Abstract
Introduction
Although messenger RNA (mRNA) vaccines have been developed and widely utilized to mitigate the coronavirus disease (COVID-19) pandemic, it is essential to describe the adverse events (AEs) following immunization. This study aimed to identify the patterns associated with serious AE reports after mRNA COVID-19 vaccination in the World Health Organization (WHO)’s global scale database (VigiBase).
Methods
This study performed a latent class analysis (LCA) of reports of serious AEs following mRNA COVID-19 vaccination from VigiBase between December 28, 2020 , and February 28, 2022 (N = 312878). The Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC) terms were selected for LCA. The reporting characteristics in accordance with the cluster were described. We used a multinomial logistic regression model to estimate the association between potential factors and each cluster.
Results
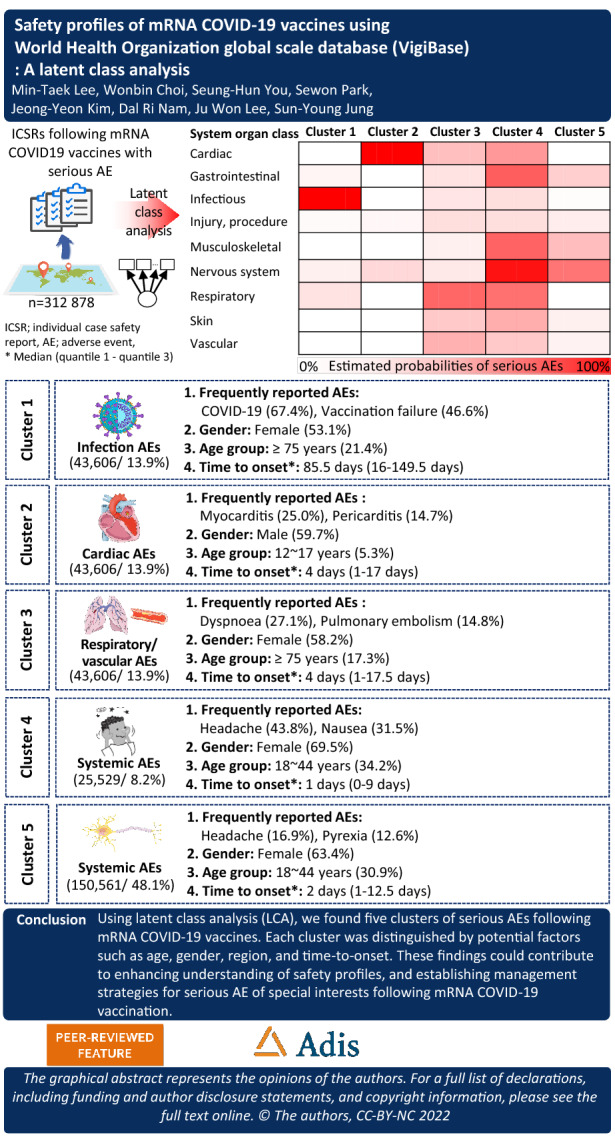
Five clusters of AE reports were distinguished through LCA: infection AEs (cluster 1), cardiac AEs (cluster 2), respiratory/thrombotic AEs (cluster 3), systemic AEs (cluster 4), and nervous system AEs (cluster 5). Compared to cluster 4, cluster 2 had a higher proportion of males (OR 2.98; 95% confidence interval (CI) 2.87–3.09), and cluster 1 had a longer time to onset than other AEs (≥ 14 days) (OR 16.2; 95% CI 15.5–16.9).
Conclusion
Using LCA, we found five clusters of serious AEs following mRNA COVID-19 vaccination. Each cluster was distinguished by potential factors such as age, gender, region, and time to onset. We suggest that monitoring should carefully consider the patterns of young males with cardiac AEs and elderly individuals with thrombosis after respiratory AEs. Our findings could contribute to enhancing understanding of safety profiles and establishing management strategies for serious AEs of special interest following mRNA COVID-19 vaccination.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00742-5.
Keywords: COVID-19, mRNA vaccine, Serious adverse event, VigiBase, Latent class analysis, Cluster
Key Summary Points
| As of November 2022, approximately 12.8 billion cumulative vaccine doses have been administered globally; this administration of COVID-19 vaccines might be expanded in the future because of variants and booster doses. |
| Thus far, myocarditis, anaphylaxis, Bell's palsy, and Guillain-Barré syndrome have been reported as AEs of mRNA COVID-19 vaccines; however, the factors associated with unknown serious adverse events (AEs) following mRNA COVID-19 vaccines have not been established. |
| Five clusters of AE reports were distinguished on LCA: infection AEs (cluster 1), cardiac AEs (cluster 2), respiratory/thrombotic AEs (cluster 3), systemic AEs (cluster 4), and nervous system AEs (cluster 5). Cardiac AEs showed a higher proportion of males and infection AEs a longer time to onset than other AEs (≥ 14 days). |
| Each cluster was distinguished by potential factors such as age group, gender, region, and time interval between vaccination date and start of reaction/event; thus, our findings could contribute to enhancing understanding of safety profiles and establishing management strategies for serious AEs of special interest following mRNA COVID-19 vaccination. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.21618561.
Introduction
Messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccines have been rapidly developed and widely utilized owing to organizations and government funding and technological collaborations in response to the COVID-19 pandemic [1]. As of November 2022, approximately 12.8 billion cumulative vaccine doses have been administered globally [2]. The administration of COVID-19 vaccines might also be expanded in the future because of variants and booster doses [3].
Authorized mRNA COVID-19 vaccines have introduced a new technology platform. Although the products have undergone clinical trials, limitations remain regarding their safety information, such as restricted trial participants who meet inclusion and exclusion criteria, short study periods, and the detection of rare events, particularly serious adverse events following immunizations (AEFIs) [4]. To overcome the limited evidence regarding the safety of COVID-19 vaccines, the vaccines are being evaluated by each country's safety system such as the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) in the USA. Vaccine manufacturers are required to submit safety update reports routinely to the national regulatory authorities [1]. Thus far, mild to moderate local or systemic AEFIs such as myocarditis [5], anaphylaxis [6], Bell's palsy [7], and Guillain-Barré syndrome (GBS) [8] have been reported. However, factors that are associated with unknown adverse events (AEs) following mRNA COVID-19 vaccination, such as age, gender, and existing diseases or high-risk subgroups, have not been well established [9–11]. The identification of patterns and factors can provide insights into preventive measures and management strategies concerning unknown AEFIs [12].
We aimed to (1) identify the patterns of serious AEFI profiles after COVID-19 vaccination within the VigiBase, the World Health Organization (WHO)’s international database of suspected adverse drug reactions, and (2) describe potential factors to identify subgroups in mRNA COVID-19 vaccine reports with similar serious AEFI profiles. To achieve these aims, we applied latent class analysis (LCA), a statistical method used to identify the relationships among a set of unobserved dichotomous or polytomous variables that can be viewed as indicators [12]. This study was conducted in accordance with the Helsinki Declaration. The study protocol was approved for exemption from review by the Institutional Review Board of Chung-Ang University (IRB number: 1041078-201903-HR-071-01), because this study analyzed a secondary database. Informed consent from subjects was waived due to the database containing anonymized data that cannot identify study subjects.
Methods
Data Source
This study used VigiBase, the WHO global database of reported potential side effects of medicinal products, which was developed and is maintained by Uppsala Monitoring Centre, between December 1, 2020, and February 28, 2022. It is the largest database of its kind in the world and is continuously updated with incoming reports, with over 30 million reports of suspected adverse effects of medicines submitted since 1968 [13]. The individual case safety reports (ICSRs) in VigiBase come from regulatory and voluntary sources containing information related to the case reports, including patient demographics, reported drugs, and AEs [14]. The VigiBase database, in addition to the ICSR database, is linked to MedDRA for AEs and the WHO Drug Dictionary (WHODrug). We used the ICSRs that included any mRNA COVID-19 vaccines as suspected drugs and serious AEFIs. A serious AE is considered to be one of the following: death, a life-threatening condition, hospitalization or prolongation of hospitalization, and chronic damage/disability. The AEFIs were coded based on the System Organ Class (SOC) and Preferred Term (PT) of the Medical Dictionary for Regulatory Activities (MedDRA) version 25.0 March 2022. Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) in terms of chemical subgroup (fourth level) and substance name.
Study Variables
Our study identified baseline reporting characteristics such as the date the reports were received, report type, gender, age group, region, notifier type, and seriousness. For mRNA COVID-19 vaccines, we included tozinameran (BNT162b2) and elasomeran (mRNA-1273). The outcome of an AE at the time of the report was categorized as unknown, recovered/resolved, recovering/resolving, not recovered/not resolved with sequelae, fatal, death–reaction may be contributory, and death–unrelated to reaction. Time to onset (TTO) is the time interval between the vaccine administration date and the start of the reaction/event (reaction start date). An ICSR might have several different outcomes and TTOs because it may contain multiple AEFIs. We selected ICSRs with a lower level of resolution of outcomes for classification [15]. For instance, if an ICSR had both a general disorder with recovery and a nervous system disorder with no recovery, we selected “not recovered” as a representative outcome. Furthermore, we regarded TTOs as outliers if they exceeded the study period. We excluded reports received with missing values of TTO and erroneous values of AEs and drugs such as “0” coded for MedDRA or drugs. We utilized vigiGrade completeness score to identify well-documented reports in VigiBase [16]. The ten dimensions that were accounted for in vigiGrade and penalized with clinical relevance in VigiBase were TTO, indication for treatment, outcome, gender, age, dose, country, notified type, report type, and free-text comments. Based on the available information in VigiBase, we estimated the completeness score considering nine dimensions excluding the free-text variable. To obtain the overall vigiGrade completeness score, we applied the average score for every reported drug–AE pair [16].
Statistical Analysis
We used LCA to identify clusters of mRNA COVID-19 vaccine reports with similar serious AEFI profiles. LCA creates latent classes based on underlying patterns that cannot be directly observed [17]. Whereas traditional cluster analysis uses the ad hoc definition of distance to form clusters, LCA is a model-based clustering method based on maximum likelihood estimation. In LCA, various goodness-of-fit diagnostics are applied to identify the optimal number of subgroups [18–20]. In our dataset, the parameters selected for a LCA indicators were MedDRA SOCs included in each ICSR [21]. The model parameters estimated were the probability of a report belonging to a specific cluster and the conditional probabilities of each SOC term given to the cluster using maximum likelihood estimation [21]. The objective of LCA is to find the smallest number of clusters that best describes the distinct characteristics. We determined the number of optimal clusters using several statistical methods and model interpretability [18]. As statistical criteria, we considered likelihood-ratio G2 statistic, Akaike's information criterion (AIC), and Bayesian information criterion (BIC) [18]. To assess model interpretability, we considered the following conditions: (1) each cluster should be distinguishable from the other clusters, (2) no cluster should be a near-zero probability, and (3) a meaningful label should be able to be assigned to each cluster [18]. We applied LCA to the most reported AEs following mRNA COVID-19 vaccination: cardiac disorders, gastrointestinal disorders, infections and infestations, injury, poisoning and procedural complications, musculoskeletal and connective tissue disorders, nervous system disorders, respiratory, thoracic and mediastinal disorders, skin and subcutaneous tissue disorders, and vascular disorders.
The difference in each latent cluster was compared using a chi-square test for categorical variables and analysis of variance (ANOVA) test for continuous variables. We also conducted multinomial logistic regression to identify the association between potential factors and the five clusters of AEs.
The database construction was carried out using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA), and LCA was performed using R-4.0.2 with poLCA package (version 1.4.1).
Results
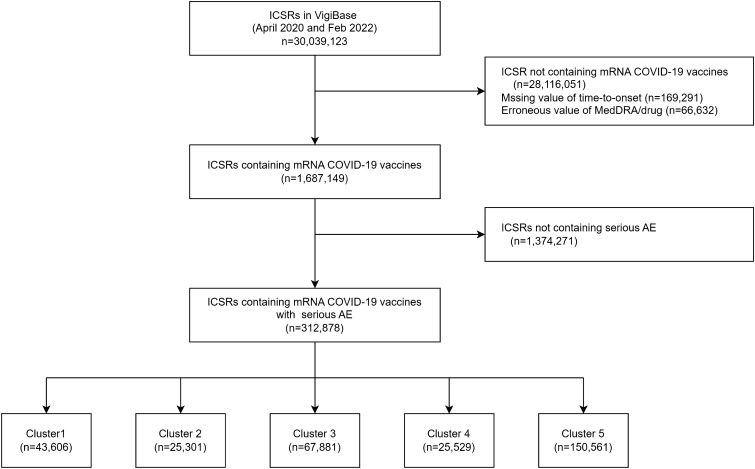
A total of 312,878 spontaneous reports on AEs after mRNA COVID-19 vaccination were identified between December 28, 2020, and February 28, 2022, in VigiBase (Fig. 1). The most frequently reported AEs in terms of SOC level were nervous system disorders (37.9%) and respiratory, thoracic, and mediastinal disorders (19.1%) (Table 1).
Fig. 1.
Flowchart of extraction of serious AE reports after mRNA COVID-19 vaccination from VigiBase. mRNA COVID-19 the messenger RNA coronavirus disease 2019, AE adverse event
Table 1.
Estimated probabilities of serious adverse events based on mRNA COVID-19 vaccine classified system organ class terms in VigiBase
| SOC | Total | Cluster 1 N = 43,606 |
Cluster 2 N = 25,301 |
Cluster 3 N = 67,881 |
Cluster 4 N = 25,529 |
Cluster 5 N = 150,561 |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Card | 52,149 (16.7) | 0 (0) | 25,301 (100) | 16,757 (24.7) | 10,091 (39.5) | 0 (0) |
| Gastr | 53,049 (17.0) | 1466 (3.4) | 0 (0) | 7218 (10.6) | 15,933 (62.4) | 28,432 (18.9) |
| Infec | 59,334 (19.0) | 43,606 (100) | 0 (0) | 8687 (12.8) | 6770 (26.5) | 271 (0.2) |
| Inj&P | 18,550 (5.9) | 857 (2.0) | 694 (2.7) | 3454 (5.1) | 3247 (12.7) | 10,298 (6.8) |
| Musc | 59,266 (18.9) | 0 (0) | 0 (0) | 5939 (8.8) | 15,340 (60.1) | 37,987 (25.2) |
| Nerv | 118,530 (37.9) | 2457 (5.6) | 3620 (14.3) | 8130 (12) | 23,634 (92.6) | 80,689 (53.6) |
| Resp | 59,644 (19.1) | 4651 (10.7) | 0 (0) | 40,699 (60.0) | 14,294 (56.0) | 0 (0) |
| Skin | 29,753 (9.5) | 0 (0) | 0 (0) | 13,961 (20.6) | 8029 (31.5) | 7763 (5.2) |
| Vasc | 32,587 (10.4) | 0 (0) | 0 (0) | 20,512 (30.2) | 5267 (20.6) | 6808 (4.5) |
COVID-19 coronavirus disease 2019, SOC system organ class, card cardiac disorders, gastr gastrointestinal disorders, infec infections and infestations, inj&p injury, poisoning, and procedural complications, metab metabolism and nutrition disorders, musc musculoskeletal and connective tissue disorders, nerv nervous system disorders, resp respiratory, thoracic and mediastinal disorders, skin skin and subcutaneous tissue disorders, vasc vascular disorders
Using the LCA, we identified five clusters of groups with similar AEFI profiles after mRNA COVID-19 administration. Although the six-cluster solution had lower AIC, BIC, and G2-statistic scores than the five-cluster solution, the difference between evaluation tool scores for the two solutions was very small. Specifically, the five-cluster solution presented lower AIC, BIC, and G2 fit statistics than the four-cluster solution. Therefore, the five-cluster solution was selected as the optimal solution in our study (Table S1).
Table 1 shows the estimated probability for each LCA indicator. Item-response probabilities were divided into the following five clusters: cluster 1: infection AEs (43,606 reports, 13.9%); cluster 2: cardiac AEs (25,301 reports, 8.1%); cluster 3: respiratory/thrombotic AEs (67,881 reports, 21.7%); cluster 4: systemic AEs (25,529 reports, 8.2%); cluster 5: nervous system AEs (150,561 reports, 48.1%). All of the clusters were reported alongside general disorders and administration site conditions (cluster 1: 66.3%, cluster 2: 29.7%, cluster 3: 44.5%, cluster 4: 81.1%, and cluster 5: 48.0%) (Table S2); the most frequently reported PTs were pyrexia (11.8%) and fatigue (10.6%) (Table 2).
Table 2.
The ten most common PTs for serious AEs after mRNA COVID-19 vaccination
| No | Total N = 312,878 |
Cluster 1 N = 43,606 |
Cluster 2 N = 25,301 |
Cluster 3 N = 67,881 |
Cluster 4 N = 25,529 |
Cluster 5 N = 150,561 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | n (%) | PT | n (%) | PT | n (%) | PT | n (%) | PT | n (%) | PT | n (%) | |
| 1 | Headache | 40,277 (12.9) | COVID-19 | 29,406 (67.4) | Myocarditis | 6327 (25.0) | Dyspnoea | 18,397 (27.1) | Headache | 11,188 (43.8) | Headache | 25,427 (16.9) |
| 2 | Pyrexia* | 37,064 (11.8) | Vaccination failure | 20,338 (46.6) | Pericarditis | 3719 (14.7) | Pulmonary embolism | 10,074 (14.8) | Nausea | 8040 (31.5) | Pyrexia | 18,904 (12.6) |
| 3 | COVID-19 | 34,339 (11.0) | Herpes zoster | 2541 (5.8) | Chest pain | 3021 (11.9) | Pyrexia | 7254 (10.7) | Fatigue | 7920 (31.0) | Fatigue | 17,716 (11.8) |
| 4 | Fatigue* | 33,124 (10.6) | Pyrexia | 2432 (5.6) | Palpitations | 2068 (8.2) | Chest pain | 6520 (9.6) | Dyspnoea | 7670 (30.0) | Myalgia | 13,373 (8.9) |
| 5 | Dyspnoea | 28,120 (9.0) | COVID-19 pneumonia | 2273 (5.2) | Myocardial infarction | 2002 (7.9) | Fatigue | 5431 (8.0) | Pyrexia | 7254 (28.4) | Nausea | 13,018 (8.6) |
| 6 | Nausea | 23,418 (7.5) | Dyspnoea | 2053 (4.7) | Atrial fibrillation | 1942 (7.7) | Cough | 5023 (7.4) | Dizziness | 6563 (25.7) | Arthralgia | 11,086 (7.4) |
| 7 | Vaccination failure* | 22,550 (7.2) | Drug ineffective | 2051 (4.7) | Tachycardia | 1754 (6.9) | Deep vein thrombosis | 4562 (6.7) | Myalgia | 5504 (21.6) | Dizziness | 11,050 (7.3) |
| 8 | Dizziness | 20,436 (6.5) | Pneumonia | 1837 (4.2) | Arrhythmia | 1727 (6.8) | Hypertension | 3387 (5.0) | Chills | 4496 (17.6) | Chills | 10,247 (6.8) |
| 9 | Myalgia | 20,359 (6.5) | Death | 1445 (3.3) | Acute myocardial infarction | 1228 (4.9) | Asthenia | 3279 (4.8) | Arthralgia | 4204 (16.5) | Pain in extremity | 9465 (6.3) |
| 10 | Chills* | 17,999 (5.8) | Cough | 1388 (3.2) | Pyrexia | 1220 (4.8) | COVID-19 | 3180 (4.7) | Malaise | 3882 (15.2) | Malaise | 8797 (5.8) |
PT preferred term, AE adverse event, COVID-19 coronavirus disease 2019, cluster 1 infection AEs, cluster 2 cardiac AEs, cluster 3 respiratory AEs, cluster 4 systemic AEs, cluster 5 nervous system AEs
*General disorders and administration site conditions
Table 3 shows the characteristics of all the five clusters. All the clusters contained a significant proportion of the number of tozinameran (76.8%) and spontaneous reports (99.1%) with a completeness score > 0.8.
Table 3.
Characteristics of serious AE clusters after mRNA COVID-19 vaccination
| Total | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | p value | |
|---|---|---|---|---|---|---|---|
| N = 312,878 | N = 43,606 | N = 25,301 | N = 67,881 | N = 25,529 | N = 150,561 | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Quarter received | < 0.001 | ||||||
| 2020.4Q* | 13 (0) | 0 (0.0.) | 0 (0.0.) | 8 (0.0) | 3 (0.0) | 2 (0.0) | |
| 2021.1Q | 20,213 (6.5) | 1409 (3.2) | 997 (3.9) | 4814 (7.1) | 1873 (7.3) | 11,120 (7.4) | |
| 2021.2Q | 48,693 (15.6) | 4139 (9.5) | 3163 (12.5) | 12,349 (18.2) | 3598 (14.1) | 25,444 (16.9) | |
| 2021.3Q | 104,809 (33.5) | 10,380 (23.8) | 9010 (35.6) | 23,929 (35.3) | 8803 (34.5) | 52,687 (35) | |
| 2021.4Q | 106,720 (34.1) | 20,472 (47.0) | 9370 (37) | 20,675 (30.5) | 8630 (33.8) | 47,573 (31.6) | |
| 2022.1Q** | 32,430 (10.4) | 7206 (16.5) | 2761 (10.9) | 6106 (9) | 2622 (10.3) | 13,735 (9.1) | |
| Report type | < 0.001 | ||||||
| Spontaneous | 310,175 (99.1) | 43,481 (99.7) | 25,207 (99.6) | 67,445 (99.4) | 25,306 (99.1) | 148,736 (98.8) | |
| Report from study | 2625 (0.8) | 121 (0.3) | 87 (0.3) | 415 (0.6) | 218 (0.9) | 1784 (1.2) | |
| Other | 70 (0.0) | 4 (0.0) | 7 (0.0) | 19 (0.0) | 5 (0.0) | 35 (0.0) | |
| Unknown | 8 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 6 (0.0) | |
| Gender | < 0.001 | ||||||
| Male | 124,849 (39.9) | 20,281 (46.5) | 15,093 (59.7) | 27,995 (41.2) | 7653 (30.0) | 53,827 (35.8) | |
| Female | 185,905 (59.4) | 23,140 (53.1) | 10,092 (39.9) | 39,501 (58.2) | 17,738 (69.5) | 95,434 (63.4) | |
| Unknown | 2124 (0.7) | 185 (0.4) | 116 (0.5) | 385 (0.6) | 138 (0.5) | 1300 (0.9) | |
| Age groups | < 0.001 | ||||||
| 0–27 days | 62 (0.0) | 2 (0.0) | 3 (0.0) | 16 (0.0) | 2 (0.0) | 39 (0.0) | |
| 28 days–23 months | 138 (0.0) | 3 (0.0) | 4 (0.0) | 27 (0.0) | 8 (0.0) | 96 (0.1) | |
| 2–11 years | 309 (0.1) | 21 (0.1) | 21 (0.1) | 72 (0.1) | 33 (0.1) | 162 (0.1) | |
| 12–17 years | 6920 (2.2) | 685 (1.6) | 1328 (5.3) | 1439 (2.1) | 567 (2.2) | 2901 (1.9) | |
| 18–44 years | 91,419 (29.2) | 11,405 (26.2) | 8474 (33.5) | 16,264 (24) | 8721 (34.2) | 46,555 (30.9) | |
| 45–64 years | 80,918 (25.9) | 11,403 (26.2) | 5346 (21.1) | 17,186 (25.3) | 7175 (28.1) | 39,808 (26.4) | |
| 65–74 years | 33,253 (10.6) | 5718 (13.1) | 2346 (9.3) | 8331 (12.3) | 2155 (8.4) | 14,703 (9.8) | |
| ≥ 75 years | 45,738 (14.6) | 9336 (21.4) | 2967 (11.7) | 11,728 (17.3) | 2144 (8.4) | 19,563 (13.0) | |
| Unknown | 54,121 (17.3) | 5033 (11.5) | 4812 (19.0) | 12,818 (18.9) | 4724 (18.5) | 26,734 (17.8) | |
| Type of mRNA vaccine | < 0.001 | ||||||
| Tozinameran | 240,330 (76.8) | 35,477 (81.4) | 19,984 (79.0) | 53,422 (78.7) | 19,146 (75.0) | 112,301 (74.6) | |
| Elasomeran | 72,446 (23.2) | 8117 (18.6) | 5311 (21.0) | 14,441 (21.3) | 6376 (25.0) | 38,201 (25.4) | |
| Both | 102 (0.0) | 12 (0.0) | 6 (0.0) | 18 (0.0) | 7 (0.0) | 59 (0.0) | |
| Seriousness | < 0.001 | ||||||
| Death | 25,157 (8.0) | 3785 (8.7) | 2849 (11.3) | 6179 (9.1) | 1487 (5.8) | 10,857 (7.2) | |
| Life threatening | 31,049 (9.9) | 1530 (3.5) | 3829 (15.1) | 11,116 (16.4) | 3439 (13.5) | 11,135 (7.4) | |
| Caused/prolonged hospitalization | 125,205 (40.0) | 14,716 (33.8) | 13,764 (54.4) | 31,013 (45.7) | 11,283 (44.2) | 54,429 (36.2) | |
| Disabling/incapacitating | 39,642 (12.7) | 1004 (2.3) | 732 (2.9) | 3908 (5.8) | 4292 (16.8) | 29,706 (19.7) | |
| Congenital anomaly/birth defect | 122 (0.0) | 2 (0.0) | 2 (0.0) | 13 (0.0) | 4 (0.0) | 101 (0.1) | |
| Other | 91,298 (29.2) | 22,564 (51.8) | 4111 (16.3) | 15,613 (23) | 5003 (19.6) | 44,007 (29.2) | |
| Unknown | 405 (0.1) | 5 (0) | 14 (0.1) | 39 (0.1) | 21 (0.1) | 326 (0.2) | |
| Region | < 0.001 | ||||||
| African | 1715 (0.5) | 103 (0.2) | 49 (0.2) | 304 (0.5) | 72 (0.28) | 1187 (0.8) | |
| Americas | 149,296 (47.7) | 16,115 (37) | 12,585 (49.7) | 36,190 (53.3) | 15,837 (62.0) | 68,569 (45.5) | |
| Southeast Asia | 30 (0) | 0 (0) | 5 (0) | 9 (0) | 1 (0) | 15 (0) | |
| European | 154,611 (49.4) | 27,080 (62.1) | 11,805 (46.7) | 29,692 (43.7) | 9008 (35.29) | 77,026 (51.2) | |
| Eastern Mediterranean | 468 (0.1) | 25 (0.1) | 52 (0.2) | 144 (0.2) | 29 (0.11) | 218 (0.1) | |
| Western Pacific | 6758 (2.2) | 283 (0.7) | 805 (3.2) | 1542 (2.3) | 582 (2.28) | 3546 (2.4) | |
| Notifier type | < 0.001 | ||||||
| Physician | 64,617 (20.7) | 20,008 (45.9) | 5428 (21.5) | 13,542 (20) | 1965 (7.7) | 23,674 (15.7) | |
| Pharmacist | 7545 (2.4) | 649 (1.5) | 613 (2.4) | 1772 (2.6) | 400 (1.57) | 4111 (2.7) | |
| Other health professional | 10,114 (3.2) | 986 (2.3) | 435 (1.7) | 2156 (3.2) | 800 (3.13) | 5737 (3.8) | |
| Lawyer | 87 (0) | 2 (0) | 16 (0.1) | 23 (0) | 6 (0.02) | 40 (0) | |
| Consumer | 82,243 (26.3) | 5975 (13.7) | 6081 (24) | 14,438 (21.3) | 6626 (25.95) | 49,123 (32.6) | |
| Unknown | 148,272 (47.4) | 15,986 (36.7) | 12,728 (50.3) | 35,950 (53.0) | 15,732 (61.6) | 67,876 (45.1) | |
| Completeness score mean (SD) | 8.3 (0.4) | 8.3 (0.3) | 8.3 (0.4) | 8.2 (0.4) | 8.2 (0.3) | 8.3 (0.4) | < 0.001 |
| Number of drugs | < 0.001 | ||||||
| Mean (SD) | 1.5 (1.5) | 1.7 (1.3) | 1.3 (1.3) | 1.5 (1.6) | 1.4 (1.4) | 1.5 (1.5) | |
| Median (Q1–Q3) | 1 (1–1) | 2 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | |
| Number of AEs | < 0.001 | ||||||
| Mean (SD) | 4.8 (5.7) | 3.1 (3.7) | 3.3 (4.0) | 5.4 (6.2) | 12.3 (9.6) | 3.9 (4.1) | |
| Median (Q1–Q3) | 3 (2–6) | 2 (2–3) | 2 (1–4) | 4 (2–7) | 10 (7–14) | 3 (1–5) | |
| TTO of AEs for each report§ | < 0.001 | ||||||
| Mean (SD) | 26.9 (53.0) | 93.4 (78.1) | 15.3 (29.6) | 21.5 (46.8) | 17.4 (46.2) | 13.7 (32.5) | |
| Median (Q1–Q3) | 4 (1–22) | 85.5 (16–149.5) | 4 (1–17) | 4 (1–17.5) | 1 (0–9) | 2 (1–12.5) | |
AE adverse event, mRNA COVID-19 messenger RNA coronavirus disease 2019, AE adverse event, SD standard deviation, TTO time to onset, cluster 1 infection AEs, cluster 2 cardiac AEs, cluster 3 respiratory AEs, cluster 4 systemic AEs, cluster 5 nervous system AEs
§Number of reports considered; class 1 (43,576 reports), class 2 (25,275 reports), class 3 (67,815 reports), class 4 (25,505 reports), class 5 (150,421 reports)
*December 2020
**January 2022
In cluster 1 (infection AEs), COVID-19 infection (67.4%) and vaccination failure (46.6%) were more likely to be included (Table 2). This cluster had the highest proportion of reports received by physicians (45.9%) and from Europe (62.1%). It had a median TTO of 85.5 (interquartile range 16–149.5) days for each report. Cluster 2 (cardiac AEs) included myocarditis (25.0%) and pericarditis (14.7%) (Table 2). Most of the reports received included males (59.7%), an age of < 18 years (5.4%), and death (11.3%). Cluster 3 (respiratory/thrombotic AEs) was distinguished from other classes in that it was composed of AEFIs of both dyspnea (27.1%) and pulmonary embolism (14.8%). It included death (9.1%), life-threatening AEs (16.4%), and AEs that caused/prolonged hospitalization (45.7%). Patients in 17.3% of AE cases in cluster 3 were ≥ 75 years old. Cluster 4 (systemic AEs) included headache (43.8%) and nausea (31.5%). Most reports included females (69.5%), and the patients were often from the Americas (62.0%). The median TTO was 1 (interquartile range 0–9) day for each report. Cluster 5 (nervous system AEs) was the largest of the five clusters (48.1%). It differed from the other clusters in that it included a high proportion of facial paralysis (3301 reports, 85.7%) and Guillain-Barré syndrome (1494 reports, 82.6%; Table S3).
Table 4 shows the results of multinomial logistic regression. We selected cluster 4 (systemic AEs) as our reference group because the AE pattern of cluster 4 was consistent with AEs from a prior study [6]. The odds ratio (OR) (95% confidence interval [CI]) of males in cluster 2 (cardiac AEs) was 2.98 (95% CI 2.87–3.09), which was the highest estimate among clusters. A significant association was observed between cluster 5 (nervous system AEs) and congenital anomaly/birth defects (OR: 4.24; 95% CI 1.56–11.5). The ORs (95% CI) of notifier type reported by physicians were as follows: cluster 1: 6.67 (95% CI 6.27–7.09), cluster 2: 2.27 (95% CI 2.13–2.42), cluster 3: 2.46 (95% CI 2.36–2.60), and cluster 5: 1.60 (95% CI 1.51–1.68). Cluster 1 showed a strong association with TTO ≥ 14 days (OR: 16.2; 95% CI 15.5–16.9).
Table 4.
Results of multinomial logistic regression on clusters with similar serious AE profile following mRNA COVID-19 vaccination compared to cluster 4 (systemic AEs)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 5 | ||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Gender (ref: female) | |||||
| Male | 1.78 (1.72–1.84) | 2.98 (2.87–3.09) | 1.47 (1.43–1.52) | 1.35 (1.31–1.39) | |
| Age groups (ref: 0–17 years) | |||||
| 18–44 years | 1.08 (0.96–1.22) | 0.65 (0.59–0.72) | 0.87 (0.79–0.96) | 0.89 (0.82–0.98) | |
| 45–64 years | 1.20 (1.06–1.36) | 0.44 (0.34–0.49) | 1.05 (0.95–1.16) | 0.94 (0.86–1.03) | |
| ≥65 years | 2.37 (2.09–2.69) | 0.51 (0.46–0.57) | 1.77 (1.60–1.96) | 1.47 (1.34–1.62) | |
| Type of mRNA (ref: tozinameran) | |||||
| Elasomeran | 0.81 (0.77–0.84) | 1.01 (0.97–1.06) | 0.92 (0.89–0.95) | 1.14 (1.10–1.18) | |
| Both | 0.56 (0.21–1.45) | 0.94 (0.31–2.84) | 1.01 (0.42–2.43) | 1.65 (0.75–3.64) | |
| Seriousness (ref: hospitalization) | |||||
| Death | 1.34 (1.25–1.44) | 1.51 (1.40–1.62) | 1.14 (1.07–1.21) | 1.26 (1.18–1.33) | |
| Life threatening | 0.38 (0.35–0.40) | 0.99 (0.94–1.04) | 1.23 (1.18–1.29) | 0.70 (0.67–0.74) | |
| Disabling/incapacitating | 0.37 (0.34–0.39) | 0.19 (0.17–0.21) | 0.43 (0.41–0.45) | 1.66 (1.59–1.72) | |
| Congenital anomaly/birth defect | 0.43 (0.08–2.40) | 0.30 (0.05–1.63) | 1.25 (0.41–3.85) | 4.24 (1.56–11.5) | |
| Other | 5.39 (5.10–5.70) | 0.53 (0.50–0.56) | 1.15 (1.10–1.21) | 1.39 (1.33–1.45) | |
| Region (ref: Americas) | |||||
| African | 1.95 (1.37–2.78) | 1.72 (1.16–2.55) | 2.00 (1.51–2.64) | 2.85 (2.20–3.69) | |
| Southeast Asia | 0.01 (< 0.01- > 99.9) | 5.17 (0.59–45.2) | 3.40 (0.43–27.1) | 2.73 (0.36–20.7) | |
| European | 1.33 (1.14–1.54) | 2.01 (1.75–2.31) | 1.13 (1.02–1.26) | 1.36 (1.23–1.50) | |
| Eastern Mediterranean | 1.14 (0.64–2.02) | 2.92 (1.80–4.74) | 1.83 (1.21–2.77) | 1.29 (0.87–1.93) | |
| Western Pacific | 0.49 (0.42–0.58) | 2.21 (1.93–2.53) | 1.17 (1.05–1.31) | 1.35 (1.21–1.49) | |
| Notifier type**** (ref: consumer) | |||||
| Physician | 6.67 (6.27–7.09) | 2.27 (2.13–2.42) | 2.46 (2.33–2.61) | 1.60 (1.51–1.68) | |
| Pharmacist | 1.63 (1.43–1.87) | 1.40 (1.22–1.60) | 1.73 (1.54–1.94) | 1.42 (1.28–1.58) | |
| Other health professional | 1.60 (1.43–1.78) | 0.62 (0.55–0.71) | 1.20 (1.10–1.31) | 0.99 (0.92–1.08) | |
| Lawyer | 0.30 (0.06–1.53) | 2.45 (0.94–6.39) | 1.67 (0.68–4.15) | 0.92 (0.39–2.18) | |
| TTO of AEs for each report§ (ref: TTO < 3) | |||||
| 3–6 days | 3.39 (3.17–3.62) | 2.49 | (2.36–2.63) | 1.78 (1.69–1.87) | 1.40 (1.34–1.47) |
| 7–13 days | 4.46 (4.17–4.77) | 2.03 | (1.91–2.16) | 1.99 (1.88–2.09) | 1.52 (1.44–1.59) |
| 14 days or longer | 16.2 (15.5–16.9) | 1.80 (1.72–1.88) | 1.67 (1.61–1.73) | 1.26 (1.22–1.30) | |
OR, odds ratio, CI, confidence interval; AE, adverse event; mRNA COVID-19; The messenger RNA coronavirus disease 2019, AE; adverse event, TTO; time-to-onset, cluster 1: infection AEs, cluster 2: cardiac AEs, cluster 3: respiratory AEs, cluster 4: systemic AEs, cluster 5: nervous system AEs, * Unknown value in gender (n): 2,124
** Unknown value in age group (n): 54,121, *** Unknown value in seriousness (n): 405, **** Unknown value in notifier type (n): 148,272, § Number of reports considered: cluster 1 (43,576 reports), cluster 2 (25,275 reports), cluster 3 (67,815 reports), cluster 4 (25,505 reports), cluster 5 (150,421 reports)
Discussion
Our study aimed to identify the patterns of serious AEFI profiles after COVID-19 vaccination with the help of VigiBase. We identified five distinguished safety profiles using LCA and described the potential factors of serious AEFI profiles, which included age, gender, region, and TTO.
We suggest that cluster 1 (infection AEs) might be related to the ineffectiveness of mRNA vaccines. The most common PTs of cluster 1 were COVID-19 infection and vaccination failure. Furthermore, cluster 1 had a median TTO of 85.5 (interquartile range: 16.0–149.5) days, and a longer TTO of AEs (≥ 14 days) (OR 16.2; 95% CI 15.5–16.9) corresponds to the results of a randomized controlled trial [5]. The efficacy of the tozinameran vaccine decreased to 90% at 2–4 months after vaccination [5]. Moreover, as the number of reports in cluster 1 has increased steadily since December 2020, a long-term study of mRNA vaccine ineffectiveness is required [22].
We identified myocarditis and pericarditis following mRNA COVID-19 vaccination in cluster 2 (cardiac AEs). This association was not reported in the trial, and several countries are required to provide continued critical data for vaccine safety monitoring [23–26]. Cluster 2 was mainly characterized by young males and is correlated with the results of previous studies [23, 27]. Given the AE cluster patterns, it is important to note that the majority of reports of death were found in young males.
Clusters 3 and 4 presented similar proportions of respiratory, thoracic, and mediastinal disorders in terms of SOCs (Table 1). However, these two clusters showed differences in the PTs of the most commonly reported type of AEs (Table 2). Cluster 3 showed higher diversity in reported respiratory symptoms compared to cluster 4. In cluster 3, the most common AEs were dyspnea (27.1%), pulmonary embolism (14.8%), and cough (7.4%) in respiratory, thoracic, and mediastinal disorders, while cluster 4 mostly included dyspnea (30.0%). Therefore, we labeled cluster 3 as “respiratory/thrombotic AEs.” Moreover, cluster 3 showed a high proportion of vascular disorders, including pulmonary embolism, deep vein thrombosis, and thrombosis, along with respiratory disorders. Previous case reports have reported thrombotic death immediately after the onset of symptoms of dyspnea [28] and deep vein thrombosis [29] following administration of mRNA COVID-19 vaccines. As a possible mechanism for thromboembolic events, massive platelet activation through platelet factor 4 has been proposed [30]. Prior studies have also reported thrombotic events after the onset of respiratory symptoms, which is consistent with cluster 3, which consisted primarily of patients ≥ 65 years (29.6% of cluster 3) [28, 29]. Thus, physicians need to monitor patients who complain of respiratory symptoms for the possibility of further thrombotic events. Cluster 4 demonstrated a shorter TTO and a higher number of AEs per report than other clusters. This could be due to the most frequent AEs in cluster 4 being headache and nausea, which are well-known acute systemic AEFIs [6]. Therefore, we named cluster 4 as “systemic AEs.” In cluster 5, reports of headache and dizziness without cardiac and respiratory disorders made up a high proportion. We identified 3301 reports of facial paralysis (85.7% of total reports of facial paralysis) and 1494 reports of Guillain-Barré syndrome (82.6% of total reports of facial paralysis), which are rare but serious events necessitating monitoring by regulatory agencies [31–33]. Therefore, we labeled cluster 5 as “nervous system disorder AEs.”
This study is meaningful because we have identified potential factors determining the patterns of serious AEFI profiles after the administration of mRNA COVID-19 vaccines on a global scale using a database containing over 30 million reports. Although LCA has been applied in many domains, only a few studies have been conducted on vaccine safety [21, 34]. In particular, this is the first study to apply LCA to identify the patterns of serious AEs after the administration of mRNA COVID-19 vaccines. Using LCA, clusters of AEFIs could be identified based on underlying patterns that cannot be directly observed. Thus, the obtained evidence on the distinct safety profiles and associated factors using a spontaneous AE database will be helpful for improving the pharmacovigilance practice of safety concerns regarding mRNA COVID-19 vaccines.
This study has several limitations. First, because we used a spontaneous reporting database, the reports are prone to a notoriety bias. The number of spontaneous reports of AEs following COVID-19 vaccination increased rapidly because of either mass media intervention or scientific communications. Considering the differential reporting rate, comparison between COVID-19 vaccines and other vaccines could result in spurious perception and unbalance in disproportionality analysis [35, 36]. Thus, clustering, without other vaccines as the denominator, could be an appropriate method of analysis of COVID-19 vaccination during the COVID-19 pandemic. Second, VigiBase is designed to identify early signs of previously unknown AEs as rapidly as possible using spontaneous reports, so some of its reports are not well documented. To overcome this limitation, we applied a completeness score to identify high spontaneous report quality. The completeness score could contribute to an important causality assessment or the detection of safety signals in accuracy. Our study utilized well-documented reports with > 0.8 completeness, as suggested by WHO [16]. Third, the present results should be interpreted with caution because there are no data on pregnancy, patient comorbidities, and the dose of the COVID-19 vaccine. Fourth, we performed all of the reports without causality assessment. In contrast, some previous studies were conducted with causality assessments for possible or greater AEs. However, VigiBase contains the essential information required for causality assessment as a variable to estimate.
Conclusion
We found five distinguished clusters of serious AEs following mRNA COVID-19 vaccination. ICSRs in each cluster shared similar AE profiles and were distinguished by potential factors such as age, gender, and TTO. Mainly, the number of COVID-19 vaccines administrated due to the pandemic resulted in a massive number of AEs following COVID-19 vaccination, which could bias safety signals. However, using LCA, it will be possible to more efficiently detect the characteristics of reported AEs by period or unexpected AEs. Therefore, our findings are important for enhancing the understanding of the benefits and risks associated with the use of vaccines and could contribute to establishing management strategies for serious AEs of special interest following mRNA COVID-19 vaccination.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This research, and the journal’s Rapid Service Fee, was supported by Government-wide R&D Fund Project for Infectious Disease Research (GFID) by Republic of Korea (grant number: HG18C0066). This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2020.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization, methodology, writing-original draft preparation, Min-Taek Lee and Sun-Young Jung; formal analysis, visualization, Min-Taek Lee; investigation, writing—review and editing, Min-Taek Lee, Wonbin Choi, Seung-Hun You, Sewon Park, Jeong-Yeon Kim, Dal Ri Nam, Ju Won Lee and Sun-Young Jung; supervision, project administration, and funding acquisition, Sun-Young Jung. All authors have read and agreed to the published version of the manuscript.
Disclosures
All named authors confirm that they have no competing interests to declare.
Prior Publication
This research was presented at 38th International Conference for Pharmacoepidemiology (ICPE), Copenhagen, Denmark, August 28, 2022.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Helsinki Declaration. The study protocol was approved for exemption from review by the Institutional Review Board of Chung-Ang University (IRB number: 1041078-201903-HR-071-01), because this study analyzed a secondary database. Informed consent from subjects was waived due to the database containing anonymized data that cannot identify study subjects.
Data Availability
The data presented in this study are available from VigiBase upon formal request to the Uppsala Monitoring Centre at the WHO Collaborating Centre for International Drug Monitoring.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuter BJ, Offit PA, Poland GA. The development of COVID-19 vaccines in the United States: why and how so fast? Vaccine. 2021;39:2491–2495. doi: 10.1016/j.vaccine.2021.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronaviris (COVID-19) Dashboard. Available online: https://covid19.who.int/?mapFilter=vaccinations accessed on 07 Nov 2022.
- 3.McIntyre PB, Aggarwal R, Jani I, et al. COVID-19 vaccine strategies must focus on severe disease and global equity. The Lancet. 2022;399:406–410. doi: 10.1016/S0140-6736(21)02835-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 5.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Li X, Sun M, et al. COVID-19 mRNA vaccines are generally safe in the short term: a vaccine vigilance real-world study says. Front Immunol. 2021;12:669010. doi: 10.3389/fimmu.2021.669010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waheed S, Bayas A, Hindi F, et al. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MS, Peer V, Magid A, et al. Gender DIFFERENCES in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines (Basel) 2022;10:233. doi: 10.3390/vaccines10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis. 2022;75:e630–e644. doi: 10.1093/cid/ciac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslak F, Gunalp A, Cebi MN, et al. Early experience of COVID-19 vaccine-related adverse events among adolescents and young adults with rheumatic diseases: a single-center study. Int J Rheum Dis. 2022;25:353–363. doi: 10.1111/1756-185X.14279. [DOI] [PubMed] [Google Scholar]
- 12.Dey A, Chakraborty A, Majumdar K, et al. Application of latent class analysis to estimate susceptibility to adverse health outcomes based on several risk factors. Int J Community Med Public Health. 2016;3:3423–3429. doi: 10.18203/2394-6040.ijcmph20164268. [DOI] [Google Scholar]
- 13.VigiBase, WHO-UMC. Available online: https://who-umc.org/vigibase/ accessed on 07 Nov 2022.
- 14.Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–419. doi: 10.1177/009286150804200501. [DOI] [Google Scholar]
- 15.di Mauro G, Zinzi A, Scavone C, et al. PCSK9 inhibitors and neurocognitive adverse drug reactions: analysis of individual case safety reports from the eudravigilance database. Drug Saf. 2021;44:337–349. doi: 10.1007/s40264-020-01021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergvall T, Noren GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37:65–77. doi: 10.1007/s40264-013-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCutcheon AL. Latent class analysis. Sage; 1987. [Google Scholar]
- 18.Lanza ST, Collins LM, Lemmon DR, et al. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Model. 2007;14:671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleland CM, Rothschild L, Haslam N. Detecting latent taxa: Monte Carlo comparison of taxometric, mixture model, and clustering procedures. Psychol Rep. 2000;87:37–47. doi: 10.2466/pr0.2000.87.1.37. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan D. The Sage handbook of quantitative methodology for the social sciences. Sage; 2004. [Google Scholar]
- 21.Ward D, Thorsen NM, Frisch M, et al. A cluster analysis of serious adverse event reports after human papillomavirus (HPV) vaccination in Danish girls and young women, September 2009 to August 2017. Euro Surveill. 2019;24:1800380. doi: 10.2807/1560-7917.ES.2019.24.19.1800380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control COVID-19 Situation Dashboard. Available online: https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html accessed on 07 Nov 2022.
- 23.Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021;6:1115–1117. doi: 10.1001/jamacardio.2021.2821. [DOI] [PubMed] [Google Scholar]
- 24.Guidance on myocarditis and pericarditis after mRNA COVID-19. Vaccines. 2021.
- 25.Updated Signal assessment report on Myocarditis, pericarditis with Tozinameran (COVID-19 mRNA vaccine (nucleoside-modified)—COMIRNATY). 2021.
- 26.Guidance on myocarditis and pericarditis after mRNA COVID-19 vaccines in Korea. 2021.
- 27.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roncati L, Manenti A, Corsi L. A three-case series of thrombotic deaths in patients over 50 with comorbidities temporally after modRNA COVID-19 vaccination. Pathogens. 2022;11:435. doi: 10.3390/pathogens11040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carli G, Nichele I, Ruggeri M, et al. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16:803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184–189. doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 31.Renoud L, Khouri C, Revol B, et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization Pharmacovigilance Database. JAMA Intern Med. 2021;181:1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emergency use authorization for an unapproved product review memorandum. Available online: https://www.fda.gov/media/144416/download accessed on 07 Nov 2022.
- 33.Selected adverse events reported after COVID-19 vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html accessed on 07 Nov 2022.
- 34.Chandler RE, Juhlin K, Fransson J, et al. Current safety concerns with human papillomavirus vaccine: a cluster analysis of reports in VigiBase((R)) Drug Saf. 2017;40:81–90. doi: 10.1007/s40264-016-0456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pariente A, Gregoire F, Fourrier-Reglat A, et al. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases the notoriety bias. Drug Saf. 2007;30:891–898. doi: 10.2165/00002018-200730100-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mettler C, Terrier B, Chouchana L. Comment on: Risk of giant cell arteritis and polymyalgia rheumatica following COVID-19 vaccination: a global pharmacovigilance study: reply. Rheumatology (Oxford) 2022;61:e103–e104. doi: 10.1093/rheumatology/keab854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graphical abstract: The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available from VigiBase upon formal request to the Uppsala Monitoring Centre at the WHO Collaborating Centre for International Drug Monitoring.