Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a gastrointestinal pathogen that causes watery diarrhea and hemorrhagic colitis and can lead to serious and even fatal complications such as hemolytic uremic syndrome. We investigated the ability of EHEC to kill host cells using three human epithelial cell lines. Analysis of phosphatidylserine expression, internucleosomal cleavage of host cell DNA and morphological changes detected by electron microscopy changes revealed evidence of apoptotic cell death. The rates and extents of cell death were similar for both verotoxin-producing and nonproducing strains of EHEC as well as for a related gastrointestinal pathogen, enteropathogenic E. coli (EPEC). The induction of apoptosis by bacterial attachment was independent of verotoxin production and greater than that produced by a similar treatment with verotoxin alone. Expression of phosphatidylethanolamine, previously reported to bind EHEC and EPEC, was also increased on apoptotic cells but with little correlation to phosphatidylserine expression. Phosphatidylethanolamine levels but not phosphatidylserine levels on dying cells correlated with EHEC binding. Cells treated with phosphatidylethanolamine-containing liposomes also showed increased EHEC binding. These results suggest that bacterial induction of apoptosis offers an advantage for bacterial attachment by augmenting outer leaflet levels of the phosphatidylethanolamine receptor.
Enterohemorrhagic Escherichia coli (EHEC) is a gastrointestinal pathogen which causes watery diarrhea and hemorrhagic colitis and in some cases can lead to serious systemic microangiopathy including hemolytic uremic syndrome (HUS) (20). EHEC adheres intimately to intestinal epithelial cells via a complex mechanism, causing rearrangement of host cell cytoskeleton and triggering a number of intracellular signals including release of inositol phosphate and cytosolic calcium (17, 18, 22, 37). It also produces a Shiga-like toxin (verotoxin) which binds globotriaosylceramide (Gb3) on the surface of eukaryotic cells and, once internalized, inhibits protein synthesis, ultimately causing cell death (28, 29). While human renal endothelial cells express Gb3, rendering them particularly sensitive to the cytotoxic effects of verotoxin (5), human intestinal cell lines have not been found to express Gb3 (4, 27), and the mechanism of systemic toxemia remains in doubt. Moreover, verotoxin-negative (VT−) strains of EHEC still cause diarrhea (43, 48), suggesting that bacterial attachment plays in an important role in the pathogenesis of this infection. In a recent study, the adherence of a selection of EHEC isolates from patients associated with an outbreak of HUS and diarrhea was compared with that of a set of nonvirulent EHEC strains isolated from the contaminated food source but not found in any of the patients. The EHEC strains from the patients adhered to a far greater extent to Henle 407 (human colonic carcinoma) cells than did the strains isolated from the food (3), suggesting that attachment is an important virulence factor.
EHEC infection is also characterized by significant colonic injury. Histopathological examination of colonic specimens from patients with EHEC infection showed focal necrosis in the superficial mucosa (14, 36, 43, 44) and some evidence of apoptosis in the colonic crypts (14). Verotoxin has been implicated in both colonic injury and HUS-associated renal injury; however, Griffin found that colonic lesions in EHEC-infected patients showed no difference associated with toxin production (14). In vitro studies have demonstrated that EHEC adheres tightly to enterocytes, resulting in destruction of the apical surface of the microvilli (8, 40). Studies on animals infected with attaching and effacing (A/E) E. coli have also found evidence of apoptotic cell death (47).
Apoptosis and necrosis in host cells has been reported for a number of gastrointestinal pathogens including enteropathogenic E. coli (EPEC) (7), Yersinia (32), Salmonella (33), Helicobacter pylori (34), and Shigella (49, 50). Cell death reported for these pathogens is generally neither purely apoptotic nor purely necrotic but rather a mixture of the two. The induction of cell death usually serves to facilitate bacterial invasion and toxin delivery or to destroy potentially dangerous macrophages and lymphocytes. Certainly, in the case of invasive pathogens such as Shigella and Salmonella, the destruction of macrophages and lymphocytes (33, 49, 50) is critical to ensuring their survival. However, the rationale for the induction of cell death by a noninvasive organism such as EHEC is less obvious.
We have previously reported that EHEC and EPEC recognize phosphatidylethanolamine (PE) and that adhesion to human epithelial cells is inhibitable by preincubation with anti-PE (2). Since the induction of apoptosis (and necrosis) disrupts phospholipid membrane asymmetry and increases the amount of outer leaflet phosphatidylserine (PS) (11, 23), it may also increase outer leaflet PE levels, thereby enhancing bacterial attachment. Indeed, surface expression of PE on a cytotoxic T-cell line, CTLL2, increases upon induction of apoptosis by the withdrawal of interleukin 2 from the culture medium (10).
Therefore, induction of apoptosis and necrosis may represent a bacterial strategy for enhancing attachment through the augmentation of outer leaflet PE. Consequently, we undertook to investigate and compare the incidence and mode of cell death induced by EHEC with another A/E pathogen, EPEC, and to examine any effect of host cell death on the level of outer leaflet PE expression. The results support the hypothesis that the induction of apoptosis and necrosis enhances bacterial attachment and outer leaflet levels of PE and may provide a vehicle for the acquisition of cell nutrients as well as toxin delivery.
MATERIALS AND METHODS
Materials.
Thin-layer chromatography plates (TLC) (SilG) were purchased from Polygram (Macherey-Nagel, Duren, Germany). Phospholipids (PE from E. coli, phosphatidylcholine [PC] from egg yolk, and PS from bovine liver) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Phospholipids labeled with NBD (4-nitrobenzo-2-oxa-1,3-diazole), C6-NBD-PE and C6-NBD-PC, were purchased from Avanti Chemicals. Rabbit antisera to (i) the outer membrane preparation (anti-OMP) of HS, a human commensal E. coli strain, and (ii) the whole cell CL56 strain of verotoxin-producing E. coli were generously provided by P. Sherman (39). Rabbit antiserum to PE from E. coli was kindly provided by B. Gold, Division of Gastroenterology, Emory University School of Medicine. Rabbit antiserum to E. coli O157 somatic antigen was purchased from Oxoid Inc. Goat anti-rabbit-fluorescein isothiocyanate conjugate (FITC-GAR), goat anti-rabbit-phycoerythrin conjugate (PE-GAR), N-ethylmaleimide (NEM), and bovine serum albumin were purchased from Sigma. Verotoxin was prepared as previously described (16).
Liposome preparation.
Small unilamellar vesicles of a single phospholipid were prepared according to a modification of the method of Kremer et al. (24). Briefly, 5 mg of standard phospholipid was dried down under nitrogen and resuspended in 500 μl of ethanol. A volume of the ethanol solution (35 to 400 μl) was injected into 10 ml of phosphate-buffered saline (PBS) or minimum essential medium (MEM) with sonication. NBD-PE liposomes were prepared by adding 2 volumes of NBD-PE to 3 volumes PE stock solution. The mixtures were dried down under nitrogen and then redissolved in the same total volume of absolute ethanol. Liposomes were then prepared in either MEM or PBS as described above.
Bacterial strains and growth conditions.
The characteristics of bacterial strains used in this study are listed in Table 1. Bacteria were stored in 40% glycerol–5% citrate at −70°C. Prior to use, each strain was subcultured on Luria or horse blood agar (PML Microbiologicals, Mississauga, Ontario, Canada). Fresh cultures obtained in this way were grown either in static, nonaerated broth cultures of Penassay (Difco) or on horse blood agar. After overnight growth at 37°C, bacteria were harvested by centrifugation at 2,500 × g for 15 min and resuspended in sterile PBS, pH 7.4. Bacterial quantitation was determined by serial dilutions plated onto MacConkey agar plates without crystal violet (PML Microbiologicals). In the case of EPEC strains, they were subcultured on tryptic soy blood agar to maximize expression of bundle-forming pili which has been implicated in epithelial cell adherence (9).
TABLE 1.
Bacterial strains used in this study
| Strain | Description | VT1 expression | Reference |
|---|---|---|---|
| CL56 | EHEC (O157:H7) | + | 21 |
| 84-289 | EHEC (O157:H7) | + | 43 |
| 85-170 | EHEC (O157:H7) | − | 43 |
| E2348/69 | Wild-type EPEC | NAa | 26 |
| HB101 | Nonpathogenic laboratory strain | NA | 30 |
NA, not applicable.
Cell culture.
The human epithelial cell line HEp-2 (American Type Culture Collection, Manassas, Va.) (25) was grown in MEM (Gibco Laboratories, Grand Island, N.Y.) supplemented with 10% decomplemented fetal calf serum (FCS; Cansera International Inc., Rexdale, Ontario, Canada), 0.5% l-glutamine (ICN Biomedicals Inc., Costa Mesa, Calif.), 0.1% sodium bicarbonate (ICN), 2% streptomycin-penicillin (ICN), and 1% amphotericin B (ICN) at 37°C in 5% CO2. The human colonic cell line Caco2 (American Type Culture Collection) was grown in MEM with Earle's salts (GibcoBRL) supplemented with 0.5% l-glutamine, nonessential amino acids, 0.1% sodium bicarbonate, 1 mM sodium pyruvate, 1 M HEPES buffer (GibcoBRL), 10% FCS and 1% gentamicin (GibcoBRL) at 37°C in 5% CO2. The human intestinal cell line T84 (American Type Culture Collection) was grown in a 1:1 solution of Ham's F-12–MEM (GibcoBRL) with 10% FCS and 1% gentamicin.
Bacterial adhesion.
Bacterial binding to HEp-2 and to Caco2 cells was assayed both by culturing and counting adherent bacteria as previously described (39) and by a modification of a flow cytometric analysis (6). For the former method, approximately 106 viable cells were infected with 108 bacteria in culture medium without supplementary antibiotics for 3 h at 37°C. Colony counts of adherent bacteria were determined by using MacConkey agar plates without crystal violet.
In the flow cytometric analysis, approximately 108 bacteria were incubated with a confluent monolayer of cells for 1 to 2 h at 37°C in a CO2 incubator. After incubation with bacteria, both detached and adherent (trypsinized) cells were harvested and then separated from nonadherent bacteria by centrifugation (1,200 rpm for 10 min at 4°C) through an isotonic 15% sucrose solution. Cells were washed three times with PBS and then incubated with a bacterium-specific antibody for 1 h at 4°C. Cells were then washed and incubated with goat anti-rabbit fluorescent label conjugate (either FITC-GAR or PE-GAR at 1/40 dilution in PBS) for 30 min at 4°C in the dark. After three washes with PBS, cell-adherent bacteria were quantified by fluorescence-activated cell sorting analysis using a FACSScan Becton Dickenson flow cytometer. All samples were analyzed with CellQuest software. Using a dot plot of forward and side scatter, the flow cytometer was gated to include only single cells and to exclude cell debris and nonadherent bacteria. The mean channel fluorescence of the cell population is directly related to the surface density of cell-adherent bacteria. For a single fluorochrome, histogram plots showing cell count versus fluorescence intensity are provided. For double fluorochromes, dot plots are provided.
Induction of apoptosis.
To assess and compare the extent and nature of cell death induced by bacterial attachment, we used verotoxin 1 (VT1) treatment as a positive control for the induction of apoptosis. Since the human epithelial cell line HEp-2 is a Gb3-positive cell line which is sensitive to verotoxin (19, 42), it was selected as a model for the study of the apoptotic response. HEp-2 cells (85% confluent monolayers) were treated with various concentrations of VT1 for periods of time ranging from 1 to 30 h, after which the extent of apoptosis was determined by surface exposure of PS, acridine orange-propidium iodide (PI) staining, internucleosomal DNA fragmentation, and electron microscopy. To study the effect of bacterial attachment, subconfluent monolayers of HEp-2, Caco2, and T84 cells were incubated with 108 bacteria at 37°C in a CO2 incubator for periods of time ranging from 2 to 24 h. Assessment of apoptosis was conducted as described for verotoxin treatment.
Assessment of apoptosis and necrosis.
The level of outer leaflet PS was assessed by flow cytometry following treatment with annexin V-FITC (Pharmingen International) as previously described (46). An early marker of apoptosis is the loss of phospholipid membrane asymmetry, which results in an increase in outer leaflet levels of PS. Annexin V-FITC is a sensitive probe for the presence of outer leaflet PS and can therefore be used to track the extent of apoptosis. However, since the translocation of PS to the external cell surface is not unique to apoptosis but also occurs during necrosis, the combination of annexin V-FITC and PI can be used to distinguish between apoptotic and necrotic cells (13). Unfixed (nonpermeabilized) cells which stain with both annexin V-FITC and PI are either late apoptotic or necrotic, having lost membrane integrity. Cells which stain only with annexin V-FITC are considered to be early apoptotic.
Analysis of internucleosomal fragmentation was conducted as described elsewhere (7). Briefly, all cells from each treatment (both detached and adherent) were harvested and gently lysed with hypotonic lysis buffer (10 mM Tris [pH 7.4], 1 mM EDTA, 0.2% Triton X-100) at 4°C for 10 min. Supernatants from a 13,700 × g 10-min centrifugation at 4°C were treated with an equal volume of 1:1 phenol-chloroform and then recentrifuged for 10 min at 13,700 × g at 4°C. Supernatants were then incubated with 1 μg of glycogen, 1/10 volume of 3 M sodium acetate, and 1 ml of absolute ethanol at −20°C overnight to precipitate cellular DNA. The DNA was pelleted by centrifugation (16,000 × g for 20 min at 4°C), washed once with 70% ethanol, and then air dried at room temperature for 30 min. The DNA pellets were dissolved in 10 μl of Tris-EDTA buffer (TE; 10 mM Tris [pH 7.4], 1 mM EDTA) to which 12 ml of RNase (20 μg/ml) in TE buffer was added and incubated for 30 min at 37°C. Samples were then incubated with 3 μl of loading buffer (50 mM EDTA, 15% [wt/vol] Ficoll, 0.25% [wt/vol] bromophenol blue) for 15 min at 65°C and run on 1.5% agarose gels.
Cell death was also analyzed by electron microscopy. Trypsinized cell monolayers and detached cells were washed twice with PBS and overlaid with 1 ml of universal fixative (1:1 formaldehyde, 1% glutaraldehyde) and postfixed in 2% osmium tetroxide. Dehydration was carried out in graded ethanol, followed by propylene oxide and embedding in Epon. One-micrometer-thick sections were stained with toluidine blue and lead citrate. Samples were analyzed under a Philips 201 transmission electron microscope (N.V. Philips, Gloeilampenfabrieken, Eindhoven, The Netherlands). Apoptosis was also assessed by acridine orange-PI staining as previously described (45).
Detection of surface PE.
Surface-exposed PE on both HEp-2 cells was assayed by immunofluorescence and flow cytometry. Adherent cells were treated with various dilutions of rabbit anti-PE antiserum for 30 min at 4°C, room temperature, or 37°C, washed three times, and then incubated with FITC-GAR (1/50 dilution) for 30 min at 4°C in the dark. For immunofluorescence microscopy, cells were washed, fixed for 10 min with formalin (1% formaldehyde in PBS), examined, and photographed under incident UV illumination using a Polyvar fluorescence microscope. Alternatively, cells were detached with trypsin-EDTA and washed, and the intensity of the fluorescein signal on the cells was assessed by flow cytometry.
Addition of exogenous PE to cells.
Confluent monolayers of HEp-2 cells were washed once with PBS and then treated either with 2 mM NEM in MEM or MEM alone for 30 min at room temperature. The cells were then washed three times with PBS and incubated with 5 ml of NBD-liposome suspensions for 30 min at 4°C. Cells were then washed three times with PBS and incubated (1 h at 37°C in a CO2 incubator) with 8 ml of MEM either alone or inoculated with 600 μl of an overnight culture of bacteria. Adherent cells were then washed three times with PBS, trypsinized, and washed again with PBS. The cell pellet was incubated with 100 μl of primary antibody (anti-O157), washed three times, and incubated with 100 μl of secondary antibody (FITC-GAR, 1/1,000 dilution). After washing, the cell pellet was resuspended in PBS and analyzed for NBD-PE and bacterial binding by flow cytometry. Since this assay involved bacterial binding to NBD-PE-treated cells, we initially assessed bacterial binding to NBD-labeled phospholipids by TLC overlay as previously described (2). All of the EHEC and EPEC strains tested recognized NBD-labeled PE to the same degree as unlabeled PE. On the other hand, none of the strains bound NBD-PC, indicating that the phospholipid binding specificity was unchanged with NBD labeling.
RESULTS
Bacterial binding induces an increase in PS exposure.
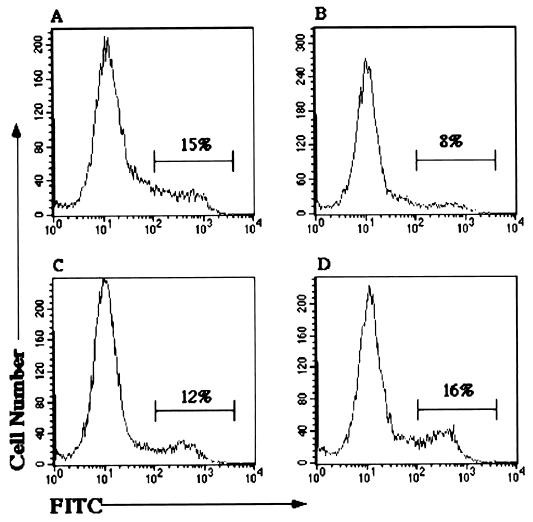
Incubation of HEp-2 cells with all three EHEC strains (CL56, 85-170, and 84-248) caused an increase in the level of outer leaflet PS as assessed by flow cytometry. Figure 1D shows that a 5-h incubation with the toxin-negative EHEC strain 85-170 resulted in high-intensity fluorescent staining with annexin V-FITC in 16% of the total cell population. Similar results were achieved with the pathogenic EPEC strain E2348/69 (Fig. 1C), the two toxigenic EHEC strains CL56 and 84-248 (results not shown), and VT1 (Fig. 1A). In contrast, incubation with the nonpathogenic HB101 produced lower PS levels (Fig. 1B), which were equivalent to those of untreated HEp-2 cells (not shown). Incubation with sterile-filtered supernatants from VT− EHEC (85-170) and VT+ EHEC (84-289) strains produced PS levels similar to those associated with untreated and VT1-treated cells respectively. Treatment with any of the EHEC strains also increased levels of outer leaflet PS in both Caco2 and T84 cells (data not shown). PS exposure in these cell lines was also independent of the production of verotoxin.
FIG. 1.
Flow cytometric analysis of outer leaflet levels of PS in HEp-2 cells. The x axis represents the staining intensity on the cells; the y axis represents the relative cell number. The percentage of cells exhibiting increased fluorescence intensity is indicated. Cells were preincubated with the following: (A) VT1 (12.5 ng/ml overnight) or 108 bacteria for 5 h; (B) HB101; (C) EPEC (E2348/69); (D) EHEC (85-170). Membrane PS was detected using annexin V-FITC. Incubation with EHEC 84-289 and CL56 produced histograms similar to panel D; PS levels for HEp-2 cells alone produced results similar to panel B.
The increase in outer leaflet PS levels correlated with the bacterium-cell incubation time, as shown in Figure 2. As the incubation time increases from 2 h to 20 h (overnight), the percentage of cells stained with high fluorescence intensity increases. These results demonstrate that incubation with both toxigenic and nontoxigenic EHEC as well as EPEC results in increased outer leaflet levels of PS, consistent with the induction of apoptosis and possibly necrosis in three epithelial cell lines.
FIG. 2.
Effect of bacterial incubation time on PS expression. HEp-2 cells were incubated with EHEC (85-170) for 2 h (dotted), 3 h (black), or overnight (grey). PS expression was determined by flow cytometric analysis of annexin V-FITC binding as for Fig. 1. Similar results (not shown) were achieved with EHEC strains 84-289 and CL56.
Bacterial binding induces apoptosis and necrosis.
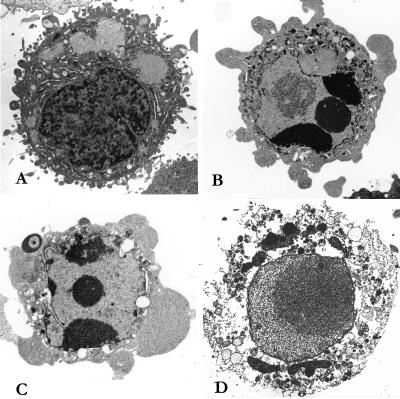
Electron microscopy of EHEC-infected HEp-2 cells revealed morphological changes consistent with induction of both apoptosis and necrosis (Fig. 3). Apoptotic cells were characterized by membrane blebbing and nuclear condensation, while necrotic cells were typically larger and lighter with plasma membrane lesions and mitochondrial abnormalities. Percentages of apoptotic cells in EHEC-infected HEp-2 (5-h incubation) were similar to those associated with VT1 overnight treatment (Table 2). Electron micrographs also indicated that EHEC targeted both apoptotic and necrotic cells in preference to viable cells. Acridine orange-PI staining of HEp-2 cells treated with VT1 or EHEC (not shown) confirmed electron microscopic evidence of apoptosis.
FIG. 3.
Electron micrographs of HEp-2 cells. (A) Viable cell (untreated); (B) apoptotic cell (treated with VT1 [12.5 ng/ml] overnight); (C) apoptotic cell (treated with CL56 for 5 h); (D) necrotic cell (treated with VT1 [12.5 ng/ml] overnight).
TABLE 2.
Levels of apoptosis and necrosis in VT1 or bacterium-treated HEp-2 cells (determined by electron microscopy)a
| Treatment | Mean % ± SD
|
|
|---|---|---|
| Apoptotic cells | Necrotic cells | |
| None | 0.85 ± 0.69 | 3.9 ± 1.0 |
| VT 1 (12.5 ng/ml overnight) | 15.8 ± 3.6 | 15.2 ± 2.8 |
| EPEC E2348/69 (5 h) | 13.5 ± 2.3 | 13.4 ± 1.4 |
| EHEC 84-289 (5 h) | 12.0 ± 1.9 | 15.0 ± 3.5 |
| EHEC 85-170 (5 h) | 10.7 ± 0 | 12.3 ± 2.5 |
| HB101 (5 h) | 6.2 ± 1.2 | 7.3 ± 0.7 |
Percentages based on a count of at least 100 cells and experiments were determined two to five times. Apoptosis was evidenced by nuclear condensation, membrane blebbing, and decreased cell volume. Necrosis was evidenced by swollen cell morphology, nuclear membrane disintegration, and plasma membrane breaks.
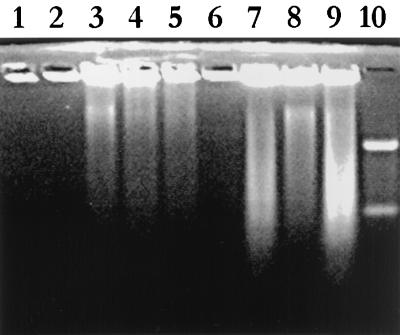
Analysis of fragmentation of internucleosomal DNA from both HEp-2 and Caco2 cells incubated with EHEC and EPEC revealed evidence of apoptotic DNA ladder formation (Fig. 4). Ladder formation was evident for HEp-2 and Caco2 cells treated overnight with VT1 or any of CL56, 85-170, 84-289, or E2348/69. There was no DNA fragmentation noted for either untreated or HB101-treated cells. Although the DNA ladders generated by bacterial incubation were not the distinctive band patterns usually associated with apoptosis, these DNA patterns were consistent with those reported for other apoptosis-inducing bacteria (7) and reflect the mixture of apoptosis and necrosis which is evident from electron microscopy. It has also been reported that internucleosomal cleavage of DNA in epithelial cell lines may be defective (35).
FIG. 4.
Analysis of internucleosomal DNA fragmentation by agarose gel electrophoresis of DNA extracts from HEp-2 cells incubated with medium (lane 1), 100 ng of VT1 (24 h) (lane 2), 100 ng of VT1 (48 h) (lane 3), 200 ng of VT1 (24 h) (lane 4), 200 ng of VT1 (48 h) (lane 5), HB101 (5 h) (lane 6), 85-170 (5 h) (lane 7), 84-289 (5 h) (lane 8), or E2348/69 (5 h) (lane 9). Lane 10, 100-kb standard.
Induction of apoptosis increases outer leaflet PE levels and EHEC binding.
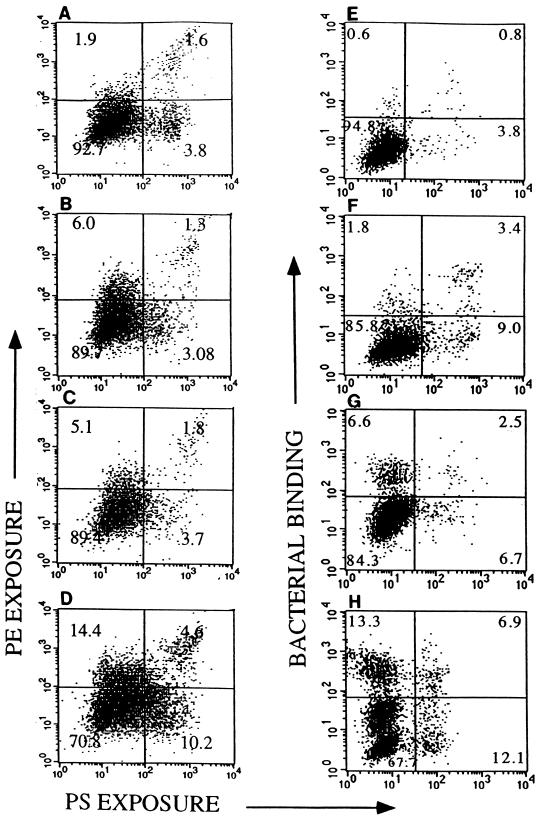
When HEp-2 cells were treated with VT1, the level of surface-exposed PE detected by immunofluorescence microscopy increased (not shown). Through the combined use of annexin V-FITC, a PE-specific antiserum, and PI, we were able to simultaneously assess the outer leaflet levels of PE and PS and to distinguish between apoptotic and necrotic cell populations following treatment with VT1 (Fig. 5A to D). Outer leaflet levels of both PE (Fig. 5A to D, upper quadrants) and PS (right-side quadrants) increased with the VT1 incubation period. However, the percentage of cells which were double labeled for PE and PS (upper right quadrants) was relatively low, suggesting that VT1 treatment of the HEp-2 cells triggers either PE or PS elevation on the cell surface. Furthermore, the double-labeled PE-PS-positive cells also stained with PI (not shown), suggesting that these cells are either late apoptotic or necrotic cells. When PI-positive cells were gated out, approximately 75% of the VT1-treated cells remained PE positive. These results suggest that PE may serve as an alternative early marker for apoptosis and may, in fact, precede PS exposure on these cells.
FIG. 5.
Flow cytometric analysis of PE and PS exposure and EHEC binding to HEp-2. Cells were preincubated for various times (0 to 20 h) with 12.5 ng of VT1 per ml in serum-free media. (A to D) Cells treated both with annexin V-FITC (to detect PS) and anti-PE (visualized with phycoerythrin-GAR conjugate) to compare PS (abscissa) and PE (ordinate) exposure with VT1 incubation time. (A) No VT1; (B) VT1, 1 h; (C) VT1, 3 h; (D) VT1, 20 h. (E to H) Cells pretreated with VT1 or medium, infected with 108 CL56, and stained with annexin V-FITC and a bacterium-specific antiserum to compare PS exposure (abscissa) with bacterial binding (ordinate). (E) Control (no VT1, no CL56); (F) VT1, 20 h (no CL56); (G) CL56 (no VT); (H) VT1 (20 h), CL56. Quadrant numbers refer to percentage of cells simultaneously stained with both fluorophores with intensities as indicated on the axes.
In a similar experiment using annexin V-FITC, EHEC-specific antisera, and PI, we assessed the level of bacterial binding versus PS exposure for apoptotic and necrotic cell populations (Fig. 5E to H). Bacterial binding (Fig. 5E to H, upper quadrants) and outer leaflet PS levels (right-side quadrants) increased after VT1 treatment in a manner very similar to that shown for PE-PS exposure (Fig. 5A to D). The percentage of cells which were double labeled for bacteria and PS (Fig. 5E to H, upper right quadrants) was similarly low, suggesting that VT1-treated cells resulted in either enhanced bacterial binding or PS exposure.
A comparison of the dot plots with respect to PE-surface exposure and bacterial binding suggests that PE exposure correlates with bacterial binding. Gating out PI-positive cells reduced the number of bacterium-adherent cells and PE-positive cells by similar amounts (not shown). The correlation between outer leaflet PE levels and bacterial binding is consistent with previous studies where we reported that binding of CL56 to HEp-2 cells could be inhibited with PE-specific antisera (2). Results similar to those shown in Fig. 5 were also achieved with EHEC strains 85-170 (VT−) and 84-289 (VT+) and EPEC strain E2348/69. Analysis of bacterial binding by the colony count method confirmed the correlation between bacterial binding and VT1 treatment: binding of CL56 to HEp-2 cells preincubated overnight with media (untreated) or VT1 (0.5 ng/ml), assessed by colony count and expressed as bacteria bound averaged over the number of cells per flask (n = 6), was 0.85 ± 0.37 or 3.04 ± 0.76, respectively.
Addition of exogenous PE augments outer leaflet PE and bacteria binding.
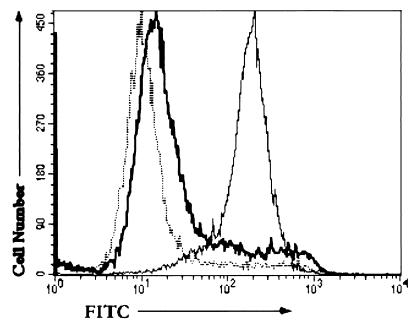
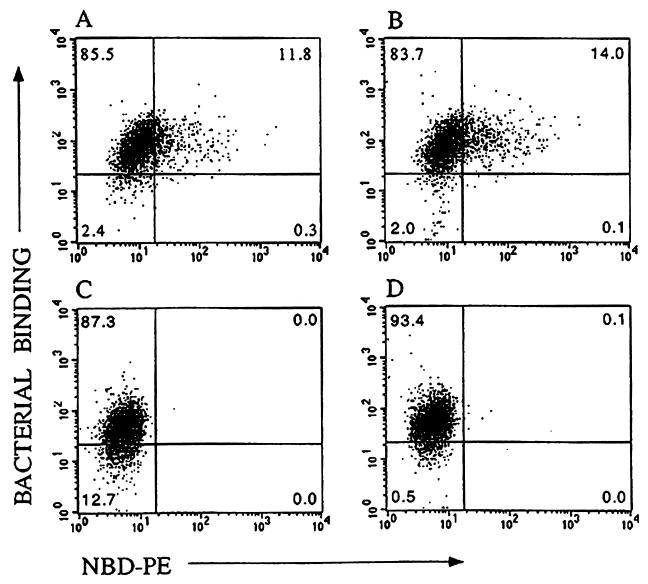
Treatment of cells with fluorescently labeled (NBD) PE-containing liposomes increased the level of outer leaflet PE and the binding of CL56 as detected by flow cytometry. Figure 6A shows that uptake of NBD-PE by HEp-2 cells is correlated with the level of binding of EHEC, with virtually all NBD-labeled cells bound by EHEC (upper right quadrant). Previous studies have shown that the majority of exogenous PE taken up by cells through liposome fusion is quickly transferred to the inner leaflet by the action of an aminophospholipid translocase (41). Pretreatment with NEM has been reported to inhibit this translocase-mediated transfer of PE (31), thereby permitting more of the PE to remain on the outer leaflet. Pretreatment of the cells with NEM resulted in a small increase in the percentage of double-stained cells (Fig. 6B, upper right-hand quadrant), confirming the correlation between outer leaflet PE levels and bacterial binding. Controls without liposome treatment showed lower levels of bacterial binding (upper quadrants) than the liposome-treated samples, with and without NEM pretreatment (Fig. 6D and C, respectively).
FIG. 6.
Bacterial binding versus NBD-PE uptake. Flow cytometric dot plots show the fluorescence intensity of NBD-PE staining on the abscissa and bacterial binding (FITC staining) on the ordinate. Bacterial binding was determined for CL56 with cells treated with NBD-PE liposomes (A), cells pretreated with NEM and then NBD-PE liposomes (B), untreated cells (C), and cells pretreated with NEM (D). Additional controls (not shown) included cells incubated with primary and/or secondary antibody and cells incubated only with NBD-PE liposomes.
DISCUSSION
We report here that EHEC induces a mixture of apoptosis and necrosis in HEp-2, Caco2, and T84 cells. Bacterial attachment was required for maximal induction of apoptosis. Both toxigenic and nontoxigenic strains of EHEC were capable of inducing similar levels of PS expression, while sterile-filtered supernatants from verotoxin-producing strains showed much lower levels and those from nontoxigenic strains produced only background levels. The extent of EHEC-induced apoptosis was similar to that triggered by EPEC in the same cell lines. A 5-h bacterial incubation with either EHEC or EPEC produced the same degree of PS expression as an overnight treatment with 12.5 ng of VT1 per ml. This toxin dosage was found to produce optimal levels of apoptosis in HEp-2 cells as ascertained by PS expression, DNA fragmentation, and electron microscopy and is in agreement with that reported by others (19).
Overnight treatment with any of the EHEC or EPEC strains resulted in cellular DNA fragmentation in both HEp-2 and Caco2 cells. These fragmentation patterns were consistent with those reported for the EPEC strain E2348/69 with T84 cells (7).
Electron micrographs of EHEC-infected cells revealed morphological changes consistent with a mixture of apoptosis and necrosis where 11 to 14% of the cells were apoptotic. EPEC-infected HEp-2 cells showed similar levels of apoptosis and necrosis. These findings are consistent with those reported for EPEC with another intestinal cell line, HeLa (7). Invasive gastrointestinal pathogens such as Salmonella and Shigella, while most efficient at killing macrophages, are less or unable to kill intestinal epithelial cells even though they are able to invade these cells (7, 33). Although we did not include macrophages in this study, others have reported that EHEC and EPEC do not kill macrophages (12).
What was striking about the electron micrographs of EHEC-infected cells was the preferential targeting by the bacteria of apoptotic and necrotic cells. Very few bacteria were attached to healthy cells. This could simply be the result of binding and simultaneous induction of cell death by the bacteria. However, the results did appear to suggest that the bacteria were preferentially adhering to dying cells. To investigate this possibility, we pretreated HEp-2 cells with VT1 and then measured bacterial binding. Adhesion of both toxigenic and nontoxigenic EHEC strains increased after VT1 treatment, confirming that the bacteria bound better to dying cells.
The rationale behind the preferential adhesion to dying cells seems, at first, counterproductive. Dying cells will be more easily sloughed off and cleared from the gastrointestinal tract, thereby eliminating bacteria. However, attachment to dying cells may facilitate nutrient access and, possibly, the delivery of verotoxin into the epithelium. The transport of verotoxin from the gut lumen to the underlying tissues and bloodstream has been the subject of considerable controversy (37) and has been postulated to be mediated alternatively by mucosal lesions, bacterium-produced intimin, or tight junctions between epithelial cells (1, 38). It is possible that bacterium-induced apoptosis/necrosis could provide entry route for verotoxin into the epithelial cell and facilitate eventual transport to the underlying tissues.
Alternatively, bacterial targeting of dying cells may simply be the result of an attachment-chain reaction in which bacteria bind to certain receptors and induce apoptosis/necrosis, which upregulates these receptors in the dying cells and thereby provides additional binding sites. This possibility prompted our investigation into the role of host membrane PE which we previously showed to be specifically recognized by both EHEC and EPEC (2). Since apoptosis disrupts phospholipid membrane asymmetry, outer leaflet levels of PE should increase in a manner similar to that already reported for PS. Bacterial recognition of PE would, therefore, explain the increased binding to apoptotic cells. Comparison of bacterial binding with PS and PE expression on verotoxin-treated cells revealed a striking similarity between bacterial binding and PE levels. PS levels, however, did not correlate with either bacterial binding or PE levels. The lack of correlation between bacterial binding and PS expression is consistent with our previous findings of no interaction between EHEC and PS in both solid-phase and liposome binding assays (2).
The low correlation in this study between PE and PS levels suggests the existence of either separate apoptotic signaling pathways or separate populations of cells expressing PE or PS. The possibility that one is associated with apoptosis and the other with necrosis was ruled out by the maintenance of this difference after exclusion of PI-positive (necrotic) cells. Future studies are needed to clarify the basis for this difference in aminophospholipid expression during apoptosis.
Most of the literature to date on bacterially induced cell death tends to emphasize the role of apoptosis over necrosis. However, neither our data nor our hypothesis excludes the involvement of necrosis, since the loss of host membrane integrity associated with necrosis would also increase the availability of host PE receptors and, therefore, bacterial binding. The electron microscopy and flow cytometry results do show a mixture of apoptosis and necrosis in the bacterium-treated cell samples. However, the extent of necrosis and the significance of its role in the host-bacterium interaction is ambiguous, since necrosis detected in vitro may not be physiological. In vivo, apoptotic cells are normally phagocytosed by macrophages, whereas in vitro, they may eventually lose their membrane integrity and appear necrotic. Therefore, while we do not exclude necrosis, the involvement of this cell death mechanism in offering a bacterial advantage is not clear.
To confirm the role of PE in bacterial attachment, HEp-2 cells were pretreated with PE-containing liposomes in an effort to augment the amount of outer leaflet PE. Although most of the exogenous PE is transferred rapidly to the inner leaflet by a lipid translocase (31), bacterial binding to PE-treated cells increased and was directly correlated with NBD-PE labeling. Inhibition of the lipid translocase by NEM (31) resulted in a further increase in both outer leaflet PE levels and EHEC binding, confirming the role of PE in bacterium-cell attachment.
In conclusion, these results clearly indicate that EHEC triggers a mixture of apoptosis and necrosis in epithelial cells, an event which is independent of toxin production. They also provide, for the first time, a rationale for EHEC and EPEC induction of apoptosis/necrosis through the augmentation of the host cell receptor candidate, PE. Host cell death as a means to amplify receptor expression can provide bacteria with only a transitory adherence advantage, given the efficient phagocytosis and sloughing off of dead cells from the gastrointestinal tract (15). Crane et al. (7) have postulated that EPEC has mechanisms to suppress apoptosis (and enhance mitosis) which could used to improve bacterial retention on the mucosal surface. Whether these events ultimately confer a bacterial advantage or provide a host defense, they offer interesting insight into the dynamic interplay between host and bacterium.
ACKNOWLEDGMENT
This work was supported by a grant from the Crohn's and Colitis Foundation of Canada (to D. Barnett Foster) and the Medical Research Council of Canada (MT13073) (to C. A. Lingwood).
We thank Beth Boyd and Anita Nutikka for their excellent technical assistance and advice.
REFERENCES
- 1.Acheson D W K, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch G T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett Foster D E, Philpott D, Abul-Milh M, Huesca M, Sherman P M, Lingwood C A. Phosphatidylethanolamine recognition promotes enteropathogenic and enterohemorrhagic E. coli host cell attachment. Microb Pathog. 1999;27:289–301. doi: 10.1006/mpat.1999.0305. [DOI] [PubMed] [Google Scholar]
- 3.Bauer M E, Welsh R A. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1996;64:167–175. doi: 10.1128/iai.64.1.167-175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björk S, Breimer M E, Hansson G C, Karlsson K-A, Leffler H. Structures of blood group glycosphingolipids in human small intestine. J Biol Chem. 1987;262:6758–6765. [PubMed] [Google Scholar]
- 5.Boyd B, Lingwood C A. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51:207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- 6.Clyne M, Drumm B. Absence of effect of Lewis A and Lewis B expression on adherence of Helicobacter pylori to human gastric cells. Gastroenterology. 1997;113:72–80. doi: 10.1016/s0016-5085(97)70082-9. [DOI] [PubMed] [Google Scholar]
- 7.Crane J K, Majumdar S, Pickhardt D F., III Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232:430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 11.Fadok V, Voelker D, Campbell P, Cohen J, Bratton D, Henson P. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 12.Fernandez-Prada C, Tall B, Elliot S, Hoover D, Nataro J, Venkatesan M. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorczyca W, Melamed M, Darzynkiewicz Z. Analysis of apoptosis by flow cytometry. In: Jaroszeski M J, Heller R, editors. Methods in molecular biology. Totowa, N.J: Humana Press; 1997. pp. 217–238. [DOI] [PubMed] [Google Scholar]
- 14.Griffin P M, Olmstead L C, Petras R E. Escherichia coli O157:H7-associated colitis. Gastroenterology. 1990;99:142–149. doi: 10.1016/0016-5085(90)91241-w. [DOI] [PubMed] [Google Scholar]
- 15.Hall P, Coates P J, Ansari B, Hopwood D. Regulation of cell number in mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 16.Huang A, DeGrandis S, Friesen J, Karmali M A, Petric M, Congi R, Brunton J L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986;166:375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismaili A, McWhirter E, Handelsman M Y, Brunton J L, Sherman P M. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect Immun. 1998;66:1688–1696. doi: 10.1128/iai.66.4.1688-1696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismaili A, Philpott D, Dytoc M T, Shermann P M. Signal transduction responses following adhesion of verotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones N L, Islur A, Mascarenhas M, Karmali M A, Sherman P M. Induction of apoptosis in human epithelial cells by Shigatoxin-1: role of globotriaosylceramide. J Pediatr Gastroenterol Nutr. 1998;26:551. [Google Scholar]
- 20.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between hemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 22.Knutton S, Baldwin T, Williams P, McNeish A. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman G, Reutelingsperger C, Kuijten G, Keehnen R, Pals S, van Oers M. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 24.Kremer J M, Esker M W, Pathmamanoharan C, Wiersema P H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977;16:3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- 25.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 26.Levine M M, Berquist J, Nalen D R, Waterman D H, Hornich R B, Young C R, Sotman S. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Bell C, Buret A, Robins-Browne R M, Stiel D, O'Loughlin E. The effect of Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 28.Lingwood C A. Verotoxins and their glycolipid receptors. In: Bell R, Hannun Y A, Merrill Jr A, editors. Advances in lipid research. San Diego, Calif: Academic Press; 1993. pp. 189–212. [PubMed] [Google Scholar]
- 29.Lingwood C A. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 30.Louie M, DeAzavedo J, Clarke R, Brunton J. Serotype distribution and sequence heterogeneity of eae gene in verotoxin-producing E. coli. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin O N, Pagano R E. Transbilayer movement of fluorescent analogues of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. J Biol Chem. 1987;262:5890–5898. [PubMed] [Google Scholar]
- 32.Monack D, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberhammer F, Wilson J, Dive C, Morris I, Hickman J, Wakeling A, Walker P, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pai C H, Gordon R, Sims H V, Bryan L E. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7: clinical, epidemiological, and bacteriological features. Ann Intern Med. 1984;101:738–742. doi: 10.7326/0003-4819-101-6-738. [DOI] [PubMed] [Google Scholar]
- 37.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philpott D J, Ackerley C A, Kiliaan A J, Karmali M A, Perdue M H, Sherman P M. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am J Physiol. 1997;273:G1349–G1358. doi: 10.1152/ajpgi.1997.273.6.G1349. [DOI] [PubMed] [Google Scholar]
- 39.Sherman P, Cockerill III F, Soni R, Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7. Infect Immun. 1991;59:890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman P, Soni R, Karmali M. Attaching and effacing adherence of vero cytotoxin-producing Escherichia coli to rabbit intestinal epithelium in vivo. Infect Immun. 1988;55:756–761. doi: 10.1128/iai.56.4.756-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sleight R G, Pagano R E. Transition movement of fluorescent phosphatidylethanolamine analogue across the plasma membrane of cultured mammalian cells. J Biol Chem. 1985;260:1146–1154. [PubMed] [Google Scholar]
- 42.Tyrrell G J, Ramotar K, Toye B, Boyd B, Lingwood C A, Brunton J L. Alteration of the carbohydrate binding specificity of verotoxins from Galα1-4Gal to GalNAcβ1-3Galα1-4Gal and vice versa by site-directed mutagenesis of the binding subunit. Proc Natl Acad Sci USA. 1992;89:524–528. doi: 10.1073/pnas.89.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzipori S, Karch H, Wachsmuth I K, Robins-Browne R M, O'Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Role of a 60-MDa plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzipori S, Wachsmuth I K, Chapman C, Birner R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic pigs. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 45.Vasconcelos A C, Lam K M. Apoptosis induced by infectious bursal disease virus. J Gen Virol. 1994;75:1803–1806. doi: 10.1099/0022-1317-75-7-1803. [DOI] [PubMed] [Google Scholar]
- 46.Vermes I, Haanen C, Steefean-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 47.Wada Y, Mori K, Iwanaga T. Apoptosis of enterocytes induced by inoculation of a strain of attaching and effacing Escherichia coli and verotoxin. J Vet Med Sci. 1997;59:815–818. doi: 10.1292/jvms.59.815. [DOI] [PubMed] [Google Scholar]
- 48.Waddell T, Lingwood C A, Gyles C L. Interaction of verotoxin VT2e with the pig intestine. Infect Immun. 1996;64:1714–1719. doi: 10.1128/iai.64.5.1714-1719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 50.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]