Abstract
Background
Pre-stenting intravascular ultrasound (IVUS) assessment is helpful for appropriate stent sizing and determination of the stent landing zone during percutaneous coronary intervention.
Aims
The aim of this meta-analysis was to investigate the effect of pre-stenting IVUS evaluation on procedural and clinical outcomes for diffuse lesions treated with drug-eluting stents (DES).
Methods
In four randomised trials comparing IVUS- and angiography-guided DES placement, a total of 1,396 patients who underwent DES implantation with IVUS guidance were identified. Pre-stenting IVUS assessment was performed in 905 patients along with post-stenting IVUS (65%; pre-stenting IVUS(+) group). Post-stenting IVUS evaluation alone was conducted on 491 patients (35%; pre-stenting IVUS(−) group).
Results
The pre-stenting IVUS(+) group had a larger angiographic minimal lumen diameter and IVUS-derived minimal stent area (MSA) than did the pre-stenting IVUS(−) group. After adjusting, these findings were consistent. The one-year composite of cardiac death, myocardial infarction, and target vessel revascularisation did not differ between the groups. In subgroup analysis, the pre-IVUS(+) group was significantly favoured over the pre-IVUS(−) group in the subset of patients with acute myocardial infarction and lesions with small vessels in terms of larger MSA, and in the subset of patients with chronic total occlusions in terms of better clinical outcomes.
Conclusions
Pre-stenting IVUS assessment prior to DES placement was associated with better acute procedural outcomes, though this did not translate into one-year clinical outcomes in the context of post-stenting IVUS assessment.
Introduction
Pre-stenting intravascular ultrasound (IVUS) assessment can provide extensive qualitative and quantitative information including the measurement of cross-sectional dimensions of the lumen and the vessel, and plaque characteristics, which is helpful for appropriate stent sizing and determination of the stent landing zone. On the other hand, post-stenting IVUS assessment can provide information regarding stent underexpansion, malapposition, or edge dissection which are the main components of stent optimisation1. The clinical usefulness of IVUS guidance during percutaneous coronary intervention (PCI) and improvement of clinical outcomes have been reported in several meta-analyses and randomised clinical trials2,3,4,5,6,7,8,9,10. However, these studies focused mainly on the role of post-stenting IVUS evaluation in relation to the final post-procedure optimisation after drug-eluting stent (DES) implantation, and did not specify the role of IVUS evaluation before stent implantation. There have been no studies to investigate the impact of pre-stenting IVUS evaluation on procedural and clinical outcomes. Therefore, this study was performed to evaluate the independent role of pre-stenting IVUS assessment in IVUS-guided PCI, comparing the acute procedural and one-year clinical outcomes after DES implantation between patients who underwent pre-stenting IVUS assessment and those who did not in the context of post-stenting IVUS assessment.
Methods
STUDY DESIGN AND POPULATION
This study included four randomised trials comparing IVUS and angiography guidance for diffuse long or chronic total occlusion lesions treated with new-generation DES: RESET IVUS (Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-eluting Stent Implantation)6, CTO-IVUS (Chronic Total Occlusion Intervention with Drug-eluting Stents Guided by Intravascular Ultrasound)7, IVUS-XPL (Impact of IntraVascular UltraSound Guidance on Outcomes of Xience Prime Stents in Long Lesions)8, and ULTRA-ZET (Intravascular Ultrasound Guided Versus Conventional Angiography Guided Strategy to Deploy Zotarolimus and Everolimus Eluting Third Generation Stents in Long Coronary Artery Lesions). Detailed explanations of these studies are shown in Supplementary Table 1. Each study included in the present analysis was approved by the institutional review board or ethics committee at each participating centre, and all patients provided written informed consent. Briefly, patients with diffuse long lesions with stent length ≥26–28 mm or chronic total occlusion treated with new-generation DES were included. All patients underwent post-stenting IVUS assessment for DES optimisation. A total of 1,396 patients were identified and divided into two groups based on whether they underwent pre-stenting IVUS evaluation - the patients who underwent pre-stenting IVUS assessment as well as post-stenting evaluation (pre-stenting IVUS(+) group) versus the patients who underwent post-stenting evaluation alone (pre-stenting IVUS(−) group). The statisticians from each trial obtained patient-level data by directly accessing the study databases. Data about the baseline characteristics of the patients, procedure information, and clinical events were collected. These data were pooled and analysed in a single data set.
QUANTITATIVE CORONARY ANALYSES, IVUS EXAMINATIONS AND IVUS ANALYSES
Detailed methods are provided in Supplementary Appendix 1.
ENDPOINTS, DEFINITIONS, AND FOLLOW-UP
The occurrence of the composite outcome of cardiac death, myocardial infarction (MI), or target vessel revascularisation (TVR) after one year was assessed. The individual components of the composite outcome were assessed. The specific endpoint definitions applied in each trial were also incorporated into the study. All deaths were considered cardiac deaths unless a definite non-cardiac cause was established11. MI after hospital discharge was defined as the presence of clinical symptoms, changes on electrocardiography, or abnormal imaging findings that indicated MI along with an increase in the creatine kinase myocardial band fraction above the upper normal limit or an increase in troponin T or I level greater than the 99th percentile of the upper normal limit, regardless of interventional procedures8. Stent thrombosis was defined as definite or possible stent thrombosis according to the Academic Research Consortium definition11. TVR was defined as repeat PCI or bypass surgery of the target vessel for either of the following reasons: 1) presence of ischaemic symptoms or positive stress test results and angiographic diameter stenosis ≥50% as measured via quantitative coronary analyses, or 2) angiographic diameter stenosis ≥70% without ischaemic symptoms or positive stress test results6,7,8. Clinical follow-up and assessment were performed in the hospital after 1, 3, 6, and 12 months via either clinic visit or telephone interview.
STATISTICAL ANALYSIS
Detailed methods are provided in Supplementary Appendix 1.
Results
BASELINE CHARACTERISTICS AND DETERMINANTS OF THE USE OF PRE-STENTING IVUS
Pre-stenting IVUS assessment was performed in 905 patients (65%; the pre-stenting IVUS(+) group), and post-stenting IVUS assessment alone was conducted in 491 patients (35%; the pre-stenting IVUS(−) group). Baseline clinical, angiographic, and procedural characteristics are presented in Table 1. The proportion of patients who maintained dual antiplatelet therapy at 12 months was similar between the groups (66% vs 65%, p=0.703). On multivariable analysis for the major determinants of the usage of pre-stenting IVUS, younger age, treatment of the left anterior descending artery, and chronic total occlusions were considered significant factors (Supplementary Table 2). The groups were comparable after inverse probability of treatment weighting adjustment (Supplementary Table 3) and after propensity score matching (Supplementary Table 4). As for procedural characteristics, the pre-stenting IVUS(+) group had a significantly greater stent diameter even with an angiographically similar reference vessel diameter than the pre-stenting IVUS(−) group. Consequently, the maximal stent diameter-to-reference vessel ratio was significantly higher in the pre-stenting IVUS(+) group than in the pre-stenting IVUS(−) group. As compared to the pre-stenting IVUS(−) group, the pre-stenting IVUS(+) group had a less frequent use of adjuvant post-dilation including high-pressure ballooning (≥15 atm) but a greater size of final balloon used.
Table 1. Clinical, angiographic, and procedural characteristics.
| Variable | Pre-stenting IVUS (+) n=905 | Pre-stenting IVUS (−) n=491 | p-value | |
| Age, years | 62±10 | 64±9 | <0.001 | |
| Male | 650 (72%) | 343 (70%) | 0.439 | |
| Body mass index, kg/m2 | 24.8±2.9 | 24.5±3.0 | 0.052 | |
| Hypertension | 569 (63%) | 318 (65%) | 0.483 | |
| Diabetes mellitus | 312 (35%) | 185 (38%) | 0.233 | |
| Current smoker | 225 (25%) | 119 (24%) | 0.796 | |
| Prior myocardial infarction | 41 (5%) | 26 (5%) | 0.523 | |
| Clinical presentation | Stable angina | 557 (62%) | 282 (57%) | 0.016 |
| Unstable angina | 269 (30%) | 142 (29%) | ||
| Acute myocardial infarction | 79 (9%) | 67 (14%) | ||
| Coronary arteries treated | Left anterior descending | 563 (62%) | 243 (50%) | <0.001 |
| Left circumflex | 111 (12%) | 99 (20%) | ||
| Right coronary artery | 231 (26%) | 149 (30%) | ||
| Chronic total occlusions | 214 (24%) | 45 (9%) | <0.001 | |
| Moderate to severe calcification | 166 (18%) | 80 (16%) | 0.337 | |
| Preprocedural quantitative coronary angiography | Reference vessel diameter, mm | 2.86±0.45 | 2.85±0.47 | 0.635 |
| Minimal lumen diameter, mm | 0.71±0.55 | 0.76±0.47 | 0.073 | |
| Diameter stenosis, % | 75.68±18.28 | 73.19±15.99 | 0.011 | |
| Lesion length, mm | 35.69±14.05 | 35.02±13.35 | 0.388 | |
| Type of drug-eluting stent | Everolimus-eluting | 541 (60%) | 388 (79%) | <0.001 |
| Zotarolimus-eluting | 257 (28%) | 90 (18%) | ||
| Biolimus-eluting | 107 (12%) | 13 (3%) | ||
| Mean stent diameter per lesion, mm | 3.08±0.37 | 3.00±0.37 | <0.001 | |
| Maximum stent diameter per lesion, mm | 3.17±0.38 | 3.05±0.05 | <0.001 | |
| Total stented length per lesion, mm | 40.09±17.18 | 37.91±15.46 | 0.016 | |
| No. of stents per lesion | 1.47±0.64 | 1.30±0.54 | <0.001 | |
| Ratio of maximal stent diameter to reference | 1.13±0.17 | 1.09±0.16 | <0.001 | |
| Adjunct post-dilation | 539 (60%) | 381 (78%) | <0.001 | |
| High-pressure adjunct post-dilation | 345 (38%) | 240 (49%) | <0.001 | |
| Final balloon size, mm | 3.17±0.45 | 3.06±0.42 | <0.001 | |
| Values are mean±standard deviation or number (%). IVUS: intravascular ultrasound | ||||
ACUTE PROCEDURAL OUTCOMES
Acute procedural angiographic and IVUS outcomes are presented in Table 2. The pre-stenting IVUS(+) group had a significantly larger final angiographic minimal lumen diameter (MLD) than the pre-stenting IVUS(−) group (2.68±0.41 vs 2.60±0.40 mm, p<0.001) (Figure 1A). The pre-stenting IVUS(+) group had a consistently larger final angiographic MLD than the pre-stenting IVUS(−) group after inverse probability of treatment weighting adjustment and after propensity score matching (Figure 1B). Similarly, the pre-stenting IVUS(+) group had a significantly greater IVUS-derived minimal stent area (MSA) than the pre-stenting IVUS(−) group (5.71±1.74 vs 5.48±1.79 mm2, mean difference=0.23 mm2, 95% confidence interval [CI]: 0.04‒0.42, p=0.020) (Figure 1C). These findings were consistent after adjustment with inverse probability weighting and with propensity score matching (Figure 1D). In the sensitivity analyses, these findings were consistent in a model accounting for trial site (5.70±1.76 vs 5.48±1.80 mm2, p=0.001), and in the population after exclusion of the patients with chronic total occlusions (5.81±1.75 vs 5.48±1.81 mm2, p=0.003). Although plaque burden (%) was similar between the groups at the proximal stent edge, the dissection at the proximal stent edge was significantly less frequent in the pre-stenting IVUS(+) group than in the pre-stenting IVUS(−) group (3% vs 6%, p=0.003) (Table 2). At the distal stent edge, there was a trend towards less plaque burden with less frequency of dissection in the pre-stenting IVUS(+) group than in the pre-stenting IVUS(−) group.
Table 2. Acute procedural angiographic and intravascular ultrasound outcomes.
| Variable | Pre-stenting IVUS (+) n=905 | Pre-stenting IVUS (−) n=491 | p-value | |
| Acute angiographic outcomes | ||||
| Post-procedural reference vessel diameter, mm | 3.06±0.43 | 2.99±0.44 | 0.002 | |
| Post-procedural minimal lumen diameter, mm | Unadjusted | 2.68±0.41 | 2.60±0.40 | <0.001 |
| IPTW-adjusted | 2.67±0.41 | 2.62±0.41 | 0.040 | |
| Propensity score-matched | 2.66±0.42 | 2.59±0.38 | 0.011 | |
| Post-procedural diameter stenosis, % | 12.39±8.49 | 12.64±8.33 | 0.597 | |
| Acute gain, mm | 1.97±0.67 | 1.84±0.57 | <0.001 | |
| Acute IVUS outcomes | ||||
| Proximal reference EEM area, mm2 | 17.74±5.13 | 16.56±4.95 | <0.001 | |
| Proximal reference lumen area, mm2 | 9.20±3.10 | 8.51±3.29 | 0.001 | |
| Plaque burden at proximal stent edge, % | 52.5±9.6 | 52.8±9.1 | 0.662 | |
| Dissection at proximal stent edge | 23 (3%) | 28 (6%) | 0.003 | |
| MSA, mm2 | Unadjusted | 5.71±1.74 | 5.48±1.79 | 0.020 |
| IPTW-adjusted | 5.73±1.76 | 5.53±1.84 | 0.041 | |
| Propensity score-matched | 5.73±1.73 | 5.47±1.73 | 0.040 | |
| Plaque burden at distal stent edge, % | 40.1±10.5 | 41.4±11.4 | 0.083 | |
| Dissection at distal stent edge | 30 (3%) | 24 (5%) | 0.146 | |
| Distal reference EEM area, mm2 | 9.91±4.08 | 10.06±4.40 | 0.568 | |
| Distal reference lumen area, mm2 | 6.11±2.03 | 5.97±2.04 | 0.206 | |
| MSA ≥distal lumen area | 531 (59%) | 279 (57%) | 0.503 | |
| Ratio of MSA to distal lumen area, % | 97±26 | 95±21 | 0.030 | |
| Ratio of MSA to mean reference lumen area, % | 76±18 | 76±17 | 0.836 | |
| Meeting the IVUS optimisation criteria | 490 (54%) | 249 (51%) | 0.220 | |
| Values are mean±standard deviation or number (%). EEM: external elastic membrane; IPTW: inverse probability of treatment weighting; IVUS: intravascular ultrasound; MSA: minimal stent area | ||||
Figure 1.

Acute procedural angiographic and IVUS outcomes. Cumulative frequency of final post-procedural MLD on angiogram (A), MLD before and after adjustment (B), cumulative frequency of final post-procedural MSA on IVUS (C), and MSA before and after adjustment (D). IPTW: inverse probability of treatment weighting; IVUS: intravascular ultrasound; MLD: minimal lumen diameter; MSA: minimal stent area; PS: propensity score
In the subgroup analyses of the difference in the final MSA between the two groups, a significant interaction was observed according to the presence of acute MI (p-interaction=0.021) and the reference vessel diameter (p-interaction=0.036) (Figure 2). In the subsets of patients with acute MI or reference vessel diameter <3 mm, the pre-stenting IVUS(+) group showed a significantly greater MSA than the pre-stenting IVUS(−) group. A significant interaction according to the presence of acute MI was noted even after adjustment for reference vessel diameter, and interaction according to the reference diameter was also noted after adjustment for lesion length (Supplementary Table 5). There were no significant interactions according to each trial (p-interaction=0.22). The between-trial forest plot for the difference in the final MSA to assess heterogeneity is presented in Supplementary Figure 1.
Figure 2.

Subgroup analyses of the difference in the final minimal stent area between the two groups. IVUS: intravascular ultrasound; LAD: left anterior descending; LCX: left circumflex; MI: myocardial infarction; MSA: minimal stent area; RCA: right coronary artery
CLINICAL OUTCOMES
Clinical outcomes one year after DES placement are presented in Table 3. The composite of cardiac death, MI, and TVR after one year was not different between the two groups (3.1% vs 3.2%; hazard ratio [HR] 0.95, 95% CI: 0.50‒1.78, p=0.867) (Figure 3A). These findings were consistent after adjustment with inverse probability weighting (Figure 3B) and with propensity score matching (Figure 3C). These findings were consistent in the population after the exclusion of patients with chronic total occlusions (3.4% vs 2.6%; HR 1.31, 95% CI: 0.64‒2.68, p=0.467), and in a model with site as a random effect (3.1% vs 3.2%; HR 0.94, 95% CI: 0.49‒1.79, p=0.889). In the occurrence of individual events, no significant difference was observed (Table 3).
Table 3. Clinical outcomes after one year.
| Clinical outcomes | Pre-stenting IVUS (+) n=905 | Pre-stenting IVUS (−) n=491 | Unadjusted HR (95% CI) | p-value | IPTW-adjusted HR (95% CI) | p-value | PS-matched HR (95% CI) | p-value |
| A composite of cardiac death, myocardial infarction and target vessel revascularisation | 27 (3.1%) | 15 (3.2%) | 0.95 (0.50–1.78) | 0.867 | 0.95 (0.52–1.74) | 0.863 | 1.05 (0.45–2.47) | 0.914 |
| Cardiac death | 2 (0.2%) | 2 (0.4%) | 0.53 (0.08–3.78) | 0.529 | 1.25 (0.18–8.60) | 0.818 | 0.97 (0.06–15.49) | 0.969 |
| Myocardial infarction | 0 | 1 (0.2%) | – | – | – | – | – | – |
| Stent thrombosis | 2 (0.2%) | 1 (0.2%) | 1.08 (0.10–11.95) | 0.948 | 1.65 (0.15–22.25) | 0.998 | 1.01 (0.07–17.84) | 0.999 |
| Target vessel revascularisation | 25 (2.9%) | 13 (2.8%) | 1.01 (0.52–1.98) | 0.975 | 0.94 (0.50–1.78) | 0.941 | 1.06 (0.43–2.60) | 0.905 |
| Values are presented as number (%). CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weighting; IVUS: intravascular ultrasound; PS: propensity score | ||||||||
Figure 3.

Kaplan-Meier survival curves for the clinical outcomes after one year. IPTW: inverse probability of treatment weighting; IVUS: intravascular ultrasound; MI: myocardial infarction; PS: propensity score; TVR: target vessel revascularisation
In the subgroup analyses of the composite of cardiac death, MI, and TVR, a significant interaction was observed according to age (p-interaction=0.029), chronic total occlusions (p-interaction=0.022) and lesion length (p-interaction=0.047) (Figure 4). In particular, in the subset of chronic total occlusion, the pre-stenting IVUS(+) compared to the pre-stenting IVUS(−) group showed a better composite outcome. The between-trial forest plot for the composite of cardiac death, MI, and TVR is presented in Supplementary Figure 2. Heterogeneity in the overall trials was observed because of the CTO-IVUS trial, which enrolled the only subset of chronic total occlusions. However, there was no significant heterogeneity after excluding the CTO-IVUS trial.
Figure 4.
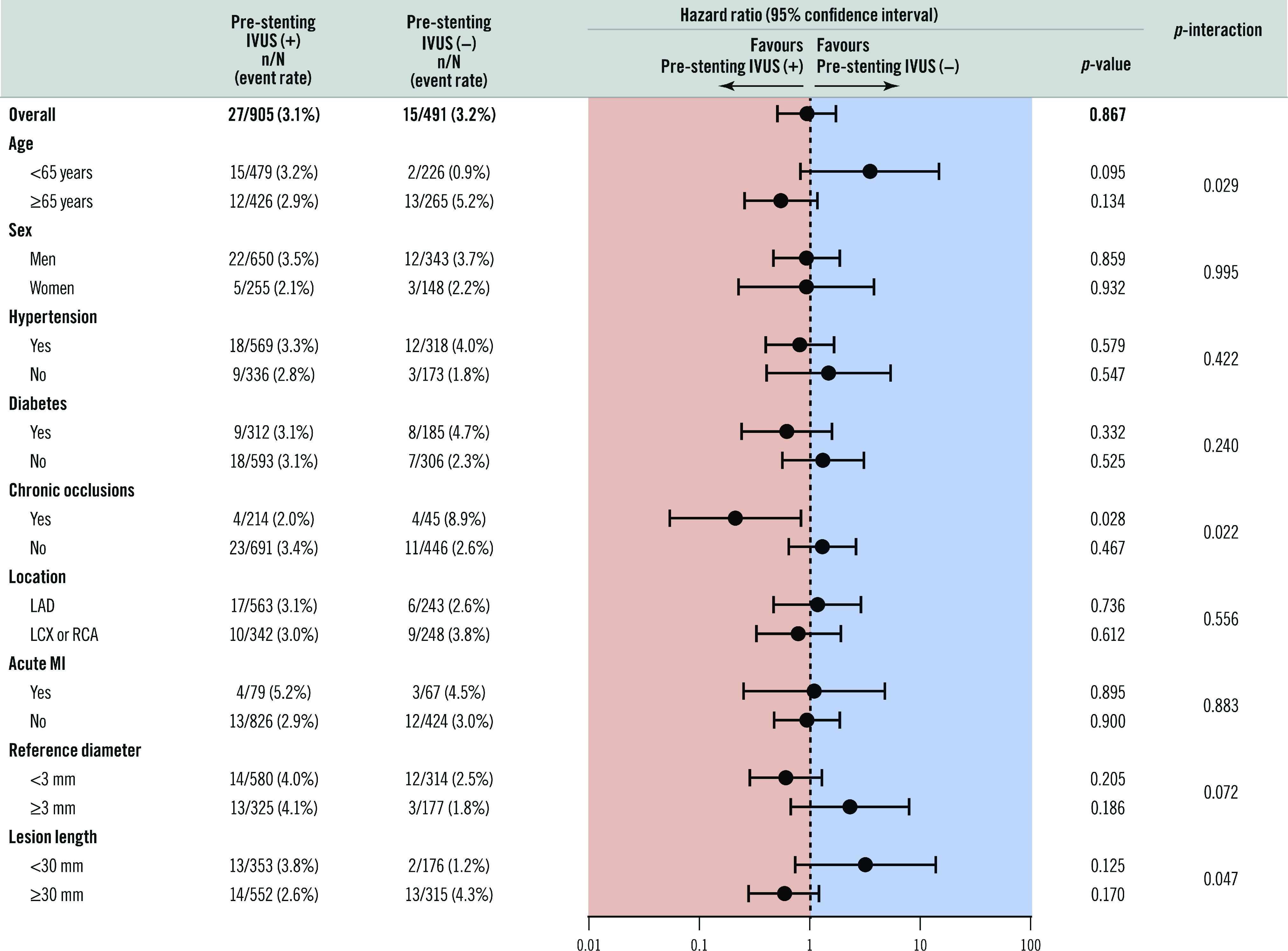
Subgroup analysis of the composite of cardiac death, myocardial infarction, and target vessel revascularisation after one year between the two groups. IVUS: intravascular ultrasound; LAD: left anterior descending; LCX: left circumflex; MI: myocardial infarction; RCA: right coronary artery
Discussion
The primary findings of this study are as follows. 1) Procedural factors, including stent size and the rate of adjunctive post-dilation, were significantly different between the pre-stenting IVUS(+) and pre-stenting IVUS(−) groups. DES with a significantly larger diameter were implanted and resulted in a less frequent adjuvant post-dilation but with a larger-sized balloon used in the pre-stenting IVUS(+) group compared with the pre-stenting(−) group. 2) The patients who underwent pre-stenting IVUS assessment had better acute procedural outcomes, such as a larger final angiographic MLD, a larger IVUS-derived MSA, and a trend for less plaque burden at stent edges with fewer dissections, thereby improving stent optimisation, compared with those who did not. Also, the benefit of greater MSA was prominent in the subsets of patients with acute MI and small vessels. 3) In terms of the occurrence of major cardiac events, no significant differences were observed in this study based on whether or not pre-stenting IVUS assessment was performed. However, in the subgroup analyses, the pre-stenting IVUS(+) group showed a better clinical outcome than the pre-stenting IVUS(−) group, especially in the subset of chronic total occlusion.
Recent randomised clinical trials and meta-analyses have shown the superiority of IVUS-guided DES implantation to angiography-guided DES implantation and have reported that the achievement of stent optimisation via post-stenting IVUS assessment can be considered the main mechanism6,7,8,9,10. However, there has been no definite evidence regarding the exact role and clinical implication of IVUS according to the procedural steps, particularly pre-stenting IVUS assessment. According to the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) trial, IVUS was used only before PCI in 7% of patients, only after PCI in 30% of patients, and both before and after PCI in 63% of patients in the IVUS-guided group12. Thus, even in patients who underwent IVUS-guided PCI, all patients did not undergo pre-stenting IVUS evaluation. Similarly to the ADAPT-DES study, approximately one third of patients who underwent IVUS guidance did not undergo pre-stenting IVUS assessment in this study. There were some specific patients and lesion subsets which favoured pre-stenting IVUS evaluation, and there were significantly different procedural factors between the pre-stenting IVUS(+) and the pre-stenting IVUS(−) groups. In particular, DES with a significantly larger diameter were inserted despite the similar vessel sizes based on quantitative coronary analyses. This resulted in a greater diameter-to-vessel ratio with less frequent adjuvant post-dilation but with a larger-sized balloon used in the pre-stenting IVUS(+) group compared with the pre-stenting(−) group. As a result, pre-stenting IVUS evaluation could cause changes in PCI strategy from stent selection to the final adjuvant post-dilation.
As for procedural outcomes, the pre-stenting IVUS(+) group had a larger final angiographic MLD and post-procedural IVUS-derived MSA than the pre-stenting IVUS(−) group. Final angiographic MLD and post-procedural MSA are the most important and well-known parameters for predicting future major adverse cardiac events13,14,15. Pre-stenting IVUS evaluation could provide information for optimised stenting - cross-sectional measurements of the vessel and lumen, longitudinal evaluation for the determination of covered lesions, and analyses of plaque characteristics and evaluation of vessel complications16. The finding of a tendency of less plaque burden with less frequency of dissection at the distal stent edge, and a significantly lower frequency of dissection at the proximal edge in the pre-stenting IVUS(+) group than in the IVUS(−) group also support the benefit of pre-stenting IVUS evaluation which can minimise the risks related to geographic miss. However, the beneficial effects of pre-stenting IVUS assessment did not improve clinical outcomes in this study. Such a result may be explained by the following: all patients underwent post-stenting IVUS assessment, which was able to evaluate optimal expansion and detect various mechanical problems. Thus, even though the pre-stenting IVUS evaluation was not performed and the implantation of DES did not initially achieve optimisation, i.e., the choice of a relatively small-sized diameter of DES, the post-stenting IVUS assessment could fix stent underexpansion and other stent-related problems by the use of different PCI strategies.
Interestingly, the difference in MSA between the pre-stenting IVUS(+) and pre-stenting IVUS(−) groups was prominent in the subsets of patients with acute MI. These results are in accordance with the findings of the ADAPT-DES study where the benefit of IVUS guidance was more prominent in patients with acute coronary syndrome13. Another subset included patients with small vessel disease. In previous studies, the discrepancy between angiography and IVUS in terms of reference diameters was greater in smaller vessels; such results support the present findings17. In addition, as for the clinical outcomes, a significant interaction was observed favouring pre-stenting IVUS evaluation for the subsets of elderly patients with chronic total occlusions or lesion length ≥30 mm. In particular, in the subset of chronic total occlusion, the pre-stenting IVUS(+) group showed a significantly better 12-month outcome than the pre-stenting IVUS(−) group. In case of PCI for chronic total occlusions, the determination of stent sizing and length is difficult due to the long-term reduced flow and negative vessel remodelling. Nonetheless, the benefit of IVUS in these procedures goes beyond optimising stent expansion (larger MSA). Particularly for the lesions with chronic total occlusions, IVUS may play a crucial role for monitoring different steps during both anterograde and retrograde PCI procedures. However, this advantage related to pre-stenting IVUS was not explored in this study since randomisation was carried out after successful wire crossing.
Limitations
This study had limitations. First, this was not a randomised trial but a post hoc analysis of pooled data. The decision to use pre-stenting IVUS guidance was at the discretion of the physician. Although the confounders were treated with the inverse probability of treatment weighting and propensity score-matched analyses, a randomised clinical trial is required. Also, data regarding the reasons for pre-IVUS assessment, which might be informative to elucidate the role of pre-IVUS assessment, were not collected. Second, the recommendations for the treatment strategies were not pre-specified according to the pre-IVUS findings. Similarly, the treatment strategies were not pre-specified in the patients who did not undergo pre-IVUS assessment. Third, this study only included diffuse long or chronic total occlusive lesions. Fourth, qualitative angiographic and IVUS assessments were not performed. Fifth, the clinical follow-up duration was relatively short, and the event rate is relatively small. Thus, a much larger number of patients and longer study duration are needed in order to demonstrate any differences in clinical outcomes. Sixth, all patients in this analysis underwent IVUS assessment after DES placement to achieve optimisation, which might have weakened the actual effect of pre-IVUS evaluation. Thus, the effect of pre-IVUS assessment on acute procedural and clinical outcomes could be underestimated in this analysis. However, assessment of IVUS only before DES placement without any after DES placement is rarely performed in real-world clinical practice.
Conclusions
The patients who underwent pre-stenting IVUS assessment in the context of post-stenting IVUS assessment had better acute procedural outcomes, such as a larger final angiographic MLD and IVUS-derived MSA, compared with those who did not. This benefit was prominent in the subsets of patients with acute MI and small vessels. Though no significant differences were observed in terms of the occurrence of major cardiac events after DES implantation based on whether or not pre-stenting IVUS assessment was performed in this study, a much larger number of patients and longer study duration would be needed in order to demonstrate any differences definitively.
Impact on daily practice
The effect of pre-stenting intravascular ultrasound (IVUS) evaluation on outcomes has not been independently evaluated. The patients who underwent pre-stenting IVUS assessment had better acute procedural outcomes, such as a larger final angiographic minimal lumen diameter and IVUS-derived minimal stent area, thereby improving stent optimisation. This benefit was prominent in the subsets of patients with acute myocardial infarction and small vessels. Thus, pre-stenting IVUS can be useful to achieve better acute procedural outcomes, particularly in patients with acute myocardial infarction and small vessels.
Supplementary data
Methods.
Between-trial forest plot for the difference in the final minimal stent area.
Between-trial forest plot for the composite of cardiac death, myocardial infarction and target vessel revascularisation.
Summary of analysed studies.
Predictors of pre-stenting intravascular ultrasound use prior to adjustment.
Clinical and angiographic characteristics after inverse probability of treatment weighting adjustment.
Clinical and angiographic characteristics after propensity score matching.
Subgroup analyses of the difference in the final minimal stent area after adjustment.
Visual summary.
Acute and one-year outcomes of pre-stenting intravascular ultrasound: a meta-analysis of randomised clinical trials.
Acknowledgments
Funding
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (no.: HI17C0882, HI16C2211, and HI15C2782), the Bio & Medical Technology Development Program of the National Research Foundation funded by the Korean government (no.: 2015M3A9C6031514), and the Cardiovascular Research Center, Seoul, Republic of Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Abbreviations
- DES
drug-eluting stent(s)
- IVUS
intravascular ultrasound
- MI
myocardial infarction
- MLD
minimal lumen diameter
- MSA
minimal stent area
- PCI
percutaneous coronary intervention
- TVR
target vessel revascularisation
Contributor Information
Sung-Jin Hong, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Daehoon Kim, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Byeong-Keuk Kim, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Chul-Min Ahn, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Jung-Sun Kim, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Young-Guk Ko, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Donghoon Choi, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Myeong-Ki Hong, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Yangsoo Jang, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea.
References
- Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, Di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656–77. doi: 10.4244/EIJY18M06_01. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855–65. doi: 10.4244/EIJV8I7A129. [DOI] [PubMed] [Google Scholar]
- Steinvil A, Zhang YJ, Lee SY, Pang S, Waksman R, Chen SL, Garcia-Garcia HM. Intravascular ultrasound-guided drug-eluting stent implantation: An updated meta-analysis of randomized control trials and observational studies. Int J Cardiol. 2016;216:133–9. doi: 10.1016/j.ijcard.2016.04.154. [DOI] [PubMed] [Google Scholar]
- Shin D-H, Hong S-J, Mintz GS, Kim J-S, Kim B-K, Ko Y-G, Choi D, Jang Y, Hong M-K. Effects of intravascular ultrasound-guided versus angiography-guided new-generation drug-eluting stent implantation: meta-analysis with individual patient-level data from 2,345 Randomized Patients. JACC Cardiovasc Interv. 2016;9:2232–9. doi: 10.1016/j.jcin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Ahn JM, Kang SJ, Yoon SH, Park HW, Kang SM, Lee JY, Lee SW, Kim YH, Lee CW, Park SW, Mintz GS, Park SJ. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–47. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Kang T-S, Mintz GS, Park B-E, Shin D-H, Kim B-K, Ko Y-G, Choi D, Jang Y, Hong M-K. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–76. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, Kim JS, Kim JS, Lee SJ, Kim HY, Hong BK, Kang WC, Choi JH, Jang Y CTO-IVUS Study Investigators. Clinical Impact of Intravascular Ultrasound-Guided, Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ Cardiovasc Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155–63. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- Tian N-L, Gami S-K, Ye F, Zhang J-J, Liu Z-Z, Lin S, Ge Z, Shan S-J, You W, Chen L, Zhang Y-J, Mintz G, Chen S-L. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409–17. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular Ultrasound-Guided Versus Angiography-Guided Implantation of Drug-Eluting Stent in All-Comers: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126–37. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: The Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study. Circulation. 2014;129:463–70. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Kim MH, Ahn TH, Ahn YK, Bae JH, Shim WJ, Ro YM, Lim DS. Multiple predictors of coronary restenosis after drug-eluting stent implantation in patients with diabetes. Heart. 2006;92:1119–24. doi: 10.1136/hrt.2005.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M-K, Mintz GS, Lee CW, Park D-W, Choi B-R, Park K-H, Kim Y-H, Cheong S-S, Song J-K, Kim J-J, Park S-W, Park S-J. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–10. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Morino Y, Ako J, Terashima M, Hassan AHM, Bonneau HN, Leon MB, Moses JW, Yock PG, Honda Y, Kuntz RE, Fitzgerald PJ. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the Sirius Trial. J Am Coll Cardiol. 2004;43:1959–63. doi: 10.1016/j.jacc.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Oh PC, Han SH. Diffuse Long Coronary Artery Disease is Still an Obstacle for Percutaneous Coronary Intervention in the Second-Generation Drug-Eluting Stent Era? Korean Circ J. 2019;49:721–3. doi: 10.4070/kcj.2019.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi K, Shannon J, Basavarajaiah S, Latib A, Al-Lamee R, Hasegawa T, Godino C, Ferraro M, Figini F, Carlino M, Montorfano M, Chieffo A, Colombo A. Discrepancies in vessel sizing between angiography and intravascular ultrasound varies according to the vessel evaluated. Int J Cardiol. 2013;168:3791–6. doi: 10.1016/j.ijcard.2013.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods.
Between-trial forest plot for the difference in the final minimal stent area.
Between-trial forest plot for the composite of cardiac death, myocardial infarction and target vessel revascularisation.
Summary of analysed studies.
Predictors of pre-stenting intravascular ultrasound use prior to adjustment.
Clinical and angiographic characteristics after inverse probability of treatment weighting adjustment.
Clinical and angiographic characteristics after propensity score matching.
Subgroup analyses of the difference in the final minimal stent area after adjustment.



