Malassezia spp. are named after Louis Charles Malassez who was the first to separate this yeast into a Genus of single cell fungi (“Saccharomyces”) from the previous inclusion of this fungus within the dermatophyte complex (1). Malassezia yeasts form a unique cluster of lipophilic fungi living almost exclusively on the skin and mucosal sites of warm-blooded vertebrates. Malassezia seem to be host-specific and in humans they also exhibit varied geographical distributions (1). Malassezia pachydermatis is the most prevalent and important commensal yeast of canine skin and mucosa.
Although these are commensal organisms, Malassezia also form a reservoir of potential pathogens in the stratum corneum or mucosae that may induce skin or ear disease whenever the homeostatic balance of yeast virulence and host immunity is disrupted in favor of the yeast. Canine Malassezia dermatitis is a routine diagnosis in small animal practice, occurring due to overgrowth and may have very deleterious effects on many animals. Yeast proliferation may be enhanced by either favorable environmental conditions (heat, humidity) and or changes in host susceptibility. Sites most frequently colonized by M. pachydermatis in healthy dogs include the peri-oral skin, chin, lip region, interdigital skin, perianal skin, and anal mucosa (2). Breed variations can be noted. For example, Basset hounds have significant colonization of M. pachydermatis in the nose, mouth, vulva, and axilla (3).
Clinical signs in dogs
Malassezia dermatitis can potentially occur in dogs of any age, sex, or breed. Some of the breeds at increased risk include West Highland white terriers, English setters, shih tzus, basset hounds, American cocker spaniels, boxers, dachshunds, poodles, and Australian silky terriers. In addition, breeds with conformations that favor skin folds are also prone to infections at intertriginous anatomical sites. There is no sex predisposition. Malassezia dermatitis typically presents as a significantly pruritic condition, although severity may vary from mild to very severe, including signs such as face rubbing, head shaking, ear scratching, paw licking or chewing, anal scooting, or generalized scratching (4). Significant pruritus may also be present due to primary disease related factors.
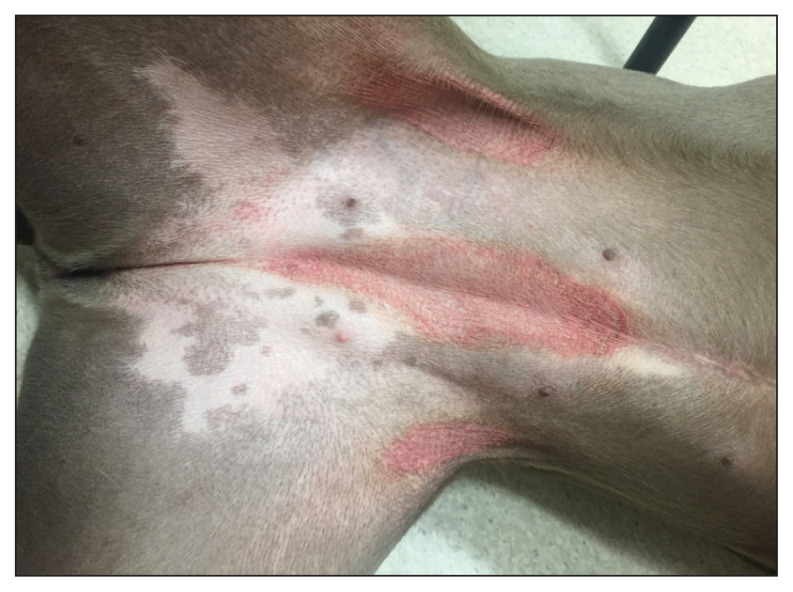
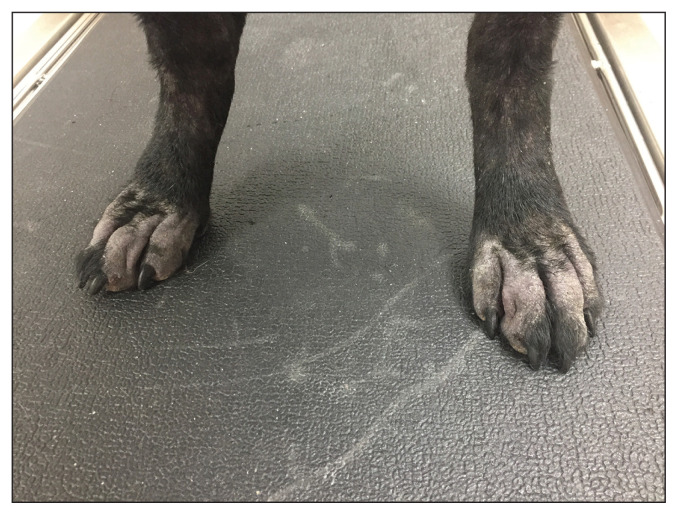
Skin lesions in canine Malassezia dermatitis can be localized or generalized. Commonly involved areas include the muzzle, lips, ventral neck, axillae, ventral abdomen (Figure 1), medial hind limbs, interdigital and pedal skin (Figure 2), perineum, external ear canal, and intertriginous areas. Paronychia may occur. Skin lesions are characterized by diffuse erythema and variable amounts of kerato-sebaceous scale that may be brown, yellow, or gray (4,5). Skin and hair coat may exhibit greasiness, alopecia, lichenification, and hyperpigmentation. Reddish brown staining of focally affected skin sites such as perioral skin, claw folds, and perivulvar skin may be noted. An offensive, rancid odor may be present. Malassezia overgrowth in the ears typically results in a pruritic, erythematous, and ceruminous otitis externa. These clinical signs and observations can be noted with pyoderma as well as many primary underlying diseases; therefore, microscopic confirmation of Malassezia involvement is required for a diagnosis.
Figure 1.
Malassezia dermatitis and canine atopic dermatitis related erythema at ventral trunk in a young canine patient.
Figure 2.
Malassezia pododermatitis including significant alopecia and superficial scale.
Predisposing factors and primary causes
Primary skin diseases that lead to increased cutaneous moisture, altered surface lipids, disruption of stratum corneum barrier function, or a combination or aberrant immune responses may encourage Malassezia overgrowth. Aberrant immune responses may also provide alterations of the skin’s surface microclimate, resulting in localized or generalized Malassezia yeast overgrowth and dermatitis (1).
Dogs with Malassezia dermatitis often have concurrent dermatoses, especially hypersensitivity disease, ectoparasitic infestation, bacterial pyoderma, endocrine conditions, cornification defects, or primary or secondary seborrhea. Pruritic inflammatory diseases may create cutaneous microclimate changes due to scratching (disruption of barrier function), licking (added moisture), or increased production of sebum (6). The presence of skin folds is a common risk factor for localized disease. In predisposed breeds, there may occasionally be no primary identifiable underlying cause and skin disease may respond completely to appropriate antifungal therapy.
Co-proliferation of Staphylococci in lesions may exacerbate clinical signs (7). An association between the onset of Malassezia dermatitis (and/or Malassezia otitis) and the recent use of antibacterial drugs is sometimes observed by practitioners and could reflect a reduction in “competition” for micro-ecological resources as the bacterial population is reduced (8).
Diagnosis
The most useful and readily available diagnostic tool for Malassezia dermatitis is cytological examination (9). Methods for cytological assessment include impression cytology using glass slides or tape, or cytological examination without impression (skin scrapings and swab samples).
There is no clear preferred cytological sampling method, as each has its own benefits and limitations (9). Direct impression with a glass slide is good for flat surfaces and where grease or wax are plentiful. Cotton swab smears are good for ears, interdigital spaces, and facial folds. Superficial scrapings are reliable but can be difficult to perform in certain areas (e.g., interdigital spaces, facial folds). Tape strips are good when the area is not overly waxy or greasy and facilitate sampling from between digits and in skin folds. More than 1 sampling technique may be utilized in a patient’s skin assessment, for thorough cutaneous cytological testing.
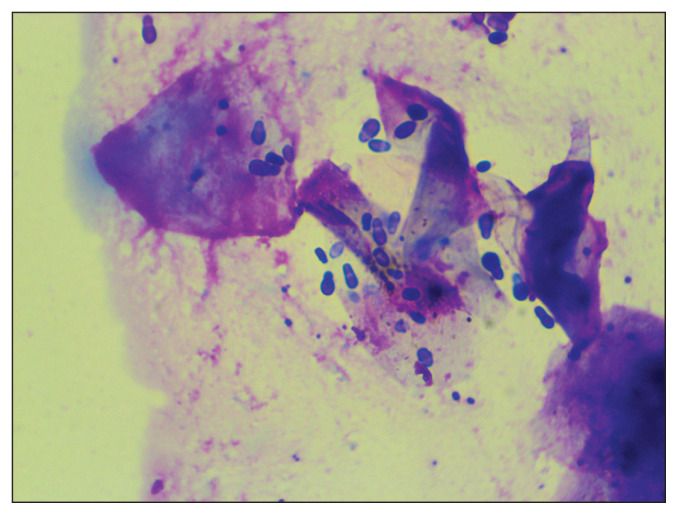
Routine cytological sampling of skin sites is inexpensive and can rapidly be achieved by patient-side light microscopical examination using a 100× (oil immersion) objective. Collected samples are stained using modified Wright Giemsa stain (“Diff-Quik”). Cotton-blue lactophenol and May-Grunwald Giemsa stains can also be used (1). During high-power (oil immersion) microscopic assessment, Malassezia yeasts are identified as round to oval or the classic peanut shape (Figure 3). Malassezia pachydermatis is characterized by monopolar budding of daughter cells from 1 site on the cell wall, formation of a prominent bud scar or collar at the site of daughter cell development, a peanut shape, and a diameter of 3 to 8 μm (9). Yeasts are often visible in clusters or adhered to keratinocytes. There is no definitive number of organisms required to diagnose Malassezia dermatitis. Varying numbers of yeast are present in different body sites, and normal numbers vary among breeds resulting in overlaps in yeast population densities in samples from clinically normal and diseased dogs (9,10). Ultimately the diagnosis of Malassezia dermatitis should rely on the combination of clinical presentation and cutaneous cytology. Even low numbers of Malassezia noted on cytology may indicate Malassezia dermatitis if samples are collected from inflamed, pruritic skin (11). Trial therapy is of minimal benefit in the absence of cytological evidence of Malassezia. Although certain lesion patterns, presence of an offensive yeasty odor, and lack of response to previous appropriate therapy may be suggestive of Malassezia dermatitis, these should not be relied upon as diagnostic criteria without pursuing cutaneous cytology to confirm Malassezia (11).
Figure 3.
Round to oval or the classic peanut shaped Malassezia yeasts are often visible in clusters or adhered to keratinocytes when viewed under 100× (oil immersion objective) microscopy.
Treatment and management
Treatment of Malassezia dermatitis typically involves topical and/or systemic antifungal medications (1). Topical treatment options are effective due to the superficial location of the yeast within the stratum corneum. Topical options include shampoos, leave on sprays, wipes, mousses, and lotions. Antifungal shampoo therapy is effective in part due to the mechanical action during bathing that helps reduce scaling and greasy exudation. Leave on topical antimicrobial products are incorporated due to ease of administration compared to bathing, as well as for effective localized treatment of sites such as lip margins, skin folds, pedal, and perianal skin. Systemic therapy may be necessary in cases in which sole topical therapy is challenging for the owner and/or the patient, or if topical therapy has been tried without adequate response. A combined topical-systemic treatment plan may be optimal in some dogs with generalized and/or severe lesions. Combined oral and topical antifungal therapy was more effective with more rapid cure than either topical or systemic treatment used alone (12).
Strong evidence is available for using 2% miconazole and 2% chlorhexidine shampoo twice weekly (1). This should be considered the topical treatment of choice when dog owners are able to administer localized or full-body topical treatments. Chlorhexidine shampoos and leave-on medicated products are available currently in varying concentrations in Canada (including 2, 3, and 4% formulations). Imidazoles such as clotrimazole, climbazole, and miconazole may be used topically in otic products, creams, or shampoos. Topical treatments are preferred to systemic treatments for long-term therapy due to a lower risk of toxicity. The frequency of topical therapy administration can often be individualized in patients based on control of underlying primary disease, skin folds, presence of seborrheic dermatitis as well as other concurrent treatments.
Systemic antifungal therapy for Malassezia yeast largely involves azole drugs including oral ketoconazole, itraconazole, and fluconazole (13–15). Itraconzole may be given once daily, or as pulse therapy (2 d on, 5 d off ) (13) based on the predicted accumulation of this lipophilic drug in the stratum corneum which can help reduce costs and potential for side-effects related to oral therapy. The allylamine derivative Terbinafine can also be administered orally when azole drugs are unavailable or not well-tolerated by the patient (16). Compounded formulations must be avoided due to unreliable bioavailability.
Clinical and cytological responses to appropriate topical and/or systemic (2 to 4 wk) antifungal therapy should be assessed based on patient needs, typically after 2 to 4 wk of selected therapy. As Malassezia yeasts are part of the normal cutaneous microflora, complete elimination of the organism is unrealistic even with effective treatment (17). Relapsing infections are common if the underlying cause for the yeast overgrowth remains undiagnosed or uncontrolled. Identification and treatment of underlying causes responsible for the proliferation of the yeast will help prevent recurrent or relapsing infections.
When the primary condition is either incurable or not definitively diagnosed, ongoing regular topical antifungal shampoo baths or pulse oral antifungal therapy have been recommended as prevention strategies to help minimize the frequency of infection relapses that cannot be managed by other means (17,18). Potential induction of antifungal drug resistance should be considered when making decisions to utilize intermittent use of oral antifungal drugs (19). Azole resistant isolates of M. pachydermatis have been described over the past few years (19,20). The reduced susceptibility of M. pachydermatis to commonly used antifungal drugs highlights the need for surveillance and vigilance for emergence of clinically relevant Malassezia resistance.
Immunological responses and hypersensitivity
The presence of Malassezia on the skin, both in normal and excessive numbers, is known to activate the skin immune system (21). Malassezia antigens can stimulate innate, antibody, and cell-mediated immune responses, as well as trigger hypersensitivity reactions (22). In animals in which an overgrowth of organisms has occurred, or in individuals that are predisposed to allergic sensitization, the ensuing inflammatory response can lead to clinical signs associated with Malassezia-related disease such as dermatitis and pruritus. Proteins from Malassezia can act as allergens in dogs predisposed to the development of atopic dermatitis (23). Malassezia hypersensitivity testing can be pursued as part of intradermal allergy testing or serum based IgE testing in atopic dogs. Malassezia immunotherapy can be initiated either as a sole allergen or in combination with other allergens (1,24).
Footnotes
The Veterinary Dermatology column is a collaboration of The Canadian Veterinary Journal with the Canadian Academy of Veterinary Dermatology (CAVD). The CAVD invites everyone with a professional interest in dermatology to join us (www.cavd.ca) for continuing education in this dynamic field. Annual membership fees are $50 for regular members and free for students.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bond R. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2020;31:27–e4. doi: 10.1111/vde.12809. [DOI] [PubMed] [Google Scholar]
- 2.Kennis RA, Rosser EJ, Olivier NB, Walker RW. Quantity and distribution of Malassezia organisms on the skin of clinically normal dogs. J Am Vet Med Assoc. 1996;208:1048–1051. [PubMed] [Google Scholar]
- 3.Bond R, Lloyd DH. Skin and mucosal populations of Malassezia pachydermatis in healthy and seborrhoeic basset hounds. Vet Dermatol. 1997;8:101–106. doi: 10.1046/j.1365-3164.1997.d01-4.x. [DOI] [PubMed] [Google Scholar]
- 4.Mason KV. Cutaneous Malassezia. In: Griffin CE, Kwochka KW, MacDonald JM, editors. Current Veterinary Dermatology. St. Louis, Missouri: Mosby Year Book; 1993. pp. 44–48. [Google Scholar]
- 5.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat, Clinical and Histopathologic Diagnosis. 2nd ed. Oxford, UK: Blackwell Publishing; 2005. pp. 142–146. [Google Scholar]
- 6.Machado ML, Ferreiro L, Ferreira RR, et al. Malassezia dermatitis in dogs in Brazil: Diagnosis, evaluation of clinical signs and molecular identification. Vet Dermatol. 2011;22:46–52. doi: 10.1111/j.1365-3164.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 7.Bond R, Rose JF, Ellis JW, Lloyd DH. Comparison of two shampoos for treatment of Malassezia pachydermatis-associated seborrhoeic dermatitis in basset hounds. J Small Anim Pract. 1995;36:99–104. doi: 10.1111/j.1748-5827.1995.tb02840.x. [DOI] [PubMed] [Google Scholar]
- 8.Bond R, Ferguson EA, Curtis CF, Craig JM, Lloyd DH. Factors associated with elevated cutaneous Malassezia pachydermatis populations in dogs with pruritic skin disease. J Small Anim Pract. 1996;37:103–107. doi: 10.1111/j.1748-5827.1996.tb02353.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller WH, Griffin CE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis, Missouri: Elsevier; 2013. pp. 243–249. [Google Scholar]
- 10.Bond R, Saijonmaa-Koulumies LEM, Lloyd DH. Population sizes and frequency of Malassezia pachydermatis at skin and mucosal sites of healthy dogs. J Small Anim Pract. 1995;36:147–150. doi: 10.1111/j.1748-5827.1995.tb02865.x. [DOI] [PubMed] [Google Scholar]
- 11.Bajwa J. Canine Malassezia dermatitis. Can Vet J. 2017;58:1119–1121. [PMC free article] [PubMed] [Google Scholar]
- 12.Bensignor E, Hahn H, Guillot J. Topical vs. systemic treatment of Malassezia dermatitis in dogs: A comparative, blinded, randomised trial. Vet Dermatol. 2012;23:84. (abstract) [Google Scholar]
- 13.Bensignor E. Oral itraconazole as a pulse therapy for the treatment of canine Malassezia dermatitis: A randomized, blinded, comparative trial. Pratique Médicale et Chirurgicale de l’Animal de Compagnie. 2006;41:69–72. [Google Scholar]
- 14.Mayer UK, Glos K, Schmid M, Power HT, Bettenay SV, Mueller RS. Adverse effects of ketoconazole in dogs–A retrospective study. Vet Dermatol. 2008;19:199–208. doi: 10.1111/j.1365-3164.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 15.Sickafoose L, Hosgood G, Snook T, Westermeyer R, Merchant S. A noninferiority clinical trial comparing fluconazole and ketoconazole in combination with cephalexin for the treatment of dogs with Malassezia dermatitis. Vet Ther. 2010;11:E1–13. [PubMed] [Google Scholar]
- 16.Guillot J, Bensignor E, Jankowski F, Seewald W, Chermette R, Steffan J. Comparative efficacies of oral ketoconazole and terbinafine for reducing Malassezia population sizes on the skin of basset hounds. Vet Dermatol. 2003;14:153–157. doi: 10.1046/j.1365-3164.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 17.Morris D. Malassezia dermatitis and otitis. Vet Clin North Am Small Anim Pract. 1999;29:1303–1310. doi: 10.1016/s0195-5616(99)50128-9. [DOI] [PubMed] [Google Scholar]
- 18.Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010;21:233–248. doi: 10.1111/j.1365-3164.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 19.Angileri M, Pasquetti M, De Lucia M, Peano A. Azole resistance of Malassezia pachydermatis causing treatment failure in a dog. Med Mycol Case Rep. 2019;23:58–61. doi: 10.1016/j.mmcr.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijima M, Kano R, Nagata M, Hasegawa A, Kamata H. An azoleresistant isolate of Malassezia pachydermatis. Vet Microbiol. 2011;149:288–290. doi: 10.1016/j.vetmic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Grice EA, Dawson TL. Host–microbe interactions: Malassezia and human skin. Curr Opin Microbiol. 2017;40:81–87. doi: 10.1016/j.mib.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Glatz M, Bosshard P, Hoetzenecker W, Schmid-Grendelmeier P. The role of Malassezia spp. in atopic dermatitis. J Clin Med. 2015;4:1217–1228. doi: 10.3390/jcm4061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T-A, Halliwell REW, Pemberton AD, Hill PB. Identification of major allergens of Malassezia pachydermatis in dogs with atopic dermatitis and Malassezia overgrowth. Vet Dermatol. 2002;13:141–150. doi: 10.1046/j.1365-3164.2002.00291.x. [DOI] [PubMed] [Google Scholar]
- 24.Aberg L, Varjonen K, Ahman S. Results of allergen-specific immunotherapy in atopic dogs with Malassezia hypersensitivity: A retrospective study of 16 cases. Vet Dermatol. 2017;28:633–e157. doi: 10.1111/vde.12475. [DOI] [PubMed] [Google Scholar]