days. This was followed by a 3-day antibiotic-free period. The mice were then infected with 109 CFU of live P. gingivalis in 100 μl of phosphate-buffered saline with 2% carboxymethylcellulose (15) placed into the esophagus and oral cavity three times at 2-day intervals. Controls included sham-infected mice which received the antibiotic pretreatment and the carboxymethylcellulose gavage but without P. gingivalis. At 47 days after the first gavage, the mice were euthanized by CO2 inhalation.
Recovery of P. gingivalis.
A sterile medium-sized paper point (Johnson & Johnson, East Windsor, N.J.) was held against the gumline of the upper molars for 5 s and then vortexed in 1 ml of prereduced brain heart infusion broth supplemented with hemin and menadione. An aliquot plated onto supplemented blood agar was incubated anaerobically for 4 weeks. P. gingivalis colonies were identified by their black pigmentation and by Gram stain (2).
P. gingivalis-specific IgG.
Blood was collected from each mouse at the time of euthanasia. Sera were stored at −70°C for later assessment of specific immunoglobulin G (IgG) antibody by enzyme-linked immunosorbent assay, as described previously (2), in polystyrene plates (Falcon, Becton-Dickinson Labware, Lincoln Park, N.J.) coated with formalin-killed whole P. gingivalis ATCC 53977. The enzyme-linked immunosorbent assay titer was defined as the reciprocal of the highest serum dilution (expressed in log2) which produced absorbance readings more than two standard deviations above background levels. Values in sham-infected mice were only slightly greater than zero and have been subtracted from the titers in infected mice shown in Fig. 4.
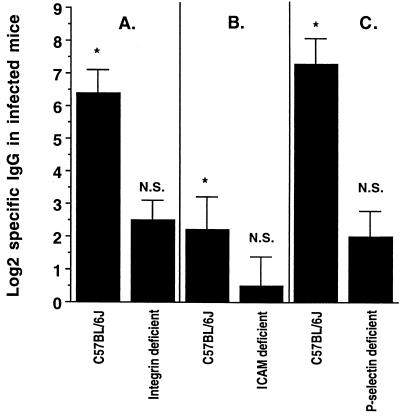
FIG. 4.
P. gingivalis-specific IgG titers in infected wild-type C57BL/6J mice and in adhesion molecule-deficient mice. Panels A, B, and C show results of separate experiments. Bars represent the means ± 1 standard error of the mean from the same eight mice as in Fig. 1 and 5. Titers were zero in sham-infected mice. *, titers in infected C57BL/6J mice are significantly greater (P < 0.05) than titers in sham-infected C57BL/6J mice and also significantly greater than titers in infected β2-integrin-deficient mice (A), ICAM-1-deficient mice (B), or P-selectin-deficient mice (C). Titers in infected adhesion molecule-deficient mice were not significantly different from the titers in sham-infected adhesion molecule-deficient mice (N.S., P > 0.05).
Alveolar bone loss.
Horizontal bone loss around the maxillary molars was assessed by a morphometric method (15). Skulls were defleshed after 10 min of treatment in boiling water under 15-lb/in2 pressure, immersed overnight in 3% hydrogen peroxide, pulsed for 1 min in bleach, and stained with 1% methylene blue. The distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at a total of 14 buccal sites per mouse. This measurement is referred to below as CEJ to ABC. Measurements were made under a dissecting microscope (magnification, ×40) fitted with a video image marker measurement system (model VIA 170; Boeckeler Instruments, Inc., Tucson, Ariz.) standardized to give measurements in millimeters. Bone measurements were made a total of three times in a random and blinded protocol by two evaluators. In some cases the CEJ-to-ABC measurements are shown directly. In other cases, data are shown as the number of millimeters of bone lost in infected animals: the “total millimeter change in bone” was calculated by subtracting the CEJ to ABC of individual mice from the mean CEJ to ABC of groups of sham-infected mice, totaled for the 14 measurement sites. Since the CEJ-to-ABC increases if bone is resorbed, this calculation gives negative values of “total millimeter change in bone” when there has been bone loss.
Statistics.
Differences between groups were evaluated by the t test (Excel; Microsoft).
RESULTS
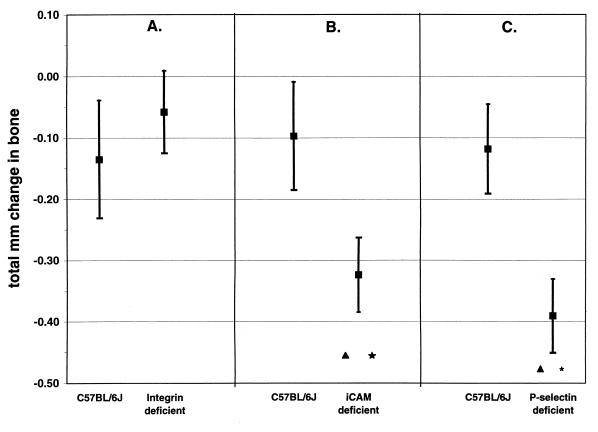
The effects of deletion of different adhesion molecules on alveolar bone loss after oral infection with P. gingivalis are shown in Fig. 1. A hypomorphic allele of CD18, leading to reduced expression of all β2-integrins, did not render mice more susceptible to bone loss after infection (P > 0.05), (Fig. 1A). A more severely hypomorphic allele of ICAM-1 (Fig. 1B) or a complete deletion of P-selectin (Fig. 1C) did, however, render C57BL/6J mice more susceptible to bone loss (P < 0.05).
FIG. 1.
Deficiency of ICAM-1 or P-selectin, but not β2-integrin, increases susceptibility to alveolar bone loss after oral infection with P. gingivalis. Data are the means and 1 standard error of the mean (n = 8) of the number of millimeters of change in the CEJ-ABC at the total of 14 sites in infected mice compared to sham-infected mice. Panels A, B, and C show results of separate experiments. (A) Neither wild-type C57BL/6J control mice nor β2-integrin-deficient C57BL/6J mice lost bone after infection. Data shown for infected mice are not significantly different from those for sham-infected mice (P > 0.05). (B) Wild-type C57BL/6J mice did not lose bone after infection, but ICAM-1-deficient C57BL/6J mice did. (C) Wild-type C57BL/6J mice did not lose bone after infection, but P-selectin-deficient C57BL/6J mice did. ∗, infected mice were different from sham-infected mice (P < 0.05); ▴, infected mice lost significantly more bone than infected wild-type C57BL/6J mice did (P < 0.05).
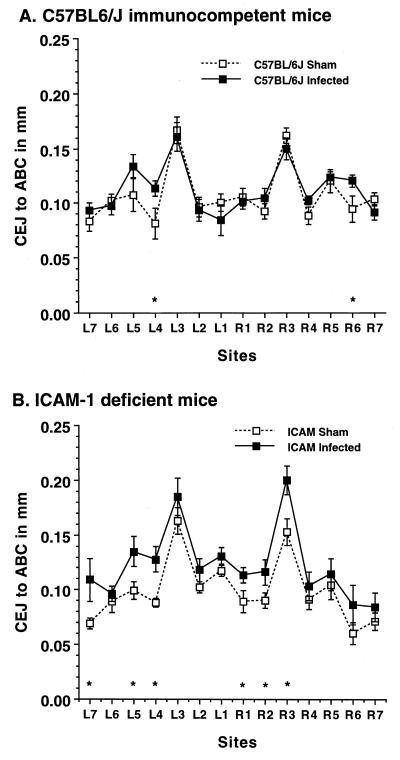
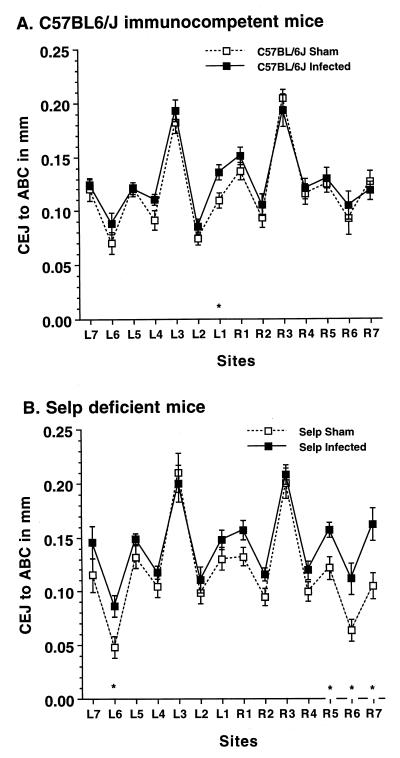
Bone loss was not evenly distributed at all sites, and deletion of ICAM-1 or P-selectin increased its distribution. Although bone loss in infected wild-type C57BL/6J mice was not significant when all 14 sites were considered (Fig. 1), there were individual sites with significant bone loss (P < 0.05), as shown in Fig. 2A and Fig. 3A. Infection of ICAM-1-deficient mice (Fig. 2B) or P-selectin knockout mice (Fig. 3B), but not of partially β2-integrin-deficient mice (data not shown), induced bone loss at a greater number of sites.
FIG. 2.
ICAM-1-deficient mice show more sites with bone loss after P. gingivalis oral infection than do wild-type C57BL/6J mice. Data are the means and 1 standard error of the mean for eight mice. ∗, infected mice different from sham-infected mice (P < 0.05).
FIG. 3.
P-selectin (Selp)-deficient mice have more sites with bone loss after P. gingivalis oral infection than do wild-type C57BL/6J mice. Data are the means ± 1 standard error of the mean for eight mice. ∗, infected mice different from sham-infected mice (P < 0.05).
Increased bone loss was not a result of any differences in alveolar anatomy in adhesion molecule-deficient mice. The CEJ to ABC at the various sites was the same in sham-infected ICAM-deficient and P-selectin-deficient mice as in sham-infected C57BL/6J mice (Fig. 2 and 3). This was also true for the β2-integrin-deficient mice (data not shown).
Deficiency of any of the three adhesion molecules decreased antibody responses to oral infection (Fig. 4). P. gingivalis-specific IgG titers were zero in sham-infected mice and were significantly (P < 0.05) elevated in infected immunocompetent C57BL/6J mice. Specific IgG titers were also zero in sham-infected β2-integrin-, ICAM-1- or P-selectin-deficient mice; however, in these mice, the titer did not increase after infection. The titers in infected adhesion molecule-deficient mice were not significantly different (P > 0.05) from those in sham-infected adhesion molecule-deficient mice.
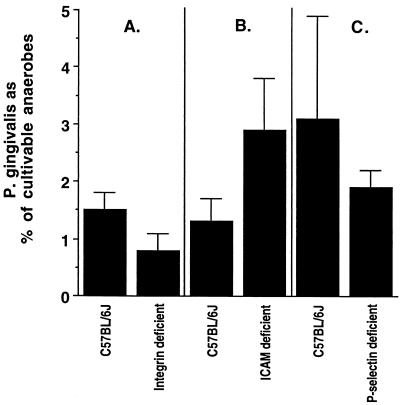
Deficiency of adhesion molecules did not change the relative P. gingivalis load. Bacteria were sampled from the oral cavity by using paper points at the end of the experiments (42 days after the final infection). There were no differences among the three experiments in Fig. 5 in the percentages of P. gingivalis in the cultivable anaerobes in infected mice. However, while we did not observe higher percentages of P. gingivalis in infected adhesion molecule-deficient mice than in infected wild-type mice, it is still possible that the total bacterial load was greater in the adhesion molecule-deficient mice.
FIG. 5.
P. gingivalis in the oral cavities of immunocompetent C57BL/6J mice or adhesion molecule-deficient mice 42 days after the final infection. Panels A, B, and C show results of separate experiments. Bars represent the means ± 1 standard error of the mean from the same eight mice as in Fig. 1 and 4. No P. gingivalis was recovered from sham-infected mice. The percentage of P. gingivalis was not significantly different (P > 0.05) in C57BL/6J mice from that in β2-integrin-deficient mice (A), ICAM-1-deficient mice (B), or P-selectin-deficient mice (C).
DISCUSSION
C57BL/6J mice are fairly resistant to alveolar bone loss after infection with P. gingivalis compared with other immunocompetent mouse strains such as BALB/c (P. J. Baker, M. Dixon, and D. C. Roopenian, submitted for publication). Among the three adhesion molecules examined, a hypomorphic β2-integrin allele was not a defect sufficient to overcome the resistance of the C57BL/6J parent strain whereas both a more severely hypomorphic allele of ICAM-1 and a complete abrogation of P-selectin made C57BL/6J mice more susceptible to bone loss. Whether differential susceptibility in the adhesion mutants is due to differences in the degree of expression or to something more specific to the different types of adhesion molecules remains to be established.
We cannot say definitively whether our results are due to changes in cell-mediated immunity or changes in innate immunity, although several lines of evidence indicate that impaired innate immunity is the more plausible explanation. β2-Integrins and ICAMs, but not P-selectin, are involved in lymphocyte activation (12). If adaptive immunity is primarily protective, strains with impaired lymphocyte activation should show increased bone loss; conversely, if specific immunity does not protect against bone loss, its abrogation would not increase bone loss. We have previously shown that the adaptive antibody response, particularly the low levels that are induced by oral infection in this model, is not protective; although antibody develops in immune normal mice before the onset of detectable bone loss, it does not prevent it (3). Here we show that although antibody responses were decreased in all three mouse strains (Fig. 4), only two of the three strains showed increased susceptibility to bone loss after oral infection (Fig. 1). In terms of cell-mediated adaptive immunity, we have previously reported that CD4+ T cells contribute to alveolar bone loss induced by oral infection with P. gingivalis (4), a finding that is consistent with the T-cell response being destructive rather than protective. If cell-mediated immunity is destructive, mice with diminished cell-mediated immunity (12, 21) should show less bone loss, not the increased bone loss we found in infected adhesion molecule-deficient mice.
An alternative explanation for our results is that susceptibility is caused by weakened innate antibacterial immune mechanisms. Our results do not correlate with the known effects of adhesion molecules on lymphocytes but are in line with the degree of inhibition of neutrophils in the three immunodeficient mouse strains. P-selectin-deficient mice exhibited increased bone loss after infection, but P-selectin is not known to be directly involved in lymphocyte activation, and mice deficient in β2-integrin, which does function in lymphocyte activation, did not show increased bone loss. Adhesion molecule deficiencies decrease the numbers of neutrophils at an infection site, and the three mouse strains we used vary in the degree of their neutrophil defects (17, 21, 28). The β2-integrin-deficient mouse strain we used has the smallest defect in neutrophil emigration and did not show altered bone loss compared to immune normal mice, while the P-selectin-deficient strain has the greatest extravasation defect and exhibited increased susceptibility to bone loss.
If neutrophils are primarily proinflammatory, a defect in adhesion molecules might be expected to decrease bone loss. On the other hand, neutrophils are thought to be protective against periodontal disease and can kill P. gingivalis (10), so that a decrease might make bone loss worse. Although disease severity increased in ICAM-1-deficient and P-selectin-deficient mice, we did not see higher percentages of P. gingivalis in infected adhesion molecule-deficient mice than in infected immunocompetent mice (Fig. 5). However, because we did not perform limiting-dilution analyses of the total oral flora, we cannot rule out the possibility that the total bacterial load was greater in the adhesion-deficient mice.
The integrins were the least inhibited of the molecules and may have been sufficient to afford protection, since we did not see increased bone loss in our partially β2-integrin-deficient mice. Patients with Mac-1 integrin deficiencies often develop periodontal disease, but the disease severity depends on the degree of the defect (26). Other contributions of the genetic background may have overcome the integrin defect. Indeed, studies have indicated that other inflammatory diseases do not result from integrin defects unless they are combined with other defects (6). P. gingivalis can use the integrins (CD11/ CD18) as attachment sites for binding to macrophages, inducing interleukin-1β and tumor necrosis factor alpha gene expression in these host cells (24). Since these cytokines induce bone resorption (5, 11, 16), integrins may tip the balance toward destructive inflammation rather than protection. If so, an integrin defect would not promote bone loss, which is in line with the lack of bone loss in our integrin-deficient mice.
In contrast to the proinflammatory activities of the integrins, soluble P-selectin is anti-inflammatory in vitro and P-selectin deficiency leads to a worsening of some experimental inflammatory diseases (20). ICAM can also be anti-inflammatory. Human rheumatoid arthritis patients have been helped by anti-ICAM antibodies (13), and the improvement was associated with decreased T-cell activity (7), which is in line with our previous finding that CD4+-T-cell deficiencies decrease alveolar bone loss (4).
These results, in addition to our findings, are most consistent with a protective role for some adhesion molecules through their regulation of innate immune mechanisms. The amplification of such innate protective immune responses without inducing destructive adaptive immunity may be a useful way to prevent periodontal disease.
ACKNOWLEDGMENTS
We thank Teresa Hopkins and Tom Sproule for their contributions to this project.
This work was supported by Public Health Service grants R29 DE10728 (to P.J.B.) and R01 AI24544 (to D.C.R.) from the National Institutes of Health and by a grant to Bates College from the Howard Hughes Medical Institute.
REFERENCES
- 1.Anderson D C, Miller L J, Schmalstieg F C, Rothlein R, Springer T A. Contributions of the Mac-1 glycoprotein family to adherence dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- 2.Baker P J, Evans R T, Roopenian D C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Baker P J, Carter S, Dixon M, Evans R T, Roopenian D C. Serum antibody response to oral infection precedes but does not prevent Porphyromonas gingivalis-induced alveolar bone loss in mice. Oral Microbiol Immunol. 1999;14:194–196. doi: 10.1034/j.1399-302x.1999.140309.x. [DOI] [PubMed] [Google Scholar]
- 4.Baker P J, Evans R T, Roopenian D C. CD4+ T cells and the proinflammatory cytokines interferon gamma and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolini D R, Nedwin T, Bringman T, Smith D, Mundy G R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1985;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 6.Bullard D C, Scharffetter-Kochanek K, McArthur M J, Chosay J G, McBride M, Montgomery C A, Beaudet A L. A polygenic mouse model of psoriasiform skin disease in CD18-deficient mice. Proc Natl Acad Sci USA. 1996;93:2116–2121. doi: 10.1073/pnas.93.5.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis L S, Kavanaugh A F, Nichols L A, Lipsky P E. Induction of persistent T-cell hyporesponsiveness in vivo by monoclonal antibody to ICAM in patients with rheumatoid arthritis. J Immunol. 1995;154:3235–3537. [PubMed] [Google Scholar]
- 8.Ebersole J L, Taubman M A, Smith D J, Frey D E, Haffajee A D, Socransky S S. Human serum antibody responses to oral microorganisms. IV. Correlation with homologous infection. Oral Microbiol Immunol. 1987;12:53–59. doi: 10.1111/j.1399-302x.1987.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 9.Genco R J. Host responses in periodontal diseases: current concepts. J Periodontol. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 10.Genco R J, van Dyke T E, Levine M J, Nelson R D, Wilson M E. Molecular factors influencing neutrophil defects in periodontal disease. J Dent Res. 1986;65:1379–1391. doi: 10.1177/00220345860650120201. [DOI] [PubMed] [Google Scholar]
- 11.Hanazawa S, Murakami Y, Hirose K, Anamo S, Ohmori Y, Higuchi H, Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991;59:1972–1979. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janeway C A, Travers P, Walport M, Capra J D. Immunobiology: the immune system in health and disease. New York, N.Y: Garland Publishing; 1999. [Google Scholar]
- 13.Kavanaugh A F, Davis L S, Nichols L A, Norris S H, Rothlein R, Scharschmidt L A, Lipsky P E. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule-1. Arthritis Rheum. 1994;37:992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]
- 14.King P D, Sandberg E T, Selvakumar A, Fang P, Beaudet A L, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1995;154:6080–6093. [PubMed] [Google Scholar]
- 15.Klausen B, Evans R T, Sfintescu C. Two complementary methods of assessing periodontal bone level in rats. Scand J Dent Res. 1989;97:494–499. doi: 10.1111/j.1600-0722.1989.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 16.Kornman K S, Crane A, Wang H-Y, di Giovine F S, Newman M G, Pirk F W, Wilson T G, Higginbottom F L, Duffy G W. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 17.Mizgerd J P, Quinlan W M, LeBlanc B W, Kutkoski G J, Bullard D C, Beaudet A L, Doerschuk C M. Combinatorial requirements for adhesion molecules in mediating neutrophil emigration during bacterial peritonitis in mice. J Leukoc Biol. 1998;64:291–297. doi: 10.1002/jlb.64.3.291. [DOI] [PubMed] [Google Scholar]
- 18.Okuda K, Takazoe I. The role of Bacteroides gingivalis in periodontal disease. Adv Dent Res. 1988;2:260–268. doi: 10.1177/08959374880020021001. [DOI] [PubMed] [Google Scholar]
- 19.Page R C, Schroeder H E. Periodontitis in man and other animals: a comparative review. S. Basel, Switzerland: Karger; 1982. [Google Scholar]
- 20.Rosenkranz A R, Mendrick D L, Cotran R S, Mayadas T N. P-selectin deficiency exacerbates experimental glomerulonephritis: a protective role for endothelial P-selectin in inflammation. J Clin Investig. 1999;103:649–659. doi: 10.1172/JCI5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sligh J E, Ballantyne C M, Rich S S, Hawkins H K, Smith C W, Bradley A, Beaudet A L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki J B. Diagnosis and classification of the periodontal diseases. Den Clin North Am. 1988;32:195–216. [PubMed] [Google Scholar]
- 24.Takeshita A, Murakami Y, Yamashita Y, Ishida M, Fujisawa S, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signalling. Infect Immun. 1998;66:4056–4060. doi: 10.1128/iai.66.9.4056-4060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Winkelhoff A J, van Steenbergen T J M, de Graaff J. Occurrence and association with disease. In: Shah H N, Mayrand D, Genco R J, editors. Biology of the species Porphyromonas gingivalis. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 33–42. [Google Scholar]
- 26.Waldrop T C, Anderson D C, Hallmon W W, Schmalsteig F C, Jacobs R L. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathological and molecular characteristics. J Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 27.Williams R C. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 28.Wilson R W, Ballantyne C M, Smith C W, Montgomery C, Bradley A, O'Brien W E, Beaudet A L. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]