Abstract
Introduction
Community acquired pneumonia (CAP) is a leading cause of under-five mortality in India and Streptococcus pneumoniae is the main bacterial pathogen for it. Pneumococcal Conjugate Vaccine 13 (PCV13) has been introduced in a phased manner, in the national immunization program of India since 2017/2018. The primary objective of this study was to evaluate the effectiveness of PCV13 on chest radiograph (CXR)-confirmed pneumonia, in children hospitalized with WHO-defined severe CAP.
Methods
This prospective, multi-site test-negative study was conducted in a hospital-network situated in three districts of Northern India where PCV13 had been introduced. Children aged 2–23 months, hospitalized with severe CAP and with interpretable CXR were included after parental consent. Clinical data was extracted from hospital records. CXRs were interpreted by a panel of three independent blinded trained radiologists. Exposure to PCV13 was defined as ≥2 doses of PCV13 in children aged ≤ 12 months and ≥ 1 dose(s) in children > 12 months of age. Our outcome measures were CXR finding of primary endpoint pneumonia with or without other infiltrates (PEP±OI); vaccine effectiveness (VE) and hospital mortality.
Results
From 1st June 2017-30th April 2021, among 2711 children included, 678 (25.0%) were exposed to PCV1. CXR positive for PEP±OI on CXR was found in 579 (21.4%), of which 103 (17.8%) were exposed to PCV. Adjusted odds ratio (AOR) for PEP±OI among the exposed group was 0.69 (95% CI, 0.54–0.89, p = 0.004). Adjusted VE was 31.0% (95% CI: 11.0–44.0) for PEP±OI. AOR for hospital mortality with PEP±OI was 2.65 (95% CI: 1.27–5.53, p = 0.01).
Conclusion
In severe CAP, children exposed to PCV13 had significantly reduced odds of having PEP±OI. Since PEP±OI had increased odds of hospital mortality due to CAP, countrywide coverage with PCV13 is an essential priority.
Introduction
Community acquired pneumonia (CAP) is a leading cause of potentially vaccine-preventable illness and death among under-five children in India. Globally, the number of deaths due to pneumonia among under-five children was 0·8 million in 2018 [1]. Nigeria had the highest number of deaths followed by India (0.13 million), Pakistan, the Democratic Republic of Congo and Ethiopia. These five countries combined account for more than half of total pneumonia deaths among the under-five children [1].
Pneumococcal conjugate vaccine (PCV) has been recommended by the World Health Organization (WHO) to prevent CAP [2]. WHO recommends its inclusion in the national immunization program (NIP) of countries with high CAP related morbidity and mortality [2,3]. In compliance with the WHO recommendations, the Government of India, introduced PCV13 (Prevnar ® by Pfizer) in the NIP on 1st June of 2017 onwards in a phased manner at select states (Bihar, Himachal Pradesh, Madhya Pradesh, Rajasthan and Uttar Pradesh) and within states select districts [4]. The dose-schedule of PCV13 in NIP of India is 6 weeks, 14 weeks and 9 months (booster dose) [4,5]. A reduction of CAP among children, after the introduction of PCV13 has been reported in different settings [6,7].
We analyzed data from an ongoing multi-site study to evaluate the effectiveness of PCV13 on chest radiograph (CXR)-confirmed pneumonia, in children hospitalized with WHO-defined severe CAP in three districts of Northern India [8]. We also compared hospital mortality among those with primary endpoint pneumonia with or without other infiltrates (PEP±OI) on CXR as a secondary objective. This work was done as part of a hospital-based surveillance on CAP among children (2–59 months) ongoing in these three districts in India since 1st January, 2015 [8].
Methods
The study analyzed data from three districts of Uttar Pradesh and Bihar states of Northern India. Uttar Pradesh is the fourth largest state by area (93,023 mi2) and is the most populous in India [9]. Bihar has an area of 36,357 mi2 and is the third most populous state [10]. This analysis reports data of Darbhnaga district of Bihar where PCV13 was introduced on 1st June, 2017 and data from Lucknow (Uttar Pradesh) and Patna (Bihar) districts where PCV13 was introduced on 1st June, 2018.
A `test-negative`study design was used in this analysis to estimate the vaccine effectiveness (VE) [11]. We used the same clinical case definition to enroll both the cases and controls [11]. However, the cases and controls differed with respect to their CXR findings [11]. A child was considered `test-positive`if the CXR finding was PEP± OI and `test -negative`if findings on CXR were either `normal`or `other infiltrates only`.
An active, hospital-based surveillance network was established for this study [8]. Hospitals included in the analysis were private and public hospitals that admitted pediatric patients. A total of 92 public and private hospitals participated from three districts whose data was analyzed. Recruitment was done by trained surveillance officers [8,12,13]. From 1st June 2017/2018-30st April 2021, surveillance officers identified children from each participating hospital by reviewing admission logbooks. Children of eligible age (2–59 months), admitted with history of fast breathing with chest in-drawing were identified from hospital records. Children were included if they were hospitalized with symptoms of WHO-defined severe CAP, were permanent resident of the project district, had illness of <14 days and were neither hospitalized nor recruited previously in the study. Excluded were those with cough for ≥14 days or prior hospitalization. Excluded from the analysis were children ≥ 24 months of age as they were not eligible for PCV13. `Pneumonia`was defined as fast breathing above age-specific cut-off (≥50 breaths/min between 2–11 months and ≥40 breaths/min between 12–59 months) with/without cough/fever [14]. Child was classified as having `severe pneumonia`in the presence of at least one of the following: (a) oxygen saturation <90% or central cyanosis or (b) severe respiratory distress (e.g., grunting, very severe chest in-drawing) or (c) signs of pneumonia with a general danger sign (inability to breast feed or drink, lethargy or reduced level of consciousness, convulsions) or (d) severe malnutrition [14].
After obtaining written, informed consent from the parents/legal guardians, trained surveillance officers interviewed them to obtain socio-demographic information. Information on age, gender, place of residence, family type, breastfeeding status and smoking status of the parents was noted. Family type was categorized into nuclear and joint. A family was considered nuclear if the family had a nuclear pair comprising of head and spouse with or without unmarried children [15]. A family that was not nuclear was considered joint [15]. Anthropometry (weight and height) were noted from the hospital records. Standardized questionnaire has been published elsewhere [12].
PCV13 status was noted from the vaccination card issued by the immunization clinic and available with the parents at the time of data collection. If the card was unavailable, the parents/caregivers were requested to provide the digital image of the card to the surveillance officer or the surveillance officer asked for the date(s) of vaccination by calling a family member at home who read it out from the vaccination card. A child was considered un-immunized in case the parents/caregivers were unable to provide the information about vaccination. Children ≤ 12 months of age who received ≥2 doses of PCV13 and children > 12 months of age who received at least one dose of PCV13 were considered `exposed to PCV`, otherwise `non-exposed to PCV`.
Clinical data was recorded by pre-existing trained hospital staff at the time of hospitalization. They noted information for the following variables: respiratory rate, heart rate, axillary temperature ≥37.5°C (yes or no with duration if yes), pallor, presence of any general danger sign (inability to breast feed or drink, lethargy or reduced level of consciousness, convulsions, vomiting everything, grunting, severe malnutrition), oxygen saturation by pulse oximetry and central cyanosis. Findings on auscultation of chest was noted by the treating physician. The data were extracted from the hospital records by project staff. Clinical outcome (discharge or death) was noted from the hospital logbook on follow-up. Detailed methodology of data collection has been published elsewhere [8,12,13].
CXRs were done on advice of the treating physician and a copy of the CXR was collected from the hospital by the surveillance officer. These CXRs were either analogue or digital. All CXRs were digitalized and stored online at www.capxrs.org. A panel of trained radiologists, using WHO methodology, interpreted CXRs. They first categorized the quality of film as interpretable or un-interpretable. Interpretable CXRs were classified as either `optimal/adequate`or `suboptimal”. Thereafter, CXRs were interpreted for radiological abnormality, and categorized as abnormal or normal. An abnormal CXR was categorized as ‘PEP only’ or ‘other infiltrates only’ or ‘both PEP and other infiltrates`[16]. Outcome of interest was the presence of PEP±OI on CXR and these were `cases`. The rest were categorized as `controls`. Standardized WHO case definition of PEP ±OI has been used [16] and has been reported elsewhere [12].
Definitions
Dependent variable was case-control status. Cases were those who had PEP±OI on CXR and controls were those who had either normal CXRs or only OI. Weight-for-age (WAZ) and height-for-age (HAZ) z-score of each child was calculated using WHO Anthro Survey Analyzer [17]. Malnutrition status were categorized as WAZ >-2SD (normal), WAZ ≤ -2SD (malnourished) and WAZ ≤ -3SD (severe malnutrition) [17]. Hypoxia was defined as oxygen saturation<90% on pulse oximetry or requiring oxygen supplementation during hospital stay [18,19]. Since respiratory syncytial virus (RSV) tends to peak in India in five months of early winter (June to October) and three months of winter (December to February), therefore these eight months were considered as RSV season [20].
Sample size
About 25% children had PEP±OI on CXR among the total children recruited in the current surveillance13. We assume that there would be a 25% reduction [21] in the occurrence of PEP±OI, in children `exposed to PCV`. For an α = 0.05 level of significance and power of 90%, and 1:3 case to controls, we required a minimum sample size of 475 in `exposed to PCV`and 1423 in `non-exposed to PCV`group. Assuming non-response rate of 10%, the required sample size was 522 in exposed and 1565 in non-exposed groups, with total sample size of 2087.
Statistical analysis
Overall descriptive statistics of independent variables, such as socio-demographic and clinical were imputed. Weight of 7.2% (196/2711) and height of 9.4% (255/2711) children were missing in our data. Missing weight and height were estimated using regression-based imputation technique [22]. Number (percentage) is being reported for categorical data. Mean and standard deviation (S.D.) or median with interquartile range (IQR) for continuous data was calculated and reported. Statistical Package of Social Science (SPSS version 24) [23] software was used to perform statistical analysis.
We compared independent variables among cases and control and also among those who were `exposed`and `non-exposed`to PCV. We used chi-square test for comparison of categorical data and student’s t-test for normal continuous data. Mann-Whitney U test was used for non-normal data. A p value of < 0·05 was taken as statistically significant using a two-tailed distribution.
A directed acyclic graph (DAG) approach was used to increase precision of the analysis [24]. Variables included in DAG were clustered in four different groups: (i) Exposure variable: Vaccination with PCV13 (ii) Outcome variable: PEP±OI (iii) Potential confounder variables: child`s age (in months), gender, birth order, immunization other than PCV, nutritional status (malnutrition), education status of parents, seasonality, resident district and year of enrollment (iv) Mediator variables: hypoxia, duration of breastfeeding ≥ 6 months or currently breastfed, any co-morbidity, use of biomass fuel for cooking. We used potential confounders in the logistic regression model as these were found to be associated with exposure to PCV.
Independent variables that had a univariate association with two-tailed p value ≤ 0·1 with outcome or were listed as potential confounders in the DAG were used in the regression model. Logistic regression was done where dependent variable was CXR abnormality (PEP±OI versus others). Unadjusted odds ratio (OR) for univariate comparison and adjusted OR (AOR) for multivariate comparison with 95% confidence interval (CI) are being reported. Also, VE against PEP ± OI was calculated by using the formula VE = (1-OR) ×100% [20].
We compared independent variables among children of CAP with and without hospital mortality and predicted hospital mortality by using step-wise logistic regression model. We calculated AOR for hospital mortality with 95% CI. Independent variable that had a univariate association with hospital mortality with a two-tailed p value ≤ 0.1 were used in logistic regression model. Unadjusted OR is also being reported.
Patient and public involvement
Patients and the public were not involved in the design, conduct, analysis or interpretation of the study.
Ethical approval
The study was ethically reviewed and approved by the Ethics Review Committee of each site. Details of ethical approval are as follows: (i) King George`s Medical University, Lucknow vide letter no. 2800 Ethics/R Cell-14 dated 22nd November, 2014 (ii) Darbhanga Medical College & Hospital, Darbhanga vide letter no. 05/IEC/DMC dated 19th February, 2015 (iii) Patna Medical College and Hospital, Patna vide letter no. nil dated 15th October, 2015. The caregivers/guardians of children signed the written, informed consent for participation in study.
Results
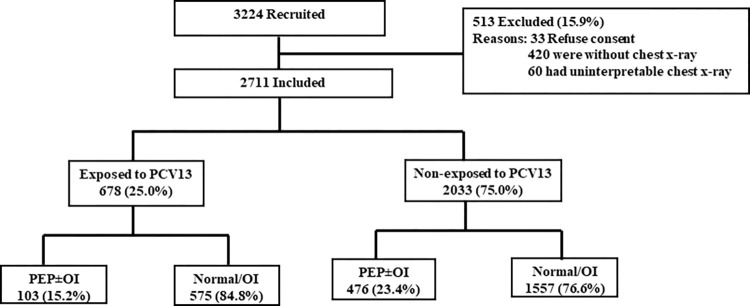
This analysis was done as part of a hospital-based surveillance on CAP among children (2–23 months) on data collected between 1st June, 2017-30th April, 2021 at Darbhanga district and between 1st June, 2018-30th April, 2021 at Patna and Lucknow districts respectively. A total of 3224 children, hospitalized with severe CAP, met the eligibility criteria. Among the eligible children, 15.9% (513/3224) were excluded. Details of excluded children are given in Fig 1. Thereafter, 2711 children were included for the analysis.
Fig 1. Flow diagram of hospitalized children (2–23 months) with community acquired pneumonia recruited from Darbhanga (1st June 2017-30th April 2021), Patna (1st June 2018 to 30th April 2021) and Lucknow (1st June 2018 to 30th April 2021).
Among the 2711 included children, 25.0% (678/2711) were `exposed to PCV`. Of these 15.2% (103/678) had PEP±OI (test-positive), and 84.8% (575/678) had normal CXRs or other infiltrates (test-negative). Among 75.0% (2033/2711) children that were `non-exposed to PCV`, 23.4% (476/2033) had PEP±OI, and 76.6% (1557/2033) had normal CXRs or other infiltrates (Fig 1).
The median age of children with CAP (n = 2711) was 6 months (IQR: 3–10) and among these 79.2% (2147/2711) were <12 months of age. Females were lesser in proportion (30.0%, 813/2711) compared to males. There was a district-wide variation among children who had PEP±OI on CXR. A significant difference was observed in socio-demographic characteristics notably in age (months), gender (female), family type, other immunization status (excluding PCV), wheezing, HAZ, WAZ, and hypoxia among those with or without PEP±OI (Table 1). In clinical features, except cough, all the variables were similar among children with or without PEP±OI. Among the general danger signs, all the variables were significantly different except inability to drink and lethargy/unconscious by PEP±OI (Table 1).
Table 1. Overall descriptive statistics and comparison of sociodemographic and clinical variables among the children with or without PEP±OI.
| Characteristics | Overall | PEP±OI | Normal/OI | |
|---|---|---|---|---|
| Sociodemographic characteristics | N = 2711 (%) |
N = 579 (%) |
N = 2132 (%) |
p value |
| Age (in months) median (IQR) | 6 (3–10) | 5 (3–10) | 6 (4–11) | <0.001 |
| Age 2–11 months n (%) | 2147 (79.2) | 471 (81.3) | 1676 (78.6) | 0.15 |
| Weight for age, mean (SD) | -1.51 (1.60) | -1.79 (1.66) | -1.43 (1.58) | <0.001 |
| Height for age, mean (SD) | -1.84 (1.77) | -2.03 (1.74) | -1.79 (1.77) | <0.001 |
| Gender (female) n (%) | 813 (30.0) | 201 (34.7) | 612 (28.7) | 0.005 |
| Residence (rural) n (%) | 1579 (58.2) | 338 (58.4) | 1241 (58.2) | 0.94 |
| aSeason (RSV) n (%) | 1886 (69.6) | 420 (72.5) | 1466 (68.8) | 0.08 |
| Biomass fuel n (%) | 1247 (46.0) | 257 (44.4) | 990 (46.4) | 0.38 |
| Pallor n (%) | 1387 (51.2) | 302 (52.2) | 1085 (50.9) | 0.59 |
| Wheezing n (%) | 2136 (78.8) | 429 (74.1) | 1707 (80.1) | 0.002 |
| Hypoxia n (%) | 1559 (57.5) | 354 (61.1) | 1205 (56.5) | 0.046 |
| cCo-morbidities n (%) | 101 (3.7) | 24 (4.1) | 77 (3.6) | 0.55 |
| bBreastfeed n (%) | 2452 (90.4) | 515 (88.9) | 1937 (90.9) | 0.17 |
| Family type (Joint) n (%) | 1914 (70.6) | 428 (73.9) | 1486 (69.7) | 0.048 |
| Mother’s education (N = 2687, row %) | ||||
| No formal education | 1094 (100.0) | 233 (21.3) | 861 (78.7) | 0.93 |
| Formal education | 1593 (100.0) | 337 (21.2) | 1256 (78.8) | |
| Father’s education (N = 2687, row %) | ||||
| No formal education | 872 (100.0) | 200 (22.9) | 672 (77.1) | 0.13 |
| Formal education | 1815 (100.0) | 370 (20.4) | 1445 (79.6) | |
| Father’s smoking (N = 2683, row %) | ||||
| Yes | 434 (100.0) | 106 (24.4) | 328 (75.6) | 0.07 |
| No | 2249 (100.0) | 462 (20.5) | 1787 (79.5) | |
| Participating sites n (%) | ||||
| Lucknow | 1007 (37.1) | 242 (41.8) | 765 (35.9) | <0.001 |
| Patna | 555 (20.5) | 145 (25.0) | 410 (19.2) | |
| Darbhanga | 1149 (42.4) | 192 (33.2) | 957 (44.9) | |
| Type of house n (%) | ||||
| Mud | 549 (20.3) | 129 (22.3) | 420 (19.7) | 0.13 |
| Bricks | 1559 (57.5) | 337 (58.2) | 1222 (57.3) | |
| Semi-constructed | 603 (22.2) | 113 (19.5) | 490 (23.0) | |
| d Year of enrollment n (%) | ||||
| 2017 | 319 (11.8) | 56 (9.7) | 263 (12.3) | 0.13 |
| 2018 | 845 (31.2) | 176 (30.4) | 669 (31.4) | |
| 2019 | 1109 (40.9) | 252 (43.5) | 857 (40.2) | |
| 2020 | 338 (12.5) | 67 (11.6) | 271 (12.7) | |
| 2021 | 100 (3.7) | 28 (4.8) | 72 (3.4) | |
| Birth order (N = 2689, row %) | ||||
| First | 984 (100.0) | 212 (37.1) | 772 (78.5) | 0.82 |
| Second | 918 (100.0) | 187 (20.4) | 731 (79.6) | |
| Third | 485 (100.0) | 103 (21.2) | 382 (78.8) | |
| More than third | 302 (100.0) | 69 (22.8) | 233 (77.2) | |
| Other immunization n (%) | ||||
| Complete for age | 2130 (78.6) | 427 (73.7) | 1703 (79.9) | 0.001 |
| Incomplete/unimmunized | 581 (21.4) | 152 (26.3) | 429 (20.1) | |
| Clinical features | ||||
| Fever | 2341 (86.4) | 504 (87.0) | 1837 (86.2) | 0.58 |
| Cough | 2684 (99.0) | 569 (98.3) | 2115 (99.2) | 0.046 |
| Fast breathing | 2365 (87.2) | 515 (88.9) | 1850 (86.8) | 0.17 |
| Difficult breathing | 2687 (99.1) | 573 (99.0) | 2114 (99.2) | 0.66 |
| General danger sign | ||||
| Inability to drink | 1386 (51.1) | 299 (51.6) | 1087 (51.0) | 0.78 |
| Vomiting everything | 1027 (37.9) | 185 (32.0) | 842 (39.5) | 0.001 |
| Convulsion | 243 (9.0) | 37 (6.4) | 206 (9.7) | 0.02 |
| Lethargy/unconscious | 1576 (58.1) | 338 (58.4) | 1238 (58.1) | 0.89 |
| Grunting | 2141 (79.0) | 438 (75.6) | 1703 (79.9) | 0.03 |
| Severe malnutrition | 454 (16.7) | 137 (23.7) | 317 (14.9) | <0.001 |
| e PCV13 immunity n (%) | ||||
| Exposed to PCV | 678 (25.0) | 103 (17.8) | 575 (27.0) | <0.001 |
| Non-exposed | 2033 (75.0) | 476 (82.2) | 1557 (73.0) |
aSeason: RSV season refers to period between June to October and December to February.
bBreastfeed: Duration of breastfeed ≥ 6 months or currently breastfeed.
dYear of enrollment refers to children enroll from 1st June 2017 to 30th April 2021.
cCo-morbidities: Congenital heart disease and history of fast breathing and cough ≥ 3 times in 6 months.
ePCV13 immunity: Children ≤ 12 months of age who received >2 PCV doses and children > 12 months of age who received at least one dose of PCV were considered `exposed to PCV`, else `non-exposed to PCV`.
We compared the socio-demographic and clinical variables among children that were or were not exposed to PCV. Significant differences were observed between those `exposed`and `non-exposed to PCV`in the socio-demographic and clinical variables. Younger children were more `non-exposed to PCV`compared to `exposed to PCV`(80.2% vs 76.3%). Children who were `non-exposed to PCV`were more likely to be females, living in rural areas, parents had no formal education, family used biomass fuel for cooking, had either incomplete immunization (excluding PCV) or were unimmunized, were hypoxic on hospitalization, had any co-morbidity or were severely malnourished as compared to the `exposed to PCV`group. There was a significant difference observed between `exposed`and `non-exposed`to PCV groups with respect to participating sites, house type, year of enrollment and CXR abnormalities (Table 2).
Table 2. Comparison of sociodemographic and clinical variables among the children exposed or unexposed to PCV13 vaccination.
| Characteristics | Exposed PCV13 |
Non-exposed PCV13 | |
|---|---|---|---|
| Sociodemographic characteristics |
N = 678 (%) |
N = 2033 (%) |
p value |
| Age 2–11 months n (%) | 517 (76.3) | 1630 (80.2) | 0.03 |
| Gender (female) n (%) | 182 (26.8) | 631 (31.0) | 0.04 |
| Residence (rural) n (%) | 369 (54.4) | 1210 (59.5) | 0.02 |
| Season (RSV) n (%) | 465 (68.6) | 1421 (69.9) | 0.52 |
| Breastfeed n (%) | 606 (89.4) | 1846 (90.8) | 0.28 |
| Family type (Joint)c n (%) | 471 (69.5) | 1443 (71.0) | 0.46 |
| Biomass fuel n (%) | 257 (37.9) | 990 (48.7) | <0.001 |
| Pallor n (%) | 355 (52.4) | 1032 (50.8) | 0.47 |
| Wheezing n (%) | 554 (81.7) | 1582 (77.8) | 0.03 |
| Hypoxiaa n (%) | 360 (53.1) | 1199 (59.0) | 0.007 |
| Co-morbiditiesb n (%) | 15 (2.2) | 86 (4.2) | 0.02 |
| Severe malnutrition n (%) | 76 (11.2) | 378 (18.6) | <0.001 |
| Mother’s education (Row %) | |||
| No formal education (n = 1094) | 210 (19.2) | 884 (80.8) | <0.001 |
| Formal education (n = 1593) | 464 (29.1) | 1129 (70.9) | |
| Father’s education (Row %) | |||
| No formal education (n = 872) | 166 (19.0) | 706 (81.0) | <0.001 |
| Formal education (n = 1815) | 507 (27.9) | 1308 (72.1) | |
| Father’s smoking (Row %) | |||
| Yes (n = 434) | 94 (21.7) | 340 (78.3) | 0.07 |
| No (n = 2249) | 579 (25.7) | 1670 (74.3) | |
| Participating sites | |||
| Lucknow | 299 (44.1) | 708 (34.8) | <0.001 |
| Patna | 51 (7.5) | 504 (24.8) | |
| Darbhanga | 328 (48.4) | 821 (40.4) | |
| Type of house n (%) | |||
| Mud | 100 (14.7) | 449 (22.1) | <0.001 |
| Bricks | 426 (62.8) | 1133 (55.7) | |
| Semi-constructed | 152 (22.4) | 451 (22.2) | |
| Birth order (Row %) | |||
| First (n = 984) | 265 (26.9) | 719 (73.1) | 0.21 |
| Second (n = 918) | 228 (24.8) | 690 (75.2) | |
| Third (n = 485) | 106 (21.9) | 379 (78.1) | |
| More than third (n = 302) | 75 (24.8) | 227 (75.2) | |
| Year of enrollment n (%) | |||
| 2017 | 15 (2.2) | 304 (15.0) | <0.001 |
| 2018 | 123 (18.1) | 722 (35.5) | |
| 2019 | 364 (53.7) | 745 (36.6) | |
| 2020 | 143 (21.1) | 195 (9.6) | |
| 2021 | 33 (4.9) | 67 (3.3) | |
| Other immunization n (%) | |||
| Complete for age | 636 (93.8) | 1494 (73.5) | <0.001 |
| Incomplete/unimmunized | 42 (6.2) | 539 (26.5) | |
| Chest x-rays findings n (%) | |||
| PEP with or without infiltrates | 103 (15.2) | 476 (23.4) | <0.001 |
| Normal/other infiltrates | 575 (84.8) | 1557 (76.6) |
aHypoxia: Oxygen saturation<90% on pulse oximetry or requiring oxygen supplementation during hospital stay.
bCo-morbidities: Congenital heart disease and history of fast breathing and cough ≥ 3 times in 6 months.
cJoint: A family that was not nuclear.
In bivariable model, unadjusted OR for `exposed to PCV`group against PEP±OI was 0.59 (95% CI 0.46–0.74) and unadjusted VE was 41.0% (95% CI, 26.0–54.0). In multivariable model, AOR was estimated by using step-wise logistic regression method, adjusted for age (in months), parent’s education, RSV season, gender, other immunization (excluding PCV), malnutrition, birth order, year of enrollment, and participating sites. We have not used co-morbidity in the model as there were collinearity with malnutrition (Unadjusted OR 2.11, 95% CI;1.29–3.44, p value = 0.003). We found that children `exposed to PCV`had reduced the odds of having PEP±OI (AOR 0.69, 95% CI, 0.54–0.89). Adjusted VE was 31.0% (95% CI, 11.0–44.0) for PEP±OI (Table 3).
Table 3. Association of chest x-ray abnormalities with PCV13, controlling for independent variables.
| Variables | Model PEP with or without other infiltrates/Normal & OIref |
|||
|---|---|---|---|---|
| Bivariable | Multivariable | |||
| Unadjusted OR (95% CI) |
p value | Adjusted OR (95% CI) |
p value | |
| Age (in months) (2–11) | 1.19 (0.94–1.50) | 0.15 | ||
| Mother’s education (without formal) | 1.01 (0.84–1.22) | 0.93 | ||
| Season (RSV) | 1.20 (0.98–1.46) | 0.08 | ||
| Exposed PCV13 | 0.59 (0.46–0.74) | <0.001 | 0.69 (0.54–0.89) | 0.004 |
| Gender (female) | 1.32 (1.09–1.61) | 0.005 | 1.24 (1.02–1.52) | 0.04 |
| Father’s education (without formal) | 1.16 (0.96–1.41) | 0.13 | 1.22 (0.99–1.51) | 0.07 |
| Other immunization (Incomplete for age) | 1.41 (1.14–1.75) | 0.001 | 1.23 (0.99–1.54) | 0.07 |
| Malnutrition status (Severe malnutrition) | 1.83 (1.45–2.31) | <0.001 | 1.72 (1.35–2.20) | <0.001 |
| Birth Order | ||||
| First | Reference | - | ||
| Second | 0.93 (0.75–1.16) | 0.53 | ||
| Third | 0.98 (0.75–1.28) | 0.89 | ||
| More than third | 1.08 (0.79–1.47) | 0.63 | ||
| Year of enrollment | ||||
| 2017 | Reference | - | ||
| 2018 | 1.24 (0.89–1.72) | 0.21 | ||
| 2019 | 1.38 (1.00–1.90) | 0.049 | ||
| 2020 | 1.16 (0.78–1.72) | 0.46 | ||
| 2021 | 1.83 (1.08–3.08) | 0.02 | ||
| Districts | ||||
| Darbhanga | Reference | - | Reference | - |
| Lucknow | 1.58 (1.28–1.95) | <0.001 | 1.70 (1.35–2.14) | <0.001 |
| Patna | 1.76 (1.38–2.25) | <0.001 | 1.65 (1.28–2.12) | <0.001 |
Abbreviations: PEP; Primary endpoint pneumonia; PCV13: Pneumococcal conjugate vaccine13; CXR: Chest x-ray; OI: Other infiltrates; OR: Odds ratio; CI: Confidence interval.
We compared the sociodemographic and clinical variables among cases of CAP with or without hospital mortality. Mortality of hospitalized child was associated with parents having no formal education (Table 4). Children having hypoxic pneumonia, any co-morbidity, severe malnutrition, and CXR abnormalities (PEP±OI) had higher odds of hospital mortality (Table 4). Unadjusted OR of hospital mortality among children with PEP±OI on CXR was 2.91 (95% CI, 1.44–5.89, p = 0.003) while the AOR of hospital mortality was 2.65 (95% CI, 1.25–5.53, p = 0.01).
Table 4. Comparison of sociodemographic and clinical variables among the cases of CAP with and without hospital mortality.
| Characteristics | Dead | Alive | |
|---|---|---|---|
| Sociodemographic characteristics |
N = 32 (%) |
N = 2679 (%) |
p value |
| Age 2–11 months n (%) | 25 (78.1) | 2122 (79.2) | 0.88 |
| Gender (female) n (%) | 13 (40.6) | 800 (29.9) | 0.19 |
| Residence (rural) n (%) | 21 (65.6) | 1558 (58.2) | 0.39 |
| Family type (Joint) n (%) | 23 (71.9) | 1891 (70.6) | 0.87 |
| Season (RSV) n (%) | 22 (68.8) | 1864 (69.6) | 0.92 |
| Breastfeed n (%) | 29 (90.6) | 2423 (90.4) | 0.97 |
| Biomass fuel n (%) | 19 (59.4) | 1228 (45.8) | 0.13 |
| Pallor n (%) | 12 (37.5) | 1375 (51.3) | 0.12 |
| Wheezing n (%) | 24 (75.0) | 2112 (78.8) | 0.60 |
| Hypoxia n (%) | 31 (96.9) | 1528 (57.0) | <0.001 |
| Co-morbiditiesa n (%) | 9 (28.1) | 92 (3.4) | <0.001 |
| Severe malnutrition n (%) | 14 (43.8) | 440 (16.4) | <0.001 |
| Mother’s education (Row %) | |||
| No formal education (n = 1094) | 19 (1.7) | 1075 (98.3) | 0.03 |
| Formal education (n = 1593) | 13 (0.8) | 1580 (99.2) | |
| Father’s education (Row %) | |||
| No formal education (n = 872) | 17 (1.9) | 855 (98.1) | 0.01 |
| Formal education (n = 1815) | 15 (0.8) | 1800 (99.2) | |
| Father’s smoking (Row %) | |||
| Yes (n = 434) | 8 (1.8) | 426 (98.2) | 0.17 |
| No (n = 2249) | 24 (1.1) | 2225 (98.9) | |
| Participating sites | |||
| Lucknow | 9 (28.1) | 998 (37.3) | 0.55 |
| Patna | 8 (25.0) | 547 (20.4) | |
| Darbhanga | 15 (46.9) | 1134 (42.3) | |
| Type of house n (%) | |||
| Mud | 7 (21.9) | 542 (20.2) | 0.97 |
| Bricks | 18 (56.3) | 1541 (57.5) | |
| Semi-constructed | 7 (21.9) | 596 (22.2) | |
| Birth order (Row %) | |||
| First (n = 984) | 11 (1.1) | 973 (98.9) | 0.46 |
| Second (n = 918) | 13 (1.4) | 905 (98.6) | |
| Third (n = 485) | 7 (1.4) | 478 (98.6) | |
| More than third (n = 302) | 1 (0.3) | 301 (99.7) | |
| Year of enrollment n (%) | |||
| 2017 | 5 (15.6) | 314 (11.7) | 0.38 |
| 2018 | 8 (25.0) | 837 (31.2) | |
| 2019 | 17 (53.1) | 1092 (40.8) | |
| 2020 | 2 (6.3) | 336 (12.5) | |
| 2021 | 0 (0.0) | 100 (3.7) | |
| Other immunization n (%) | |||
| Complete for age | 22 (68.8) | 2108 (78.7) | 0.17 |
| Incomplete/unimmunized | 10 (31.3) | 571 (21.3) | |
| PCV13 status | |||
| Exposed to PCV13 | 4 (12.5) | 674 (25.2) | 0.10 |
| Non-exposed to PCV13 | 28 (87.5) | 2005 (74.8) | |
| Chest x-rays findings n (%) | |||
| PEP with or without infiltrates | 14 (43.8) | 565 (21.1) | 0.002 |
| Normal/other infiltrates | 18 (56.3) | 2114 (78.9) |
aCo-morbidities: Congenital heart disease and history of fast breathing and cough ≥ 3 times in 6 months.
Discussion
In this prospective, multi-site, test negative study, we found that exposure to PCV13 had significantly reduced the odds of having PEP±OI on CXRs. Adjusted VE for PEP±OI was found to be 31.0% (95% CI, 11.0–44.0). CXRs were evaluated by a panel of trained external radiologists using standard WHO-methodology of CXR interpretation [13]. The study therefore had internal as well and external validity and can be compared to studies that used similar methodology [25,26].
The single, largest infectious cause of death among children worldwide is pneumonia, caused by viruses, bacteria and fungi [27]. Streptococcus pneumoniae (SP) is the most common bacterial cause of CAP in children [27]. However, there is a dearth of data on invasive bacterial pathogens, isolated by culture of sterile body fluids, causing pneumonia. Etiological association between microorganism(s) and pneumonia has been assessed by molecular techniques over the last one decade. A case-control, multi-site study (including India as a site) reported that adjusted population attributable fraction for association of SP with pneumonia was 42.2% (95 CI: 35.5%-48.2%), followed by respiratory syncytial virus or RSV (18.2%; 95% CI: 17.4%-19.0%), and rhinovirus (11.2%; 95% CI, 7.5%-14.7%) [28]. Similar findings were reported by primarily single-center studies conducted in India [29]. A case-control study entitled Pneumonia Aetiology Research for Child Health (PERCH) investigated the etiology for CAP using molecular as well as culture methods [30]. PERCH, a multi-centric study conducted in seven countries (Gambia, Kenya, Mali, South Africa, Zambia, Thailand, and Bangladesh), RSV as the most common cause, specifically for children <6 months of age [30]. Bacterial pathogens, including SP, caused substantial proportion of fatality among children, which can be prevented by early access to treatment and increased coverage with PCV13 [29]. This also makes a case for early and widespread use of PCV13 vaccine against SP [29].
In our study, among the 2711 included children, 25.0% (678/2711) were `exposed to PCV13`. This happened because the Government of India introduced PCV13 in a phased manner across the country from 1st June in 2017 onwards and even within allotted districts the vaccine coverage was taking time to optimize [4]. A WHO-UNICEF Survey has reported that in 2018 and 2019 only 44% and 57%, of the surviving infants from the target population in India, had received all the three doses of PCV13 [31].
Effectiveness of PCV vaccine to reduce radiological pneumonia among young children from other countries has been widely reported in scientific literature [7,17,32]. A South African trial reported that PCV9 reduced the incidence of first episodes of radiologically confirmed alveolar consolidation by almost one fourth in children without human immunodeficiency virus (HIV) infection [32]. This is slightly lower than 31% odds of reduction in PEP±OI in our study. Since our study area has a low prevalence of HIV [33], subjects were not tested for it. A population-based surveillance in Gambia on children (2–59 months) reported about 24% reduction in the incidence of radiological pneumonia with consolidation in children aged 2–59 months following the introduction of PCV [17]. The same study reported VE increased with greater numbers of doses [17].
In our analysis, we found that presence of PEP±OI was associated with almost three times the odds of hospital mortality in severe CAP. In a trial of PCV9 in South Africa, there was an insignificant reduction in all-cause as well as pneumonia specific mortality among the HIV infected as well as uninfected children [32]. In contrast, a population-based case-control study from Chile reported VE estimates of PCV10 to be 71.5 on pneumonia deaths and 34.8 on all-cause deaths [34]. Similar findings were reported from a time-series analysis conducted in Peru against pneumonia-related mortality [35]. Hence, the introduction of PCV would reduce the proportion of PEP±OI in children with CAP and hospital mortality.
We observed that no formal education of parents, presence of hypoxic pneumonia, co-morbidities, severe malnutrition, and CXR abnormalities (PEP±OI) were significantly associated with hospital mortality. Our results are in concurrence with those by PERCH multi-centric study that also reported that those with consolidation on CXR were at significantly higher risk of death than those with a normal CXR or with other infiltrates only [36]. Hypoxic pneumonia is a known marker of severe pneumonia and a precursor to mortality [20]. Similar to our study, previous studies have established association of hypoxic pneumonia with mortality [20,37,38] and found that PCV13 is effective against the hypoxic pneumonia [20] and hence cause-specific [39,40] and all-cause mortality [39,40].
In the current study, etiology of CAP was not investigated. RSV is an important, perhaps the leading cause of CAP. Since RSV peaks between June to October months in India and shows smaller peak in December, January and February months [22], therefore RSV seasonality was controlled in our analysis. Also, the coverage of PCV13 is likely to increase with increase in number of years since its introduction. Increase in coverage is known to provide indirect protective benefit [41], hence the year of enrollment was a confounder in our analysis. In our study, there was a gradual increase in the number of enrolled children as between 1st June, 2017 to 1st June, 2018 only Darbhanga site was enrolling children; however between 1st June 2018 to 30th April, 2021 all three sites (Darbhanga, Lucknow and Patna) enrolled. Between 1st March, 2020 to 30th April, 2021 there was a dip in number of enrolled children from all sites due to COVID-19 pandemic. Gender disparity in health care-seeking for females is common in India [13], and in other South Asian countries [42]. Gender was therefore kept as a confounder in our analysis. Previous studies have reported that child`s age (<1 year) [43], maternal education [43,44], incomplete immunization/un-immunized [43,44], malnutrition [44,45] and resident district [46] were important risk factors of CAP in resource-deprived settings and were therefore confounders in our study.
Strengths and limitations of the study
The study has several strengths. This analysis was done on data collected from a multi-site hospital-based surveillance for radiological pneumonia in which standardized definition of CAP was used for recruitment [14]. Uniform methodology of data captured by trained surveillance officers ensured quality. We also used WHO-recommended methodology for interpreting CXRs [16] which was done by a panel of trained radiologists [13], who had been a part of the study since its inception in 2015. This ensured internal and external validity. Use of test-negative design helped to reduce bias associated with confounding by health-care-seeking behavior [11]. DAG approach reduced bias, improved transparency and increased precision on our analysis [24]. Use of data of three representative sites and standardized methods will help in extrapolating our results to other similar sites in Northern India. The study had multiple hospitals in the surveillance network where uniform treatment protocols could not be followed. The different treatment protocols in such settings might have influenced the mortality and is hence a limitation. Also, longitudinal follow-up of the recruited children was not done. We also did not investigate for the etiological agents of CAP.
Conclusion
Introduction of PCV vaccine has been found to reduce the proportion of cases with radiological pneumonia in previous [13] as well as current study. In our test-negative study design, VE of PEP ±OI was 31.0%. Since the PEP±OI was associated with increased odds of hospital mortality due to CAP, countrywide coverage with PCV13 is an essential priority.
Supporting information
(TIF)
Acknowledgments
We gratefully appreciate the efforts of the management, physicians and nurses from the network hospitals in four districts who provided cooperation during data collection. We also express our gratitude towards the caregivers who provided consent for participation in this study. We would like to acknowledge Dr Keith Klugman, Dr Gail Rodgers and Dr. Prachi Vora from Bill & Melinda Gates Foundation for their valuable feedback through all stages of the study.
Data Availability
All data relevant to the study are included in the article.
Funding Statement
The work was supported by Bill & Melinda Gates Foundation (https://www.gatesfoundation.org/) via Grant No: OPP1189869/INV-006521 KGMU. The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
References
- 1.Fighting for breath call to action: End childhood pneumonia deaths (Report). The Global Forum on childhood pneumonia. 2020. Available at: https://stoppneumonia.org/wp-content/uploads/2019/11/Fighting-for-Breath-briefing-8th-pp-low-res_rev-20-Nov.pdf Assessed on: August 1, 2021.
- 2.WHO Publication. Pneumococcal vaccines WHO position paper—2012—recommendations. Vaccine. 2012. Jul 6;30(32):4717–8. doi: 10.1016/j.vaccine.2012.04.093 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2013). Introduction of pneumococcal vaccine PCV13: a handbook for district and health facility staff. World Health Organization. Available at: https://apps.who.int/iris/handle/10665/90380. [Google Scholar]
- 4.Ministry of Health and Family Welfare. Government of India Introduction of pneumococcal conjugate vaccine (PCV): National Operational Guidelines. Available at: https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Operational_Guidelines_for_PCV_introduction.pdf Accessed: 18 July, 2021.
- 5.Verma R, Khanna P. Pneumococcal conjugate vaccine: A newer vaccine available in India. Hum Vaccin Immunother. 2012. Sep 16;8(9):1317–20. doi: 10.4161/hv.20654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gönüllü E, Soysal A, Yıldız I, et al. Impact of the 13-valent pneumococcal conjugate vaccine on the incidences of community-acquired pneumonia and pneumonia-related hospitalizations in children≤ 5 years after its implementation into the national immunization program of Turkey. Hum Vaccin Immunother. 2020. Oct 2;16(10):2504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silaba M, Ooko M, Bottomley C, et al. Effect of 10-valent pneumococcal conjugate vaccine on the incidence of radiologically-confirmed pneumonia and clinically-defined pneumonia in Kenyan children: an interrupted time-series analysis. Lancet Glob Health. 2019. Mar 1;7(3):e337–46. doi: 10.1016/S2214-109X(18)30491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awasthi S, Singh JV, Kohli N, et al. Hospital-based surveillance for radiological pneumonia in children under 5 years of age in Uttar Pradesh and Bihar. Pediatr Infect Dis. 2016. Jun 30;8(2):52–7. [Google Scholar]
- 9.Annual health survey (AHS) factsheet 2012–13 Uttar Pradesh [Internet]. New Delhi: Office of Registrar General & Census Commissioner, Ministry of Home Affairs, Government of India; 2010–2011. Available at: http://www.censusindia.gov.in/vital_statistics/AHSBulletins/AHS_Factsheets_2012-13/FACTSHEET-UTTAR_PRADESH.pdf. Accessed: February 2, 2022.
- 10.Annual health survey (AHS) factsheet 2012–13 Bihar [Internet]. New Delhi: Office of Registrar General & Census Commissioner, Ministry of Home Affairs, Government of India; 2010–2011. Available at: http://www.censusindia.gov.in/vital_statistics/AHSBulletins/AHS_Factsheets_201213/FACTSHEET-Bihar.pdf. Accessed: February 2, 2022.
- 11.Chua H, Feng S, Lewnard JA, Sullivan SG, Blyth CC, Lipsitch M, et al. The Use of Test negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of Methodology. Epidemiology. 2020. Jan;31(1):43–64. doi: 10.1097/EDE.0000000000001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi S, Pandey CM, Verma T, Mishra N, Lucknow CAP Group (2019). Incidence of community acquired pneumonia in children aged 2–59 months of age in Uttar Pradesh and Bihar, India, in 2016: An indirect estimation. PloS one. 2019 Mar 20;14(3):e0214086. doi: 10.1371/journal.pone.0214086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awasthi S, Rastogi T, Mishra N, Chauhan A, Mohindra N, Shukla RC, et al. Chest radiograph findings in children aged 2–59 months hospitalised with community-acquired pneumonia, prior to the introduction of pneumococcal conjugate vaccine in India: a prospective multisite observational study. BMJ Open. 2020. May 7;10(5):e034066. doi: 10.1136/bmjopen-2019-034066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. World Health Organization; 2005. [Google Scholar]
- 15.Household Structures in India. Census of India. Occasional Paper No.1. Social Studies Division, Office of the Registrar General, India.
- 16.Mahomed N, Fancourt N, de Campo J, de Campo M, Akano A, Cherian T, et al. Preliminary report from the World Health Organisation Chest Radiography in Epidemiological Studies project. Pediatr Radiol. 2017. Oct;47(11):1399–1404. doi: 10.1007/s00247-017-3834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Anthro Survey Analyser and other tools: World Health Organization. Available: https://www.who.int/tools/child-growth-standards/software Accessed on: 10 September 2022.
- 18.Weaver R, Nguyen CD, Chan J, Vilivong K, Lai JYR, Lim R, et al. The effectiveness of the 13-valent pneumococcal conjugate vaccine against hypoxic pneumonia in children in Lao People’s Democratic Republic: An observational hospital-based test-negative study. Lancet Reg Health West Pac. 2020. Sep 6;2:100014. doi: 10.1016/j.lanwpc.2020.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi S, Rastogi T, Pandey AK, Roy C, Mishra K, Verma N, et al. Epidemiology of Hypoxic Community-Acquired Pneumonia in Children Under 5 Years of Age: An Observational Study in Northern India. Front. Pediatr. 9:790109. doi: 10.3389/fped.2021.790109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghia C, Rambhad G. Disease Burden Due to Respiratory Syncytial Virus in Indian Pediatric Population: A Literature Review. Clinical Medicine Insights Pediatrics. 2021. Jul 6;15:11795565211029250. doi: 10.1177/11795565211029250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackenzie GA, Hill PC, Sahito SM, et al. Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies. Lancet Infect Dis. 2017. Sep 1;17(9):965–73. doi: 10.1016/S1473-3099(17)30321-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. The American Statistician. 2007. Feb 1;61(1):79–90. doi: 10.1198/000313007X172556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SPSS Inc. Released 2009. SPSS for Windows [software], Version 26.0. Chicago, SPSS Inc. Available: www.spss.com.
- 24.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008. Dec;8(1):1–5. doi: 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCollum ED, Ahmed S, Roy AD, Chowdhury NH, Schuh HB, Rizvi SJR, et al. Effectiveness of the 10-valent pneumococcal conjugate vaccine against radiographic pneumonia among children in rural Bangladesh: A case-control study. Vaccine. 2020. Sep 29;38(42):6508–16. doi: 10.1016/j.vaccine.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baqui AH, McCollum ED, Saha SK, Roy AK, Chowdhury NH, Harrison M, et al. Pneumococcal Conjugate Vaccine impact assessment in Bangladesh. Gates Open Res. 2018. Apr 26;2:21. doi: 10.12688/gatesopenres.12805.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virkki R, Juven T, Rikalainen H, Svedström E, Mertsola J, Ruuskanen O. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002. May;57(5):438–41. doi: 10.1136/thorax.57.5.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bénet T, Sánchez Picot V, Messaoudi M, Chou M, Eap T, Wang J, et al. Microorganisms Associated With Pneumonia in Children <5 Years of Age in Developing and Emerging Countries: The GABRIEL Pneumonia Multicenter, Prospective, Case-Control Study. Clinical Infectious Diseases. 2017. Aug 15;65(4):604–612. doi: 10.1093/cid/cix378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav KK, Awasthi S. The current status of community-acquired pneumonia management and prevention in children under 5 years of age in India: a review. Therapeutic Advances in Infectious Disease. 2016. Jun;3(3–4):83–97. doi: 10.1177/2049936116652326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pneumonia Etiology Research for Child Health (PERCH) Study Group Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019. August 31; 394(10200):757–779. doi: 10.1016/S0140-6736(19)30721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. India: WHO and UNICEF estimates of immunization coverage: 2019 revision. Available at: https://www.who.int/immunization/monitoring_surveillance/data/ind.pdf. Accessed on: 1st Feb 2022.
- 32.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003. Oct 2;349(14):1341–8. doi: 10.1056/NEJMoa035060 [DOI] [PubMed] [Google Scholar]
- 33.Nath A. Pediatric HIV in India: Current scenario and the way forward. Indian J Public Health. 2017. Apr-Jun;61(2):124–130. doi: 10.4103/ijph.IJPH_314_15 [DOI] [PubMed] [Google Scholar]
- 34.Diaz J, Terrazas S, Bierrenbach AL, Toscano CM, Alencar GP, Alvarez A, et al. Effectiveness of the 10-Valent Pneumococcal Conjugate Vaccine (PCV-10) in Children in Chile: A Nested Case-Control Study Using Nationwide Pneumonia Morbidity and Mortality Surveillance Data. PLoS One. 2016. Apr 8;11(4):e0153141. doi: 10.1371/journal.pone.0153141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez V, Michel F, Toscano CM, Bierrenbach AL, Gonzales M, Alencar AP, et al. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: Time series analyses. Vaccine. 2016. Sep 7;34(39):4738–4743. doi: 10.1016/j.vaccine.2016.07.027 [DOI] [PubMed] [Google Scholar]
- 36.Fancourt N, Deloria Knoll M, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, et al. Chest Radiograph Findings in Childhood Pneumonia Cases From the Multisite PERCH Study. Clin Infect Dis. 2017. Jun 15;64(suppl_3):S262–S270. doi: 10.1093/cid/cix089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998. Mar;18(1):31–40. doi: 10.1080/02724936.1998.11747923 [DOI] [PubMed] [Google Scholar]
- 38.Usen S, Weber M, Mulholland K, Jaffar S, Oparaugo A, Omosigho C, et al. Clinical predictors of hypoxaemia in Gambian children with acute lower respiratory tract infection: prospective cohort study. BMJ. 1999. Jan 9;318(7176):86–91. doi: 10.1136/bmj.318.7176.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker-Dreps S, Amaya E, Liu L, Moreno G, Rocha J, Briceño R, et al. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J. 2014. Jun;33(6):637–42. doi: 10.1097/INF.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 40.Kleynhans J, Tempia S, Shioda K, von Gottberg A, Weinberger DM, Cohen C. Estimated impact of the pneumococcal conjugate vaccine on pneumonia mortality in South Africa, 1999 through 2016: An ecological modelling study. PLoS Med. 2021. Feb 16;18(2):e1003537. doi: 10.1371/journal.pmed.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varghese R, Veeraraghavan B, Jeyaraman Y, Kumar G, Arora NK, Balasubramanian S. Pneumococcal conjugate vaccine rollout in India: Expectations and challenges. Indian J Med Microbiol. 2019. Apr-Jun;37(2):141–146. doi: 10.4103/ijmm.IJMM_19_320 [DOI] [PubMed] [Google Scholar]
- 42.Ismail SA, McCullough A, Guo S, Sharkey A, Harma S, Rutter P. Gender-related differences in care-seeking behaviour for newborns: a systematic review of the evidence in South Asia. BMJ Glob Health. 2019. May 9;4(3):e001309. doi: 10.1136/bmjgh-2018-001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah N, Ramankutty V, Premila PG, Sathy N. Risk factors for severe pneumonia in children in south Kerala: a hospital-based case-control study. J Trop Pediatr. 1994. Aug;40(4):201–6. doi: 10.1093/tropej/40.4.201 [DOI] [PubMed] [Google Scholar]
- 44.Hoang VT, Dao TL, Minodier P, Nguyen DC, Hoang NT, Dang VN, et al. Risk Factors for Severe Pneumonia According to WHO 2005 Criteria Definition Among Children <5 Years of Age in Thai Binh, Vietnam: A Case-Control Study. J Epidemiol Glob Health. 2019 Dec;9(4):274–280. doi: 10.2991/jegh.k.191009.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azab SF, Sherief LM, Saleh SH, Elsaeed WF, Elshafie MA, Abdelsalam SM. Impact of the socioeconomic status on the severity and outcome of community-acquired pneumonia among Egyptian children: a cohort study. Infect Dis Poverty. 2014. Apr 24;3:14. doi: 10.1186/2049-9957-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl B, Knoll MD, Shet A, Gupta M, Kumar R, Liu L, et al. National, regional, and state-level pneumonia and severe pneumonia morbidity in children in India: modelled estimates for 2000 and 2015. Lancet Child Adolesc Health. 2020. Sep;4(9):678–687. doi: 10.1016/S2352-4642(20)30129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All data relevant to the study are included in the article.