Abstract
Introduction
Pre-exposure prophylaxis (PrEP) is effective for HIV prevention, but the PrEP care continuum also involves improving PrEP awareness, uptake, adherence, and retention in care. Users’ awareness is often compromised because of vulnerability factors and risk behaviors, such as chemsex practice or specific substance use, which could lead to risk compensation. Correct adherence and retention in care are essential to achieve the full effectiveness of PrEP. This study describes changes in users’ risk behaviors and sexually transmitted infections (STIs), as well also PrEP care continuum details.
Methods
This was a descriptive single-center retrospective study including adults at high HIV risk screened between November 2019 and June 2021 in the PrEP program of our hospital. Demographic, behavioral, STI, adherence, and retention in care variables were assessed. Data were collected from medical records and self-report questionnaires.
Results
A total of 295 people were included, 94% men and 5% transgender women, with a mean age of 34 years (SD 10) and 10% sex workers. At baseline, 55% disclosed chemsex practice and 3% slamming. During follow-up, condom use for anal intercourse decreased from 41% to 13% (p ≤ 0.0001) and one HIV infection was detected; other risk behaviors and STIs remained stable. Chemsex, group sex, fluid exchange, and condomless anal intercourse were related to STI risk. Adherence was correct in 80% of users, and retention in care was 57%. Discontinuations and loss to follow-up were high, mainly affecting transgender women, sex workers, and people practicing fisting.
Conclusion
PrEP program implementation in our hospital was adequate, since it allowed, in a population at high HIV risk, overall users’ risk behaviors and STIs to remain stable, with only one HIV diagnosis during the follow-up. We should target specific strategies to improve adherence and retention in care, as vulnerable subgroups at higher risk of loss to follow-up are identified.
Keywords: PrEP, Retention in care, PrEP care continuum, Chemsex, Risk behaviors, STIs, HIV
Key Summary Points
| Why carry out this study? |
| PrEP was implemented in Hospital Clínic of Barcelona in November 2019 as a comprehensive multidisciplinary strategy to prevent HIV infection and to attend to other individual and behavioral factors that may influence PrEP effectiveness. |
| We hypothesized that our comprehensive strategy with targeted interventions would lead to safer sexual and drug consumption behaviors among users without more sexually transmitted infections (STIs) beyond the prevention of HIV. |
| What was learned from this study? |
| Our PrEP program was adequate since sexual practices and drug use remained stable during the follow-up, with only one HIV diagnosis. Although users continued to engage in risk behaviors, the rate of STIs did not increase. |
| We need to focus on interventions to improve adherence and retention in care as both were poor in our cohort, with high loss to follow-up and discontinuation rates. |
| It is essential to promote comprehensive multidisciplinary targeted interventions in PrEP programs to address factors that may influence HIV acquisition and to provide users with an optimal PrEP care continuum. |
Introduction
Daily oral administration of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC or Truvada®) for pre-exposure prophylaxis (PrEP) is effective in preventing human immunodeficiency virus (HIV) infection among gay, bisexual, and other men who have sex with men (GBMSM) who engage in high-risk behaviors [1–5]. In developed countries, new HIV infections occur mainly among GBMSM, representing 55% of all new diagnoses in Spain by 2020 [6, 7]. Beyond decreasing the incidence of HIV infection, the PrEP care continuum includes addressing other factors that may influence HIV acquisition and the success of the prevention strategy. Therefore, the PrEP care continuum involves improving PrEP awareness, uptake, adherence to medication, and retention in care [8, 9].
PrEP is offered to GBMSM whose risk behaviors potentially expose them to HIV, but not all eligible GBMSM take PrEP [10, 11]. PrEP awareness is essential to enhance users’ self-perceived HIV risk and enables health professionals to identify people at higher risk of HIV acquisition [8]. Users’ awareness is often compromised as a result of vulnerability factors [10, 11]. Indeed, changes in sexual behaviors regarding condomless practices [12] and increasing rates of sexually transmitted infections (STIs) after PrEP prescription have been reported in multiple studies [13–15], probably related to the phenomenon of risk compensation [16, 17]. However, an increase in STI incidence after PrEP initiation has not been observed in all cohorts, so there are discrepancies about this association [18, 19]. Chemsex is the intentional use of psychoactive drugs to facilitate, enhance, and prolong sexual encounters. People who engage in chemsex have a five times higher risk of contracting HIV [20] as a result of risky behaviors, such as condomless sex, intravenous drug use, or prolonged traumatic anal sex with multiple partners [21, 22]. These practices also confer a higher risk for bacterial STIs [23]. The chemsex phenomenon is rapidly spreading in European countries [24–26], and Barcelona is the Spanish city with the highest prevalence [27–30]. PrEP could be offered as an additional HIV and STI prevention strategy to people who engage in chemsex as part of a comprehensive risk reduction program [31–33].
PrEP facilitation, linkage, and prescription are all involved in PrEP uptake [8]. Once PrEP is initiated, adherence to TDF-FTC is essential to achieve the HIV preventive efficacy of the PrEP strategy [34]. Low adherence is related to loss to follow-up [35] and intermittent retention in care [36], which could lead to potential ongoing HIV risk. Retention in care is an opportunity to enhance PrEP awareness, access to medication, and adherence. Multiple variables may influence retention [36], so addressing these factors through tailored biobehavioral interventions may improve the PrEP care continuum.
Hospital Clínic of Barcelona (HCB) is a tertiary hospital located in the center of Barcelona. The HIV unit of HCB is a Spanish referral center for the management of HIV infection and STIs. In September 2019, the Ministry of Health of Spain approved the inclusion of PrEP in the portfolio of public health services [37]. Two months later, PrEP was implemented in HCB as a global program that includes HIV and STI prevention, behavioral risk reduction, and psychological advice.
This study describes the evolution of risk behaviors and STIs among users after 1 year follow-up since they started PrEP at HCB and reports adherence and retention in care details. The evaluation of these data will allow the identification of possible areas for improvement for an optimal PrEP care continuum.
Methods
Study Design and Participants
A descriptive single-center retrospective study of the PrEP program was conducted at HCB. All adults at high HIV risk screened between November 2019 and June 2021 for participating in the PrEP program at HCB were included in this study and their first year of follow-up within the program was evaluated. Inclusion in the PrEP program was stopped because of the COVID-19 pandemic during the first 4 months of 2020; only follow-up visits were conducted for people already recruited. PrEP was offered on a daily regimen to GBMSM, transgender women, and sex workers at high risk of acquiring HIV, according to the protocol approved by the hospital, which was based on the Spanish Ministry of Health guidelines [37]. People who attended STI and PEP consultations were also identified to receive PrEP. All candidates were evaluated for inclusion and exclusion criteria, and people who agreed to receive PrEP signed an informed consent form to use their PrEP-related data for research purposes. The study was evaluated and accepted by the Ethics Committee of the HCB (HCB/2021/0072).
Our protocol established a quarterly follow-up after the baseline visits (screening and basal visit with PrEP prescription). In addition to general laboratory tests and STI screening, counseling on sexual practices, drug use, and STI prevention was provided by specialized physicians and nurses. For counseling to be as optimal as possible, we tried to create an environment of trust where the user could feel free to communicate their doubts and risk behaviors without being judged, understanding their social situation, and always respecting their choices. We tried to facilitate decision-making and problem-solving, providing them with strategies to reduce risk behaviors. In the case of explaining intravenous drug use, the use of sterile material was reinforced. In relation to STIs, advice and counseling were provided to avoid transmission. Psychological support was offered to those who requested it and referral was made to psychiatric units or specific NGO programs in those cases that required it. At each visit, adherence to PrEP was reinforced and the wish to discontinue was assessed among those who disclosed it.
Study Objectives
The primary objective of this study was to describe changes in sexual practices, drug use, and STIs of users after 12 months of follow-up since their inclusion in our hospital-based PrEP program. Secondary objectives were related to data on the PrEP care continuum: adherence to PrEP, retention in care, and re-engagement. Assessing these data allowed us to evaluate whether the multidisciplinary approach and counseling provided to users were optimal and also highlighted the weaknesses that PrEP programs should improve to enhance users’ PrEP care continuum. This study also characterized demographic data, new HIV diagnoses, and PrEP-related adverse effects and identified factors and subject profiles associated with higher STI and loss to follow-up risk.
Variables
Data were collected using the information obtained from medical records and three self-report questionnaires (sexual practices, drug use, and adherence-related surveys).
Demographic data included age, gender, origin, and educational level. Risk behaviors were evaluated using data on sexual practices (current relationship status, type of sex encounters, and condom use) and drug consumption (type of drug, route of administration, frequency, chemsex practice details, and users’ feelings).
STI data were obtained from triple-site PCR samples (pharynx, urine, and rectum smear), serology (HIV, hepatitis A virus (HAV), hepatitis B virus, hepatitis C virus (HCV), and syphilis) and rapid HIV tests. Possible variables associated with increasing STI risk were assessed [24, 38]: chemsex, polydrug consumption (use of three or more drugs at the same time), condomless anal sex, group sex, fisting, slamming, double penetration, fluid exchange, and sex toy use.
PrEP-related adverse effects were obtained from medical records. Adherence to medication was assessed by the Morisky–Green test: users who answered “No” to the four questions were considered good adherers [39]. People who did not attend two consecutive visits were considered discontinuations. Reasons for stopping PrEP were reviewed in clinical records; those who did not return to the appointments for unknown reasons were defined as lost to follow-up. Subject profiles at higher risk of loss to follow-up were assessed using demographic data, drug consumption characteristics, sexual behavior data (type of practice, chemsex engagement), and STI variables. Retention in care was assessed after analyzing the percentage of users who attended all follow-up visits. Individuals who stopped PrEP and later attended an appointment were defined as re-engagement.
Statistical Analysis
All study variables were collected in an electronic case report form (eCRF) implemented in the REDCap system (Research Electronic Data Capture tool) hosted at HCB. Data management and statistical analysis were performed using Stata (StataCorp. 2021. Stata: Release 17. Statistical Software. College Station, TX: StataCorp LLC). Qualitative variables were described using absolute frequencies and percentages, and they were compared between groups using the chi-squared test or Fisher’s exact test. Quantitative variables were reported as the means and standard deviations (SDs) or medians and interquartile ranges (IQRs), and we compared between groups using the t test or the Wilcoxon rank-sum test. Changes in the incidence rate of events over follow-up were analyzed using a Poisson regression model with the visit time (baseline, month 3, 6, 9, and 12) as categorical covariate. The goodness-of-fit of the model was assessed using the Pearson’s chi-squared statistic. To assess the impact of different risk variables on the likelihood of presenting at least one STI, mixed effects logistic regression models were estimated, reporting the odds ratios (ORs), the 95% confidence intervals (CIs), and p values. All tests were two-tailed, and the significance level was set at less than 0.05.
Results
Study Participants
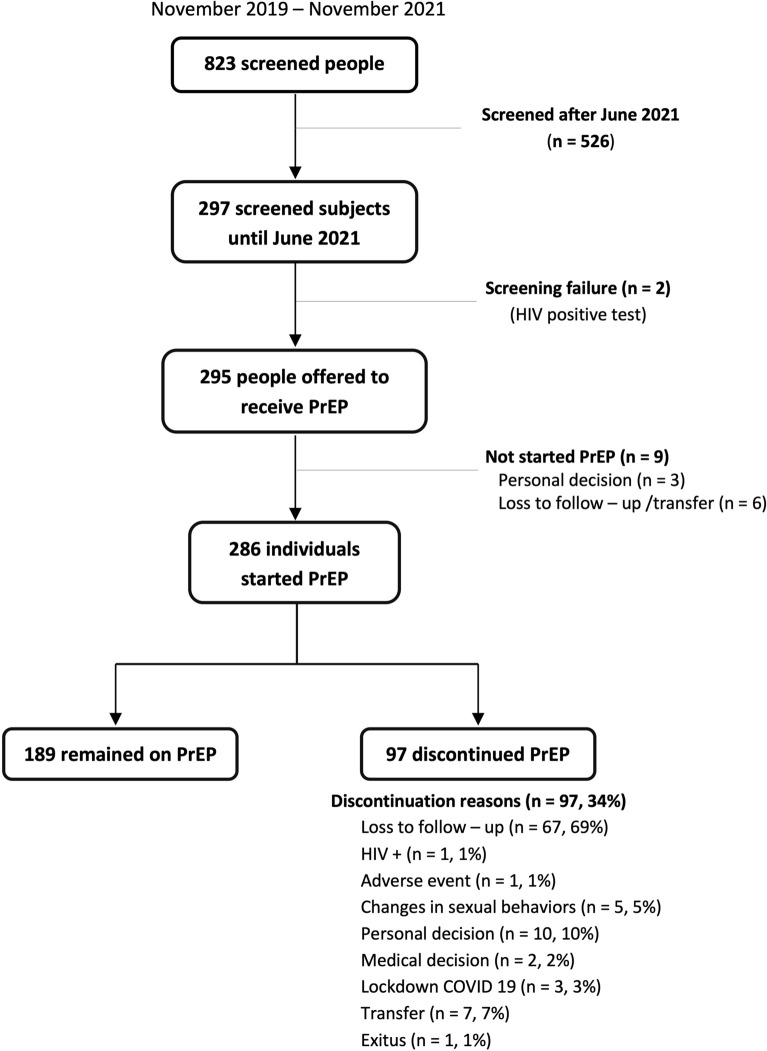
Between November 2019 and November 2021, a total of 823 people were screened for PrEP at HCB. The 297 subjects screened until June 2021 were included in the current study. Two men were excluded because of an HIV-positive rapid test, so PrEP was offered to 295 individuals. The flowchart is shown in Fig. 1.
Fig. 1.
Flowchart of the study
The baseline characteristics of the 297 participants are detailed in Table 1. Referring to demographic data, 94% were men (n = 278) and 5% were transgender women (n = 14) with a mean age of 34 years (SD 10). Almost half of the participants (n = 133, 48%) were originally from Spain, followed by Central and South America (n = 110, 39%). Regarding educational level, 71% (n = 154) had university studies. There were 29 sex workers (10%).
Table 1.
Baseline characteristics of 297 screened people who had a potential follow-up of at least 12 months
| Demographic data | Number (%) |
|---|---|
| Age | 34 (10)a |
| Sex | |
| Male | 278 (94%) |
| Female | 5 (2%) |
| Transgender | 14 (5%) |
| Origin | |
| Spain | 133 (48%) |
| Europe, except Spain | 33 (12%) |
| North America | 3 (1%) |
| Central-South America | 110 (39%) |
| Educational level | |
| Primary | 3 (1%) |
| Secondary | 61 (28%) |
| University | 154 (71%) |
| Referral source | |
| PEP or STI consultations | 115 (40%) |
| Primary care center | 3 (1%) |
| Community center, NGO | 18 (6%) |
| Personal decision | 152 (53%) |
| Inclusion criteria | |
| Multiple sexual partners | 275 (94%) |
| Untreated HIV-positive partner | 8 (3%) |
| Recent bacterial STI | 93 (32%) |
| Sex worker | 29 (10%) |
| Intravenous drug use | 2 (1%) |
| PEP in the last year | 194 (67%) |
| Previous PrEP | 43 (15%) |
| Exclusion criteria | |
| Positive HIV serology | 2 (1%) |
| HBV infection | 0 |
| Positive pregnancy test | 0 |
| PrEP contraindications | 0 |
PEP post-exposure prophylaxis, STI sexually transmitted infection, NGO non-governmental organization, HBV hepatitis B virus
aMean age (standard deviation)
Sexual Practices
Table 2 shows users’ sexual practices. Most of the users were GBMSM. At baseline, 74 subjects (26%) had a current partner; 72% of participants were in an open relationship (n/N = 51/71). The median number of sexual partners in the preceding 3 months was 10 (IQR 4, 20), with no changes after PrEP prescription until the 12-month visit (p = 0.226). A total of 179 users (n/N = 179/286, 63%) reported having had group sex at the screening visit. This practice remained stable during the follow-up (p = 0.536). Regarding potentially traumatic practices, double penetration was observed in 18% of users (n = 53), fisting in 14% (n = 41), and sex toy use in 39% (n = 114). Although condom use in anal intercourse decreased during the 12 months follow-up (p ≤ 0.0001), the use of condoms for double penetration (p = 0.093), gloves for fisting (p = 0.888), and protected sex toy use (p = 0.585) remained stable.
Table 2.
Changes in sexual practices and most frequent sexually transmitted infections during the study period. Association between specific sexual practices and increased risk of sexually transmitted infections
| Screening | Month 3 | Month 6 | Month 9 | Month 12 | P valued | ||
|---|---|---|---|---|---|---|---|
| Relationship statusa | |||||||
| Steady partner | 74 (26%) | 53 (29%) | 55 (27%) | 56 (27%) | 56 (30%) | 0.956 | |
| Open relationship | 51 (72%) | 32 (64%) | 36 (68%) | 38 (72%) | 36 (67%) | 0.985 | |
| Group sexa | 179 (63%) | 105 (56%) | 110 (52%) | 109 (52%) | 105 (57%) | 0.536 | |
| Number of sexual partners in previous 3 monthsb | 10 (4; 20) | 6 (3; 12) | 6 (3; 15) | 6 (3; 16) | 6.5 (4; 15) | 0.226 | |
| Type of sexual practicesa | |||||||
| Oral | 287 (99%) | 186 (99%) | 207 (99%) | 208 (100%) | 184 (98%) | 1.000 | |
| Condom use | 17 (6%) | 6 (3%) | 5 (2%) | 6 (3%) | 4 (2%) | 0.823 | |
| Anal | 262 (91%) | 169 (89%) | 196 (93%) | 190 (92%) | 176 (95%) | 0.982 | |
| Condom use | 108 (41%) | 33 (20%) | 36 (18%) | 34 (18%) | 22 (13%) | < 0.0001 | |
| Double penetration | 53 (18%) | 32 (17%) | 26 (12%) | 24 (12%) | 25 (14%) | 0.228 | |
| Condom use | 22 (42%) | 6 (19%) | 6 (23%) | 4 (17%) | 3 (13%) | 0.093 | |
| Fisting | 41 (14%) | 20 (11%) | 14 (7%) | 12 (6%) | 18 (10%) | 0.277 | |
| Glove use | 10 (24%) | 3 (16%) | 4 (29%) | 4 (33%) | 4 (22%) | 0.888 | |
| Sex toy use | 114 (39%) | 63 (34%) | 54 (25%) | 52(25%) | 52 (28%) | 0.021 | |
| Condom use | 28 (25%) | 15 (24%) | 10 (19%) | 8 (16%) | 8 (16%) | 0.585 | |
| Share | 11 (10%) | 10 (16%) | 12 (23%) | 12 (24%) | 7 (14%) | 0.266 | |
| Cleaning | 101 (89%) | 51 (82%) | 46 (85%) | 48 (94%) | 44 (86%) | 0.973 | |
| Fluid exchange (semen) | 135 (79%) | 106 (84%) | 101 (78%) | 102 (78%) | 105 (84%) | 0.465 | |
| At least one STI at any locationa | 85 (31%) | 64 (32%) | 70 (33%) | 64 (30%) | 64 (35%) | 0.924 | |
| Rectal gonorrheaa | 24 (9%) | 14 (7%) | 28 (13%) | 18 (9%) | 20 (11%) | 0.364 | |
| Rectal non-LGV chlamydiaa | 16 (6%) | 14 (7%) | 19 (9%) | 15 (7%) | 19 (10%) | 0.540 | |
| Pharynx gonorrheaa | 30 (11%) | 23 (11%) | 18 (8%) | 28 (12%) | 23 (12%) | 0.699 | |
| Acute syphilisa | 25 (10%) | 6 (7%) | 7 (9%) | 6 (6%) | 12 (10%) | 0.654 | |
| Association with increased STI riska–c | OR (95% CI) | P valuee | |||||
| Chemsex | 44 (64%) | 38 (67%) | 40 (63%) | 34 (59%) | 37 (61%) | 1.83 (1.28, 2.63) | 0.0010 |
| Polyconsumption | 30 (63%) | 23 (53%) | 30 (67%) | 25 (63%) | 24 (59%) | 1.29 (0.83, 2.01) | 0.2598 |
| Unprotected anal sex | 50 (69%) | 47 (87%) | 54 (89%) | 50 (96%) | 54 (92%) | 2.28 (1.43, 3.65) | 0.0006 |
| Group sex | 38 (69%) | 33 (70%) | 35 (67%) | 23 (55%) | 33 (79%) | 1.82 (1.19, 2.76) | 0.0053 |
| Fisting | 7 (9%) | 9 (16%) | 5 (8%) | 3 (5%) | 4 (7%) | 0.67 (0.37, 1.22) | 0.1898 |
| Slamming | 2 (5%) | 1 (3%) | 2 (5%) | 1 (3%) | 1 (3%) | 14.35 (1.69, 122.18) | 0.0148 |
| Double penetration | 17 (21%) | 14 (24%) | 13 (20%) | 5 (9%) | 9 (15%) | 1.57 (0.98, 2.52) | 0.0593 |
| Fluid interchange (semen) | 40 (49%) | 36 (62%) | 36 (55%) | 34 (58%) | 37 (61%) | 1.45 (1.03, 2.04) | 0.0342 |
| Unprotected toy use | 7 (25%) | 4 (20%) | 4 (24%) | 1 (10%) | 1 (6%) | 0.80 (0.34, 1.87) | 0.6098 |
STI sexually transmitted infection, LGV lymphogranuloma venereum
aNumber (%)
bMedian (IQR)
cOdds ratio (95% CI)
dPoisson regression model
eMixed-effects logistic regression models
Drug Consumption and Chemsex
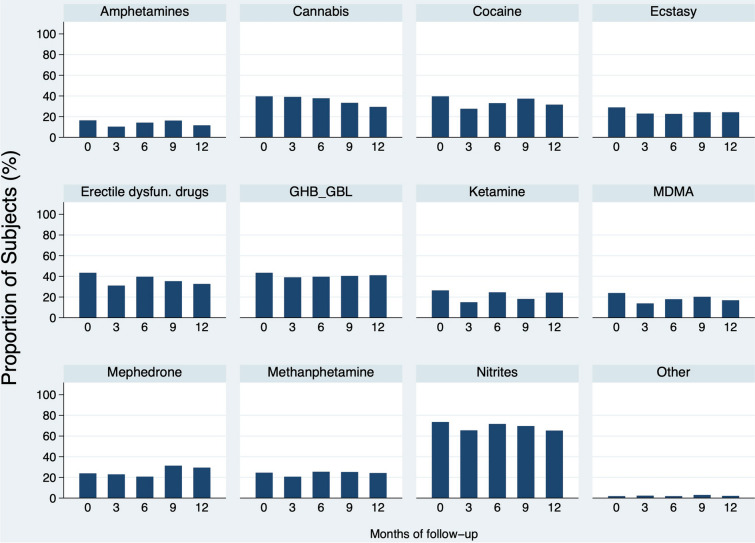
Data on substance use are reported in Table 3. In the whole cohort, 184 subjects (n/N = 184/292, 63%) reported the use of drugs in the last year, and 87% of them (n = 160, 55% of all users) disclosed engaging in chemsex practice, for more than a year in 35% of the individuals (n/N = 51/146). Slamming was reported by 3% of users (n/N = 3/105). Twenty percent of people who engaged in chemsex (n/N = 30/153) disclosed polydrug consumption. Nitrites (n = 117, 74%) were the most commonly used substances, followed by GHB/GBL (n = 69, 43%), erectile dysfunction drugs (n = 69, 43%), cannabis (n = 63, 40%), and cocaine (n = 63, 40%). A quarter of users used ketamine (n = 42) and methamphetamine (n = 39). The most prevalent combinations were cannabis with nitrites and GHB/GBL with methamphetamine, followed by cocaine with GHB/GBL and cocaine with nitrites. Sex group was disclosed by 74% of users (n/N = 117/159). During the follow-up period, the global chemsex rate remained stable (p = 0.320), and slamming, group sex, and polydrug consumption did not change either (p = 0.967, p = 0.924, p = 0.853). The most frequent drugs and their follow-up evolution are detailed in Fig. 2.
Table 3.
Chemsex data: evolution of sexualized drug use and users’ feelings
| Screening | Month 3 | Month 6 | Month 9 | Month 12 | P valuec | |
|---|---|---|---|---|---|---|
| General drug usea | ||||||
| Yes | 184 (63%) | 112 (59%) | 125 (58%) | 119 (57%) | 113 (59%) | 0.916 |
| Chemsex | 160 (87%) | 89 (79%) | 106 (85%) | 100 (84%) | 96 (85%) | 0.320 |
| Polyconsumptiona | 91 (59%) | 44 (51%) | 65 (63%) | 60 (61%) | 57 (62%) | 0.853 |
| Group sexa | 117 (74%) | 61 (71%) | 69 (66%) | 63 (66%) | 67 (72%) | 0.924 |
| Routea | ||||||
| Oral | 121 (83%) | 57 (76%) | 74 (79%) | 66 (73%) | 59 (84%) | 0.539 |
| Inhaled | 117 (81%) | 54 (71%) | 71 (76%) | 72 (80%) | 51 (70%) | 0.018 |
| Sniffed | 81(64%) | 37 (55%) | 61 (67%) | 58 (66%) | 55 (70%) | 0.035 |
| Sublingual | 21 (19%) | 4 (7%) | 10 (13%) | 12 (17%) | 7 (11%) | 0.137 |
| Rectal | 14 (14%) | 6 (10%) | 12 (16%) | 12 (16%) | 8 (13%) | 0.750 |
| Intravenous | 3 (3%) | 2 (3%) | 2 (3%) | 2 (3%) | 3 (5%) | 0.967 |
| Experiencesa | ||||||
| Bad experiencesb | 57 (36%) | 19 (24%) | 24 (22%) | 15 (15%) | 21 (23%) | 0.001 |
| Sober sex | 147 (93%) | 80 (92%) | 97 (92%) | 94 (96%) | 894 (94%) | 0.725 |
| Worriesa | 70 (44%) | 19 (22%) | 27 (25%) | 20 (20%) | 26 (28%) | 0.003 |
| Consumption management | 53 (79%) | 14 (82%) | 18 (69%) | 15 (75%) | 24 (92%) | 0.919 |
| Sexuality | 54 (82%) | 15 (83%) | 19 (70%) | 16 (80%) | 20 (83%) | 0.982 |
| STIs | 63 (93%) | 16 (89%) | 20 (74%) | 16 (80%) | 21 (81%) | 0.911 |
| Need of helpa | 35 (22%) | 7 (8%) | 7 (7%) | 11 (11%) | 16 (17%) | 0.004 |
| Consumption management | 29 (94%) | 7 (100%) | 5 (71%) | 10 (91%) | 14 (93%) | 0.982 |
| Sexuality | 23 (72%) | 6 (86%) | 4 (67%) | 9 (82%) | 7 (47%) | 0.777 |
| STIs | 29 (88%) | 4 (57%) | 2 (33%) | 7 (64%) | 9 (64%) | 0.541 |
STI sexually transmitted infection
aNumber (%)
bLoss of consciousness, hallucinations, paranoia
cPoisson regression model
Fig. 2.
Drug use data. Representation of the most consumed substances and the trend of drug use throughout the PrEP program (proportion of subjects reporting the consumption of a specific drug at each visit). Amphetamines, p = 0.691; cannabis, p = 0.688; cocaine, p = 0.560; ecstasy, p = 0.850; erectile dysfunction drugs, p = 0.508; GHB/GBL, p = 0.986; ketamine, p = 0.318; MDMA, p = 0.464; mephedrone, p = 0.530; methamphetamine, p = 0.964; nitrites, p = 0.925
Regarding users’ baseline chemsex-related feelings, 36% of people who engaged in chemsex (n/N = 57/158) explained having had bad experiences, almost half (n/N = 70/158, 44%) affirmed being worried about sexualized drug use, and 22% (n/N = 35/158) disclosed that they needed help. Worries and needs were mainly related to drug use management and to self-perceived STI acquisition risk. Concerns about chemsex practice decreased by almost half during the first year of follow-up (p = 0.003), as did the need for help (p = 0.004).
Sexually Transmitted Infections
At baseline, approximately one-third of participants had at least one STI at any of the three locations (N = 85, 31%). Gonococcal proctitis (n = 24, 9%), pharyngitis (n = 30, 11%), and rectal non- lymphogranuloma venereum chlamydia (n = 16, 6%) were the most frequent infections. During the study period, the global STI rate remained stable at approximately 35% (p = 0.924), without statistically significant changes in the locations of PCR-positive samples. Data on the STIs rate are presented in Table 2.
Regarding serology tests, acute syphilis was diagnosed in 10% (n = 25) of the included participants. Seventy-nine percent of the users (n = 219) were immunized for HBV, 89% (n = 246) for HAV, and one user tested positive for HCV serology with negative RNA. No changes in syphilis rate (p = 0.654) and no acute HCV infections were observed during the follow-up.
We found that people who engaged in chemsex (OR 1.83, 95% CI 1.28; 2.63; p = 0.0010), group sex (OR 1.82, 95% CI 1.198; 2.76; p = 0.0053), fluid exchange (OR 1.45, 95% CI 1.03; 2.04; p = 0.0342), and condomless anal intercourse (OR 2.28, 95% CI 1.43; 3.65; p = 0.0006) were at higher risk of presenting STIs.
HIV Seroconversion
Three HIV infections were observed during the study period. Two of them were diagnosed at screening, and the third at the first month visit. All three were GBMSM with a mean age of 38 years (SD 7). They all had multiple sexual partners and recent negative HIV tests. The main difference between cases was that the patient who was not detected at screening probably became infected during the first month after PrEP initiation and had a resistance test with the M184V mutation.
PrEP Adherence, Adverse Events, and Retention in Care
PrEP was offered to 295 subjects, but 9 individuals did not start taking it, 3 of them for personal reasons, and 6 were lost to follow-up/transfer (Fig. 1). Finally, 286 subjects received PrEP at least once, mostly on a daily regimen (n = 278, 97%). Adherence was good in approximately 80% of users, and this rate remained stable during the follow-up (p = 0.940).
A total of 136 adverse events were observed, mainly involving the gastrointestinal tract (n = 44, 34%): 10 users (n = 11%) reported diarrhea and 14 (n = 12%) disclosed nausea. Eighty-eight percent (n = 119) were mild in severity, and 97% of the cases had ad integrum recovery. Of all adverse events, 29 (21%) were considered to be possibly related to PrEP; however, only one led to therapy being stopped (non-serious acute diarrhea). Acute kidney failure was observed in one individual.
During the study period, 97 participants discontinued PrEP (n/N = 97/286); 67 of them were lost to follow-up (n/N = 67/97). Reasons for discontinuation are detailed in Fig. 1. The median duration of PrEP among users who were lost to follow-up was longer (12.0 months; IQR 5.7, 17.4) than in those who discontinued for other reasons (3.4 months; IQR 2.1, 6.0) (p < 0.0001).
Transgender women (62% vs. 22%, p = 0.0029), sex workers (41% vs. 22%, p = 0.0173), and people who practiced fisting (10% vs. 25%, p = 0.0316) were at higher risk of loss to follow-up. We did not identify any demographic data, STI, chemsex practice, type of drug, or other risk behaviors related to a higher risk of loss.
In the whole cohort, 57.3% of participants (n/N = 169/295) were retained in care after 12 months of follow-up (Fig. 3). There were two main periods when users stopped PrEP (Fig. 4). At an early stage, a total of 40 screened people (14%) did not return to the 3-month appointment; 18 of them came back later to the 6-month visit, but the remaining 22 (7.5%) only attended the screening or the screening and basal visits (9 screened people decided not to start PrEP, and 13 participants started PrEP in the basal visit but did not return to the following appointments) (Figs. 1 and 3). People also discontinued during the study period, since 90 users (31% of the whole cohort) did not attend the 12-month visit (Fig. 4).
Fig. 3.
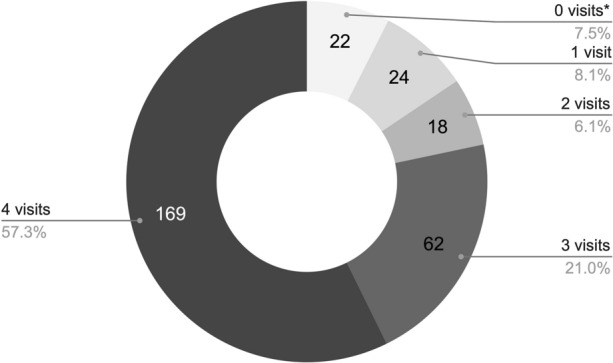
Retention in care details. User distribution by number of visits performed. *Refers to people who attended only the screening or the screening and basal visits
Fig. 4.
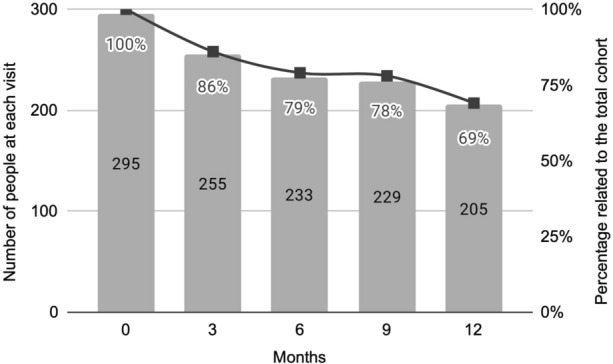
Number of users attended at each visit. Numbers and percentages are not consecutive from one visit to another
PrEP was started for the second time among 15 subjects (7 people discontinued, 46%), and 2 of them received PrEP for a third time.
Discussion
PrEP was designed at HCB as a tailored biobehavioral program to prevent HIV infection and improve users’ global health care. This study assessed changes in risk behaviors and STIs in our cohort and described the PrEP care continuum of the users, which included PrEP awareness, uptake, adherence, and retention in care [8, 9]. Each step was an opportunity to detect weaknesses that need to be reinforced to provide users with optimal care.
This was the largest Spanish cohort of users included exclusively in a hospital-based PrEP program [38, 40]. Regarding the influence area of the hospital, the percentage of transgender women and sex workers was higher than in other studies, including national series [38, 40], increasing users’ vulnerability [15]. Our cohort was largely composed of individuals derived from NGOs or community centers and STI and PEP consultations. Subjects at high risk of HIV acquisition were detected in those units, so PrEP was offered and prioritized to this group. Forty percent attended on their own, a fact that reflects the high initial awareness of users.
The prevalence of potentially traumatic sexual practices (double penetration, fisting, and sex toy use) and group sex were higher than in previous published studies [31]. After starting PrEP, an increase in condomless anal sex was observed, as described in other studies [12, 14, 15, 38, 40]. However, the use of protection during other high-risk sexual practices did not change. Therefore, the risk reduction advice provided at each visit to increase users’ self-perceived HIV risk and avoid the potential phenomenon of risk compensation was effective. We consider that the aim of PrEP programs is not to change sexual behaviors, since high-risk practices are performed on an individual basis and are often conditioned by vulnerability factors. PrEP programs should strengthen behavioral risk reduction interventions and enhance tailored approaches to sexual practices to improve PrEP awareness and to promote a healthy sexual life with less HIV acquisition risk [41].
The recent increase in people who engage in chemsex in several cities in Europe [24–26, 42], including in Barcelona [27–30], may have influenced our users’ elevated substance consumption data. The chemsex rate in our cohort was higher than that described in other international studies, as we found that 87% of people who used drugs engaged in chemsex (n/N = 160/292, 55% of the whole cohort), compared to 38.5% detected in a multicenter English study [31] or 42.6% in a Paris cohort [15]. Specific substance consumption (GHB/GBL, methamphetamine, cocaine, ketamine, or mephedrone) and their combination (polydrug use) were also much more prevalent compared to other cohorts [22, 25, 40]. Methamphetamine use has increased worldwide among GBMSM [24, 28, 38], also among PrEP users who combine methamphetamine, erectile dysfunction drugs, and Truvada® (“the MTV generation”) [43]. Its consumption potentially leads to physical, mental health, and social problems [44]. In our cohort, methamphetamine consumption was almost double that in other groups [22, 25]. A more comprehensive and multidisciplinary approach to chemsex could avoid considerable individual and public health issues beyond HIV transmission [45, 46].
PrEP users likely identified our consultations as a safe place to express their concerns and needs. Almost half of the screened people disclosed being worried about chemsex practice-related issues. This rate decreased significantly during the follow-up, once doubts and fears were addressed at their appointments. The need for help also decreased since they felt accompanied. Although data on chemsex practice and substance consumption remained stable during the study period, alleviating their concerns led to a decrease in problematic sexualized drug use.
Our baseline STI rate was almost twice as high as that in other international series [12–15, 47], partly explained by the proportion of people referred from STI and PEP consultations, but also by their high-risk behaviors. There are discrepancies about the incidence of STI in PrEP programs, as some cohorts have reported an increase after PrEP initiation [13–16, 40], whereas other publications have found the opposite [18]. A positive fact to be emphasized in our study was that, despite the decrease in condom use for anal intercourse and the persistence of other high-risk behaviors during the follow-up, no more STIs were diagnosed. This was probably due to the effective STI management in our program with quarterly screening tests, targeted early antibiotic therapy, and counseling. An association between higher STI risk and specific sexual practices (chemsex, group sex, fluid exchange, and condomless anal intercourse) was observed, so we consider it important to focus on people with these behaviors to enhance STI prevention. Regarding HCV, a high incidence among PrEP users has been reported by different study groups [48]. Nevertheless, in our cohort, no acute HCV infections were diagnosed during follow-up, despite people engaging in high-risk practices (3% intravenous drug use, approximately 15–20% ulcerative proctitis, and 14% of users who practice fisting). This fact may be explained by the counseling provided at each visit regarding safe injecting drug use and by the widespread treatment of HCV in recent years in our country, which has decreased HCV transmission among the GBMSM community. PrEP programs are also an opportunity to achieve the World Health Organization (WHO) HCV goal for 2030 [49].
In line with previous studies, our program also showed that PrEP is an effective strategy to prevent HIV infections. Only one seroconversion was detected in a man with high-risk behaviors during the follow-up [50]. Starting Truvada® in previously infected people could lead to drug resistance and reduce future treatment options [51, 52]. Therefore, in addition to HIV serology, molecular studies could be performed on all PrEP candidates with high-risk behaviors or in those suspected of being infected.
Transgender women and sex workers are less likely to adhere to PrEP owing to individual factors and sociostructural barriers to PrEP uptake [53–58]. The high proportion of these subgroups in our cohort may have partly influenced our adherence rate, as 80% of individuals took PrEP correctly, compared with 90% shown in previous publications [4, 38, 47]. Chemsex practice has not been described as negatively affecting adherence to PrEP [31–33, 43, 59]. However, some authors reported that specific substance use, especially methamphetamine and GBL/GHB consumption, could lead to poor PrEP adherence and frequent forgetfulness [60, 61]. Thus, we believe that the moderate use of these drugs in our cohort may also have decreased the overall adherence rate. The use of additional interventions to detect correct medication intake deserves a reflection [35, 54, 55], particularly in vulnerable subgroups (transgender women, sex workers, and people who use illicit drugs).
We would like to highlight the elevated rate of PrEP discontinuation in our cohort, almost double compared to previous publications [12, 15, 40, 62]. Additionally, there was a higher loss to follow-up rate [15, 35, 36, 40], since 69% of discontinuations were due to this reason. The already mentioned complicated access to the health care system and the lower adherence to PrEP in transgender women and sex workers increase the risk of PrEP program discontinuation [40, 57, 58]. Indeed, in our cohort, these subgroups, together with people who practice fisting, were associated with a higher loss to follow-up rate. Other vulnerability factors and additional risk behaviors of our users, such as specific drug consumption or chemsex practice [60, 61, 63], may also have influenced PrEP awareness and thus increased the risk of leaving the strategy. An interesting finding observed in the current study was that people who were lost to follow-up remained in PrEP almost four times longer (12 months; IQR 5.7, 17.4) than those who discontinued for other reasons (3.4 months; IQR 2.1, 6.0). The first group probably stopped PrEP because they lost motivation, so it would be interesting to find tools to promote the enthusiasm of the users for the program. The high discontinuation and loss to follow-up rates affected our retention in care data, which were lower than those described by some authors [4, 15, 40], but similar to those in other publications [9, 36]. However, cohorts with our similar retention rate had more re-engagement than ours: less than 3% of subjects re-engaged during the study period compared to 27% observed in a US cohort [9]. Persistence in care is essential to achieve the comprehensive effectiveness of PrEP programs, so identifying loss to follow-up patterns would also be useful for the development of tailored interventions to improve retention in care.
Limitations
This study has some limitations that must be considered. First, our cohort may have selection bias, as almost one-third of the participants were referred from STI and PEP consultations, so these people probably adopted more baseline risk behaviors. The second limitation is the limited number of users included in the analysis. Despite the 823 screening visits carried out between November 2019 and November 2021, only 297 users were included in the study, because until September 2021 the inclusion of people was institutionally limited to approximately 200 subjects. Moreover, given the limited resources provided by government entities, not all key populations listed in the national Ministry of Health guidelines are included in the PrEP program. In addition, the high rate of loss to follow-up could have hindered the detection of statistically significant changes in the analyzed variables. Finally, during the first 4 months of 2020, the COVID-19 pandemic stopped new PrEP admissions, and some participants may have had difficulty accessing hospitals.
Conclusions
Although our PrEP cohort included people at high HIV risk with vulnerable subgroups and users who engaged in high-risk behaviors, the implementation of the hospital-based PrEP program was adequate, as it maintained stable risk practices and STIs with a low rate of new HIV diagnoses. The counseling provided allowed people to feel safer with fewer concerns related to chemsex use and drug consumption. Even though users remained engaged in high-risk sexual practices, tailored interventions allowed the STI rate not to increase. Our PrEP program needs to focus on improving users’ adherence and retention in care, as both were not optimal with high rates of loss to follow-up and discontinuations, especially among transgender women, sex workers, and people who practice fisting.
PrEP programs need a tailored approach to address additional factors that may influence HIV acquisition, such as drug use or sexual behaviors and other STIs, as well as strategies to enhance adherence and retention in care. The next challenge for PrEP programs should be to intensify comprehensive biobehavioral interventions to improve users’ care continuum. This would provide individual and public health benefits beyond HIV prevention.
Acknowledgements
We thank all users for their participation; without them, this study would not have been possible.
Funding
The authors have not received any related funding for the study. The rapid service fee was supported by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception, design, data collection and interpretation. The first draft of the manuscript was written by Ainoa Ugarte and Montserrat Laguno. All authors read and approved the final manuscript.
Disclosures
The authors do not have any conflicts of interest.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of the Hospital Clínic of Barcelona (HCB/2021/0072). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All participants provided informed consent to participate in the study and for publication of the information obtained in the final manuscript.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Ainoa Ugarte and Lorena de la Mora are co-first authors.
Josep Mallolas and Montserrat Laguno are co-seniors authors.
Ainoa Ugarte, Lorena de la Mora, Josep Mallolas, and Montserrat Laguno contributed equally.
Contributor Information
Ainoa Ugarte, Email: UGARTE@clinic.cat.
Lorena de la Mora, Email: DELAMORA@clinic.cat.
David García, Email: DAGARCIAH@clinic.cat.
María Martínez-Rebollar, Email: REBOLLAR@clinic.cat.
Elisa de Lazzari, Email: ELAZZARI@recerca.clinic.cat.
Berta Torres, Email: BTORRES@clinic.cat.
Alexy Inciarte, Email: AJINCIAR@clinic.cat.
Juan Ambrosioni, Email: AMBROSIONI@clinic.cat.
Iván Chivite, Email: ICHIVITE@clinic.cat.
Estela Solbes, Email: ESOLBES@clinic.cat.
Nicolás de Loredo, Email: DELOREDO@clinic.cat.
Guillermo Federico Del Carlo, Email: GFDELCARLO@clinic.cat.
Ana González-Cordón, Email: AGONZAL1@clinic.cat.
José Luis Blanco, Email: JBLANCO@clinic.cat.
Esteban Martínez, Email: ESTEBANM@clinic.cat.
Josep Mallolas, Email: MALLOLAS@clinic.cat.
Montserrat Laguno, Email: MLAGUNO@clinic.cat.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina J-M, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 4.Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73:540–546. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volk JE, Marcus JL, Phengrasamy T. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Joint United Nations Programme on HIV/AIDS (UNAIDS). “Key population” section. https://www.unaids.org/en/resources/fact-sheet. Accessed 6 Oct 2022.
- 7.Epidemiological surveillance of HIV and AIDS in Spain 2020. Information system on new HIV diagnoses. National registry of AIDS cases. Last update June 2021. https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/Informe_VIH_SIDA_WEB.pdf. Accessed 6 Oct 2022.
- 8.Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31:731–734. doi: 10.1097/QAD.0000000000001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao J, Montgomery MC, Williams R, et al. Loss to follow-up and re-engagement in HIV pre-exposure prophylaxis care in the Unites States, 2013–2019. AIDS Patient Care STDS. 2021;35(7):271–277. doi: 10.1089/apc.2021.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iniesta C, Folch C, Meyer S, Vázquez M, Casanoba J, Días A. Would eligible gay, bisexual and other men who have sex with men use PrEP? Awareness, knowledge, eligibility and intention to use PrEP among EMIS-2017 participants in Spain. Prev Med. 2022;156:106962. doi: 10.1016/j.ypmed.2022.106962. [DOI] [PubMed] [Google Scholar]
- 11.Iniesta C, Álvarez-Del Arco D, García-Sousa LM, et al. Awareness, knowledge, use, willingness to use and need of pre-exposure prophylaxis (PrEP) during World Gay Pride 2017. PLoS ONE. 2018;13(10):e0204738. doi: 10.1371/journal.pone.0204738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoornenborg E, Coyer L, van Laarhoven A, et al. Change in sexual risk behaviour after 6 months of pre-exposure prophylaxis use: results from the Amsterdam pre-exposure prophylaxis demonstration project. AIDS. 2018;32:1527–1532. doi: 10.1097/QAD.0000000000001874. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523–530. doi: 10.1097/QAD.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y-H, Snowden JM, McFarland W, Raymond HF. Preexposure prophylaxis (PrEP) use, seroadaptation, and sexual behavior among men who have sex with men, San Francisco, 2004–2014. AIDS Behav. 2016;20:2791–2797. doi: 10.1007/s10461-016-1357-2. [DOI] [PubMed] [Google Scholar]
- 15.Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine fon HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161–2169. doi: 10.1097/QAD.0000000000001939. [DOI] [PubMed] [Google Scholar]
- 16.Montaño AM, Dombrowski JC, Dasgupta S, et al. Changes in sexual behavior and STI diagnoses among MSM initiating PrEP in a clinic setting. AIDS Behav. 2019;23:548–555. doi: 10.1007/s10461-018-2252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gafos M, Horne R, Nutland W, et al. The context of sexual risk behaviour among men who have sex with men seeking PrEP, and the impact of PrEP on sexual behaviour. AIDS Behav. 2019;23:1708–1720. doi: 10.1007/s10461-018-2300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina JM, Ghosn J, Delaugerre C, et al. Incidence of HIV infection with daily or on-demand oral PrEP with TDF/FTC in France. CROI 2021, Conference on Retroviruses and Opportunistic Infections, March 6–10, 2021. Abstract 148.
- 19.Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396(10246):239–254. doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pakianathan M, Whittaker M, Lee MJ, et al. Chemsex and new HIV diagnosis in gay, bisexual and other men who have sex with men attending sexual health clinics. HIV Med. 2018;19:485–490. doi: 10.1111/hiv.12629. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald N, Elam G, Hickson F, et al. Factors associated with HIV seroconversion in gay men in England at the start of the 21st century. Sex Transm Infect. 2008;84:8–13. doi: 10.1136/sti.2007.027946. [DOI] [PubMed] [Google Scholar]
- 22.Sewell J, Cambiano V, Speakman A, et al. Changes in chemsex and sexual behaviour over time, among a cohort of MSM in London and Brighton: findings from the AURAH2 study. Int J Drug Policy. 2019;68:54–61. doi: 10.1016/j.drugpo.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 23.John SA, Parsons JT, Rendina HJ, Grov C. Club drug users had higher odds of reporting a bacterial sexually transmitted infection compared to non-club drug users: results from a cross-sectional analysis of gay and bisexual men on HIV pre-exposure prophylaxis (PrEP) Sex Transm Infect. 2019;95(8):626–628. doi: 10.1136/sextrans-2018-053591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings ('chemsex’) and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect. 2015;91(8):564–568. doi: 10.1136/sextrans-2015-052052. [DOI] [PubMed] [Google Scholar]
- 25.Sewell J, Cambiano V, Miltz A, et al. Changes in recreational drug use, drug use associated with chemsex, and HIV-related behaviours, among HIV-negative men who have sex with men in London and Brighton, 2013–2016. Sex Transm Infect. 2018;94(7):494–501. doi: 10.1136/sextrans-2017-053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt AJ, Bourne A, Weatherburn P, et al. Illicit drug use among gay and bisexual men in 44 cities: findings from the European MSM Internet Survey (EMIS) Int J Drug Policy. 2016;38:4–12. doi: 10.1016/j.drugpo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 27.De la Mora L, Laguno M, de Lazzari E, et al. Vulnerability conditions in a cohort of men who have sex with men who engage in chemsex in Barcelona city: a cross-sectional study. Sex Res Soc Policy. 2022 doi: 10.1007/s13178-022-00702-1. [DOI] [Google Scholar]
- 28.EMIS – 2017. The European Men-Who-Have-Sex-With-Men Internet Survey. Published August 2019. https://www.ecdc.europa.eu/sites/default/files/documents/European-MSM-internet-survey-2017-findings.pdf. Accessed 10 Sept 2022.
- 29.Spanish observatory of drugs and addictions 2020. Alcohol, tobacco and illegal drugs in Spain. Published 2015. https://pnsd.sanidad.gob.es/profesionales/sistemasInformacion/informesEstadisticas/pdf/2020OEDA-INFORME.pdf. Accessed 10 Sept 2022.
- 30.Guerras JM, Hoyos J, García de Olalla P, et al. Comparison of polydrug use prevalences and typologies between men who have sex with men and general population men, in Madrid and Barcelona. Int J Environ Res Public Health. 2021;18(21):11609. doi: 10.3390/ijerph182111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Halloran C, Rice B, White E, et al. Chemsex is not a barrier to self-reported daily PrEP adherence among PROUD study participants. Int J Drug Policy. 2019;74:246–254. doi: 10.1016/j.drugpo.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores Anato JL, Panagiotoglou D, Greenwald ZR, et al. Chemsex practices and pre-exposure prophylaxis (PrEP) trajectories among individuals consulting for PrEP at a large sexual health clinic in Montréal, Canada (2013–2020) Drug Alcohol Depend. 2021;1(226):108875. doi: 10.1016/j.drugalcdep.2021.108875. [DOI] [PubMed] [Google Scholar]
- 33.Roux P, Fressard L, Suzan-Monti M, et al. Is on-demand HIV pre-exposure prophylaxis a suitable tool for men who have sex with men who practice chemsex? Results from a substudy of the ANRS-IPERGAY trial. J Acquir Immune Defic Syndr. 2018;79(2):e69–e75. doi: 10.1097/QAI.0000000000001781. [DOI] [PubMed] [Google Scholar]
- 34.Powell VE, Gibas KM, DuBow J, Krakower DS. Update on HIV preexposure prophylaxis: effectiveness, drug resistance, and risk compensation. Curr Infect Dis Rep. 2019;21:28. doi: 10.1007/s11908-019-0685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinelli MA, Glidden DV, Anderson PL, et al. Brief report: short-term adherence marker to PrEP predicts future nonretention in a large PrEP demo project: implications for point-of-care adherence testing. J Acquir Immune Defic Syndr. 2019;81(2):158–162. doi: 10.1097/QAI.0000000000002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doblecki-Lewis S, Liu AY, Feaster DJ, et al. Patterns and correlates of participant retention in a multi-city pre-exposure prophylaxis demonstrataion project. J Acquir Immune Defc Syndr. 2018;79(1):62–69. doi: 10.1097/QAI.0000000000001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manual for the implementation of an HIV pre-exposure prophylaxis program in Spain. PrEP Working Group. Division of HIV, STI, Viral Hepatitis and Tuberculosis Control. Ministry of Health. Last updated December 2021. https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/PrEP/Manual_PrEP_FINAL.pdf. Accessed 20 Sept 2022.
- 38.Ayerdi Aguirrebengoa O, Vera García M, Arias Ramírez D, et al. Low use of condom and high STI incidence among men who have sex with men in PrEP programs. PLoS ONE. 2021;16(2):e0245925. doi: 10.1371/journal.pone.0245925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Iniesta C, Coll P, Barberá MJ, et al. Implementation of pre-exposure prophylaxis programme in Spain. Feasibility of four different delivery models. PLoS ONE. 2021;16(2):e0246129. doi: 10.1371/journal.pone.0246129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillis A, Germain J, Hope V, McVeigh J, Van Hout MC. Pre-exposure prophylaxis (PrEP) for HIV prevention among men who have sex with men (MSM): a scoping review on PrEP service delivery and programming. AIDS Behav. 2020;24(11):3056–3070. doi: 10.1007/s10461-020-02855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosinska M, Gios L, Nöstlinger C, et al. Prevalence of drug use during sex amongst MSM in Europe: results from a multi-site bio-behavioural survey. Int J Drug Policy. 2018;55:231–241. doi: 10.1016/j.drugpo.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Hammoud MA, Vaccher S, Jin F, et al. The new MTV generation: using methamphetamine, Truvada, and Viagra to enhance sex and stay safe. Int J Drug Policy. 2018;55:197–204. doi: 10.1016/j.drugpo.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Gavín P, Arbelo N, Monràs M, et al. Methamphetamine use in chemsex and its consequences on mental health: a descriptive study. Rev Esp Salud Publica. 2021;31(95):e202108108. [PubMed] [Google Scholar]
- 45.Whitlock GG, Protopapas K, Bernardino JI, et al. Chems4EU: chemsex use and its impacts across four European countries in HIV-positive men who have sex with men attending HIV services. HIV Med. 2021;22(10):944–957. doi: 10.1111/hiv.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahan VM, Moreno C, Al-Tayyib A, et al. Pre-exposure prophylaxis awareness and use among cisgender men who have sex with men and use methamphetamine in 3 western US cities. Sex Transm Dis. 2020;47(4):217–223. doi: 10.1097/OLQ.0000000000001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lal L, Audsley J, Murphy DA, et al. Medication adherence, condom use and sexually transmitted infections in Australian preexposure prophylaxis users. AIDS. 2017;31(12):1709–1714. doi: 10.1097/QAD.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 48.Hoornenborg E, Coyer L, Boyd A, et al. High incidence of HCV in HIV-negative men who have sex with men using pre-exposure prophylaxis. J Hepatol. 2020;72:855–864. doi: 10.1016/j.jhep.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. World Health Organization. https://cscfsvpm01.csc.es/Data$/ugarte/Downloads/9789240053779-eng.pdf. Accessed 22 Sept 2022.
- 50.Chivite I, Riera-Monroig J, Ambrosioni J, Laguno M. HIV infection in the setting of PrEP: development of antiretroviral resistance and breakthrough infection. Report of two cases in real-life. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40(5):280–281. doi: 10.1016/j.eimc.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Ambrosioni J, Petit E, Liegeon G, Laguno M, Miró JM. Primary HIV-1 infection in users of pre-exposure prophylaxis. Lancet HIV. 2021;8(3):e166–e174. doi: 10.1016/S2352-3018(20)30271-X. [DOI] [PubMed] [Google Scholar]
- 52.Gibas KM, van den Berg P, Powell VE, Krakower DS. Drug resistance during HIV pre-exposure prophylaxis. Drugs. 2019;79:609–619. doi: 10.1007/s40265-019-01108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downing J, Yee K, Sevelius JM. PrEP use and adherence among transgender patients. AIDS Behav. 2022;26(4):1251–1259. doi: 10.1007/s10461-021-03482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Footer KHA, Lim S, Rael CT, et al. Exploring new and existing PrEP modalities among female sex workers and women who inject drugs in a U.S. city. AIDS Care. 2019;31(10):1207–1213. doi: 10.1080/09540121.2019.1587352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Bassel N, Wechsberg WM, Shaw SA. Dual HIV risk and vulnerabilities among women who use or inject drugs: no single prevention strategy is the answer. Curr Opin HIV AIDS. 2012;7(4):326–331. doi: 10.1097/COH.0b013e3283536ab2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarus L, Deering KN, Nabess R, Gibson K, Tyndall MW, Shannon K. Occupational stigma as a primary barrier to health care for street-based sex workers in Canada. Cult Health Sex. 2012;14(2):139–150. doi: 10.1080/13691058.2011.628411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarwell M, John SA, Westmoreland D, et al. PrEP uptake and discontinuation among a US national sample of transgender men and women. AIDS Behav. 2021;25(4):1063–1071. doi: 10.1007/s10461-020-03064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valente PK, Mimiaga MJ, Chan PA, Biello KB. Health service- and provider-level factors influencing engagement in HIV pre-exposure prophylaxis care among male sex workers. AIDS Patient Care STDS. 2021;35(8):279–287. doi: 10.1089/apc.2021.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maxwell S, Shahmanesh M, Gafos M. Pre-exposure prophylaxis (PrEP) uptake and adherence experiences of gay and bisexual men who engage in chemsex: a qualitative study. Int J Drug Policy. 2022;103:103630. doi: 10.1016/j.drugpo.2022.103630. [DOI] [PubMed] [Google Scholar]
- 60.Grov C, Rendina HJ, John SA, Parsons JT. Determining the roles that club drugs, marijuana, and heavy drinking play in PrEP medication adherence among gay and bisexual men: implications for treatment and research. AIDS Behav. 2019;23(5):1277–1286. doi: 10.1007/s10461-018-2309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hojilla JC, Vlahov D, Glidden DV, et al. Skating on thin ice: stimulant use and suboptimal adherence to pre-exposure prophylaxis. J Int AIDS Soc. 2018 doi: 10.1002/jia2.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coyer L, van den Elshout MAM, Achterbergh RCA, et al. Understanding pre-exposure prophylaxis (PrEP) regimen use: switching and discontinuing daily and event-driven PrEP among men who have sex with men. EClinicalMedicine. 2020;1(29–30):100650. doi: 10.1016/j.eclinm.2020.100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viamonte M, Ghanooni D, Reynolds JM, Grov C, Carrico AW. Running with scissors: a systematic review of substance use and the pre-exposure prophylaxis care continuum among sexual minority men. Curr HIV/AIDS Rep. 2022;19(4):235–250. doi: 10.1007/s11904-022-00608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.