Introduction
Insect-borne diseases transmitted by mosquitoes, such as malaria, dengue, Zika, and lymphatic filariasis, remain among the most prevalent infectious diseases worldwide [1]. For example, the incidence of dengue infection has increased significantly in recent decades to more than 390 million cases per year, of which 96 million have clinical manifestations [2]. At the same time, the remarkable progress in malaria control programs has now staggered, and, in 2021, Plasmodium falciparum malaria incidence increased to more than 600,000 deaths [3]. While the deployment of insecticide-based strategies dramatically reduced the toll of insect-borne diseases in several regions, it resulted in widespread insecticide resistance in natural populations [4]. Thus, the development of new strategies to reduce disease transmission is greatly needed.
The immune response of an insect vector against a pathogen is a major determinant of vector competence, defined as the ability of a vector to transmit disease. Insect immunity is regulated by several different signaling pathways such as the JNK, JAK-STAT, Toll, IMD, and RNAi, which activate final effectors that limit pathogen development and replication [5,6]. Thus, immune priming and other mechanisms of immune memory that result in long-term enhancement of mosquito immunity have gained attention as important mechanisms to reduce disease transmission [7]. Here, we review recent discoveries on the molecular mechanisms mediating insect immune priming and its possible role in modulating the transmission of vector-borne diseases.
Insects rely on an innate immune system that can activate a priming response
Like other invertebrates, insect defenses rely on their innate immune system, which shares some conserved features with that of vertebrates [8]. For decades, insects were thought to lack the ability to “learn” from previous exposure to pathogens because they do not have a classic adaptive immune system. However, this view has now been challenged by several studies demonstrating that insects can enhance their immune competence by activating a priming response. Immune priming has been defined as a functional state in which cells undergo long-lasting changes that enhance their response to a subsequent infection [9]. One of the first descriptions of innate immune priming in insects was in the cockroach Periplaneta americana [10] where the authors showed that immunization with killed Pseudomonas aeruginosa protected against infection with live bacteria. Similar events were later reported in several other insects (Table 1) [11–15], and in some cases, immune enhancement was shown to last for weeks [12]. For example, in P. americana, protection against P. aeruginosa was observed up to 14 days after priming [10], and a sublethal dose of Streptococcus pneumoniae also protected Drosophila flies for 14 days [13]. In these systems, however, it was not possible to demonstrate whether the observed protection was due to a long-lasting initial immune response or to the ability to mount a stronger immune response to the second challenge.
Table 1. Previous reports of insect immune memory have identified evidence for specific, nonspecific, transgenerational, and long-term immune memory depending on the insect and pathogen model.
| Reference | Invertebrate Species | Pathogen | Specific Memory | Nonspecific Memory | Transgenerational Memory | Memory Across Life Stages |
|---|---|---|---|---|---|---|
| [27] | Aedes aegypti | DENV | Yes | No | No | Yes |
| [24] | Aedes aegypti | Escherichia coli | Yes | No | No | Yes |
| [70] | Anabrus simplex | Metarhizium acridum | Yes | No | No | No |
| [71] | Anopheles albimanus | Plasmodium berghei | No | Yes | No | No |
| [26] | Anopheles gambiae | Escherichia coli | Yes | No | No | No |
| [37] | Anopheles gambiae | Plasmodium berghei | No | Yes | No | No |
| [72] | Anopheles gambiae | Escherichia coli, Enterobacter sp. and Staphylococcus aureus | Yes | Yes | No | Yes |
| [31] | Apis mellifera | Paenibacillus larvae | Yes | No | Yes | No |
| [15] | Bombus terrestris | Pseudomonas fluorescens, Paenibacillus alvei and Paenibacillus larvae | Yes | No | No | No |
| [14] | Bombus terrestris | Crithidia bombi | No | Yes | Yes | No |
| [14] | Bombus terrestris | Arthrobacter globiformis | Yes | No | Yes | No |
| [73] | Bombus terrestris | Arthrobacter globiformis | No | Yes | Yes | No |
| [18] | Bombyx mori | Photorhabdus luminescens | Yes | No | No | No |
| [74] | Caenorhabditis elegans | Pseudomonas aeruginosa | Yes | No | No | No |
| [28] | Daphnia magna | Pasteuria ramosa | Yes | No | Yes | No |
| [13] | Drosophila melanogaster | Streptococcus pneumoniae | Yes | No | No | No |
| [16] | Drosophila melanogaster | Sindbis virus | Yes | No | No | No |
| [35] | Drosophila melanogaster | Drosophila C virus | Yes | No | No | Yes |
| [32] | Drosophila melanogaster, Aedes aegypti | Sindbis virus, Drosophila C virus, cricket paralysis virus, flock house virus | Yes | No | Yes | No |
| [11] | Galleria mellonella | Photorhabdus luminescens | Yes | No | No | No |
| [20] | Galleria mellonella | Photorhabdus luminescens | No | Yes | No | No |
| [25] | Gryllus campestris | Serratia marcescens (LPS) | Yes | No | No | Yes |
| [23] | Haliotis diversicolor | Vibrio harveyi | Yes | No | No | No |
| [75] | Litopenaeus vannamei | Vibrio alginolyticus and Vibrio harveyi | Yes | Yes | No | No |
| [76] | Litopenaeus vannamei | Bacillus subtilis | No | Yes | No | No |
| [21] | Manduca sexta | Escherichia coli | No | Yes | No | No |
| [77] | Manduca sexta | Micrococcus luteus | Yes | No | Yes | No |
| [10] | Periplaneta americana | Pseudomonas aeruginosa | Yes | Yes | No | No |
| [30] | Plodia interpunctella | Plodia interpunctella granulosis virus | Yes | No | Yes | No |
| [78] | Drosophila melanogaster | Pseudomonas aeruginosa | Yes | No | No | No |
| [12] | Tenebrio molitor | LPS | No | Yes | No | No |
| [79] | Tenebrio molitor | Staphylococcus aureus, Bacillus thuringiensis, Escherichia coli and Serratia entomophila | Yes | No | Yes | No |
| [80] | Tenebrio molitor | LPS (Escherichia coli) | Yes | No | Yes | No |
| [81] | Tenebrio molitor | Arthrobacter globiformis, Bacillus subtilis, Escherichia coli and Serratia entomophila | No | Yes | Yes | No |
| [29] | Tenebrio molitor | Escherichia coli (LPS) | Yes | No | Yes | No |
| [17] | Tribolium castaneum | Bacillus thuringiensis | Yes | No | No | No |
| [82] | Tribolium castaneum | Bacillus thuringiensis and Escherichia coli | Yes | Yes | Yes | No |
| [19] | Tribolium castaneum | Escherichia coli, Bacillus thuringiensis thuringiensis and Bacillus subtilis | Yes | No | No | No |
| [83] | Tribolium castaneum | Bacillus thuringiensis | No | Yes | Yes | Yes |
| [84] | Tribolium castaneum. | Bacillus thuringiensis | Yes | No | No | No |
| [85] | Tribolium confusum | Gregarina minuta | Yes | No | No | Yes |
Depending on the model, the effect of priming can be pathogen specific [13,15–19] or nonspecific [20–23]. In P. americana, the long-lasting (14 days) protection against P. aeruginosa after priming with killed P. aeruginosa was reduced to 3 days when insects were challenged with a different bacterial species [10]. Priming can also extend across different life stages. For example, priming larvae can enhance adult immunity [24–27], and even transgenerational immune priming (TGIP) has been described in several models [28–32]. Interestingly, stress such as that inflicted by tissue injury [22], larval competition [33], or nutritional restriction [34] can also result in priming-like phenotypes, suggesting that understanding the mechanisms of these responses might provide further insights into the molecular regulators of immune priming. In summary, a plethora of evidence (Table 1) has confirmed that insect immunity shares memory-like components. However, the molecular mechanisms and immune pathways underlying these responses have only been established in a very limited number of model systems.
Antiviral immune priming in Drosophila
Drosophila hemocytes enhance antiviral immunity in adult flies by taking up dsRNA and generating viral DNA (vDNA) that serves as a template to synthesize secondary viral siRNAs (vsRNA), which are delivered to other tissues by exosome-like vesicles [16]. Oral infection of fly larvae with Drosophila C virus (DCV) enhanced survival to a lethal challenge with the same virus as adults, although there was no difference in viral load, suggesting that previous exposure to the virus enhanced tolerance to infection in adult flies [35]. A strong TGIP was triggered when adult female flies were infected with positive single-strand RNA viruses. This enhanced antiviral immunity was passed to the offspring for up to five generations in a sequence-specific and RNA-dependent manner. Interestingly, TGIP was not mediated by the RNAi pathway [32]. Strong TGIP was also documented in the progeny of Aedes aegypti female mosquitoes infected with the chikungunya virus [32]. vDNA was suggested to be an important component of the antiviral immune memory, as it has been isolated in adult flies infected during larval stages [35] and in the progeny of infected adult females [32]. However, the mechanism of vDNA transfer and amplification and the cells involved remain to be determined.
Plasmodium infection primes the Anopheles immune system
Plasmodium-induced enhanced immunity in Anopheles is a well-characterized model that provides some insights into the mechanism of insect priming. Briefly, Plasmodium infection results in a hemocyte-dependent state of enhanced immunity to subsequent infections [36]. Early work established that an increase in the proportion of circulating granulocytes—dynamic phagocytic hemocytes—mediates antiplasmodial immune memory [37]. This response is permanent, and the gut microbiota is required both to establish and recall the priming response, demonstrating that it is not due to a long-lasting immune response to the initial infection [37]. Later studies revealed remarkable coordination of insect immunity involving several different tissues and cell types, with eicosanoid lipids (prostaglandins and lipoxins) as key systemic signaling molecules.
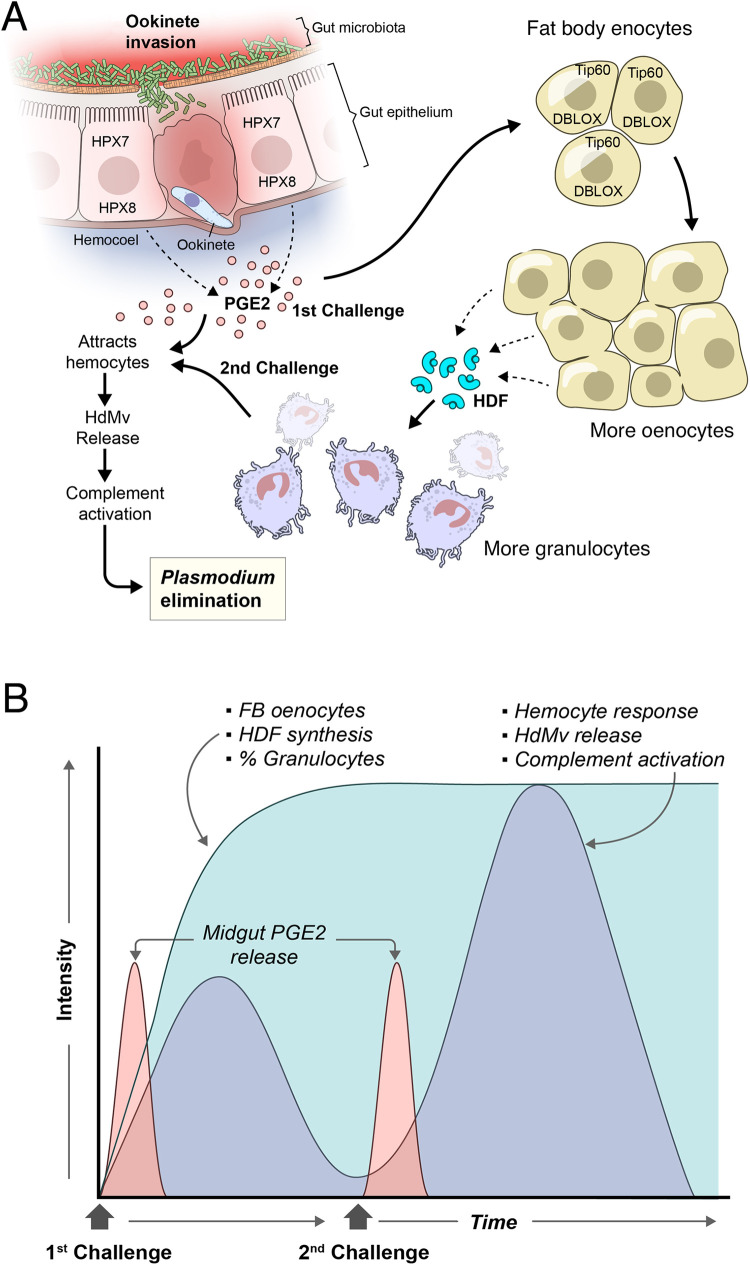
Plasmodium infection induces the expression of two heme-peroxidases (HPX)—HPX7 and HPX8—which mediate prostaglandin E2 (PGE2) synthesis by the midgut, when the microbiota comes in contact with midgut cells during ookinete midgut invasion (Fig 1A) [38]. This systemic PGE2 release triggers the production of a hemocyte differentiation factor (HDF), that promotes hemocyte differentiation into granulocytes. HDF is a complex of lipoxin A4 (LXA4) bound to Evokin, a lipid carrier of the lipocalin family [39]. More recently, LXA4 synthesis was shown to require the activity of a third HPX—double-peroxidase (DBLOX)—produced by oenocytes, a subpopulation of fat body cells that proliferates in primed mosquitoes (Fig 1A) [40]. DBLOX and Evokin expression remain high after infection. The observation that a single systemic injection of PGE2 also triggers a long-lasting increase in DBLOX expression, and HDF release, suggested that epigenetic factors could be important mediators of immune priming. A functional screening in which all mosquito histone acetyltransferase (HATs) were silenced revealed that the HAT Tip60 is, indeed, essential for priming [40].
Fig 1. Immune priming in An. gambiae mosquitoes.
(A) Plasmodium infection induces the expression HPX7 and HPX8 that mediate PGE2 synthesis by the midgut following microbiota contact with epithelial cells during ookinete invasion. The release of PGE2 triggers the production of the HDF by DBLOX-positive fat body oenocytes that proliferate following a Tip60-dependent mechanism. At the hemolymph, HDF induces the proliferation of circulating granulocytes, which are attracted to the midgut during reinfection following the PGE2 signal. Granulocyte release microvesicles (HdMv) at the site of recruitment, which mediates complement-like activation and Plasmodium elimination. Thus, the intensity of the mosquito immune response to Plasmodium can be enhanced by a previous infection. (B) Upon PGE2-dependent priming, the production of HDF in response to ookinete midgut invasion is constitutively enhanced following the first challenge, and this induces a constitutive increase in the proportion of circulating granulocytes. After the initial challenge, hemocyte association with the mosquito midgut goes back to basal levels. However, reprogramming of hemocytes during this first exposure results in enhanced hemocyte recruitment and a stronger immune response to a subsequent infection [37–40,43]. DBLOX, double-peroxidase; FB, fat body; HDF, hemocyte differentiation factor; HdMv, hemocyte-derived microvesicle; PGE2, prostaglandin E2.
Ookinete invasion causes irreversible damage and invaded cells activate a strong nitration response as they undergo apoptosis [41,42]. Mosquito hemocytes are attracted to the basal surface of the midgut by PGE2, and undergo apoptosis when they come in contact with a nitrated surface, releasing microvesicles that promote mosquito complement-mediated elimination of ookinetes [43]. A mosquito hemocyte atlas was recently established using single-cell transcriptomics, and it identified new subpopulations of granulocytes that express specific markers [44]. Studies are underway to define the role of different hemocyte subpopulations in antiplasmodial responses and how priming affects hemocyte differentiation.
Trained immunity as a memory feature of innate immunity
Recently, the concept of immune training (or trained immunity) has emerged as a key component of vertebrate innate immunity [45]. Like immune priming, trained immunity enhances the immune response to a second challenge. In trained immunity, transcription of immune effectors returns to a basal state after the primary challenge, but the final effector cells respond better to subsequent infections [9].
Immune training of monocytes by fungi infection comprises one of the best-studied models of trained immunity. Briefly, the presentation of fungal β-glucans induces an epigenetic reprogramming that increases cytokine release in response to a second exposure [46]. Similar to immune priming models, immune training lacks specificity, as exposure to fungal molecules also results in a protection against bacterial infections [47]. Similarly, bacille Calmette-Guérin (BCG) vaccines offer nonspecific protection against several different infections [48], and a role of BCG vaccine-induced trained immunity against SARS-COV-2 has been proposed [49]. At the molecular level, trained immunity is characterized by a shift in energy metabolism, including an increased rate of glycolysis. This metabolic shift is dependent on the mevalonate-induced TOR-HIF1α pathway [47,50]. Accordingly, inhibition of the TOR pathway during in vivo Candida mice infection prevented immune training and resulted in increased mortality during a subsequent immune challenge [47].
While the distinctive features of trained immunity are yet to be better described in insect models, insects also undergo some metabolic shifts following infection, like those in immune-trained vertebrate cells. For example, Drosophila macrophages switch to aerobic glycolysis when mounting an antibacterial defense [51]. Furthermore, this metabolic switch is also HIF1α dependent and is required for the survival of infected flies [51]. Interestingly, while HDF production and the proportion of circulating granulocytes remain constitutively elevated following an initial Plasmodium infection (Fig 1B), the ability of hemocytes to mount a more effective response to a second Plasmodium challenge is dependent on the presence of the bacterial gut microbiota both at the time when priming response is established and to elicit a stronger response to the second challenge [37]. Overall, this indicates that hemocytes do not remain constantly activated after the initial challenge, but rather mount a stronger response to a second ookinete midgut invasion in the presence of the gut microbiota (Fig 1B). This is in agreement with the observation that hemocyte mRNAs (TEP1, LRIM1) associated with the midgut went back to basal levels 7 days after the first infection, before the second challenge, but reach reached very high levels 24 hours after the second challenge, indicative of enhanced hemocyte recruitment to the midgut surface [37].
Can immune memory impact vector-borne disease transmission?
Despite its remarkable plasticity, activation of vector immunity often restricts infection under tolerable levels, instead of completely eliminating the pathogen [52]. Nevertheless, refractory or quasi-refractory populations are observed worldwide [53], and experimental infections of field-caught mosquitoes reveal remarkable variability between individuals. While stochastic variations [54] and genetic components [55,56] are important factors driving this heterogeneity, differences in individual life histories also shape host immunological status [57–60].
Immune priming, immune tolerance, and immune training are likely to be important modulators of individual vector competence. Recently, features of trained immunity were described in nonimmune vertebrate cells, such as epithelial and mesenchymal cells [61], and the role of the gut microbiota has been proposed [62]. Interestingly, the vector gut microbiota is also a major driver of mosquito immunity [63]. It remains to be established whether insect immune priming and trained immunity share some regulatory signaling pathways, such as those activated by the gut microbiota.
Thus, priming the immune system of insect vectors is a potential strategy to reduce disease transmission. Manipulation of larvae would be an interesting strategy to reduce vectorial capacity, as infection or immune challenge of larvae, nutritional manipulation, and intraspecific competition have been shown to enhance immunity in adult stages [26,27,64] and logistics to target larval breeding sites are well established [65–67]. While the epigenetic regulation of immunometabolism is an important factor driving susceptibility in vertebrates (and a target for therapeutics), it has only recently started to be investigated in models of disease vectors [40,68,69]. Further identification of molecular markers will make it possible to establish the frequency and intensity of such events in natural populations.
Acknowledgments
We thank Ryan Kissinger from the NIH Rocky Mountain Laboratories for the illustrations.
Funding Statement
FMG was supported by grants from the Instituto Serrapilheira (G-1912-32058) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (E-26/210.331/2022). CB-M and AM-C were supported by the Intramural Research Program of the Division of Intramural Research Z01AI000947, NIAID, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. World malaria report 2021. World Health Organization; 2021. [Google Scholar]
- 4.Rivero A, Vézilier J, Weill M, Read AF, Gandon S. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 2010;6:e1001000. doi: 10.1371/journal.ppat.1001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Xu Y, Wang X, Gu J, Yan G, Chen X-G. Antiviral systems in vector mosquitoes. Dev Comp Immunol. 2018;83:34–43. doi: 10.1016/j.dci.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 6.Rosales C. Cellular and molecular mechanisms of insect immunity. In: Shields VDC, editor. Insect Physiology and Ecology. London, England: InTech; 2017. [Google Scholar]
- 7.Lanz-Mendoza H, Garduño JC. Insect innate immune memory. Advances in Comparative Immunology. Cham: Springer International Publishing; 2018. p. 193–211. [Google Scholar]
- 8.Buchmann K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front Immunol. 2014;5:459. doi: 10.3389/fimmu.2014.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol. 2021;22:2–6. doi: 10.1038/s41590-020-00845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulhaber LM, Karp RD. A diphasic immune response against bacteria in the American cockroach. Immunology. 1992;75:378–381. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu G, Xu L, Yi Y. Galleria mellonella larvae are capable of sensing the extent of priming agent and mounting proportionatal cellular and humoral immune responses. Immunol Lett. 2016;174:45–52. doi: 10.1016/j.imlet.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 12.Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor Proc Biol Sci. 2003;270:2475–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadd BM, Schmid-Hempel P. A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biol Lett. 2009;5:798–801. doi: 10.1098/rsbl.2009.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047 [DOI] [PubMed] [Google Scholar]
- 16.Tassetto M, Kunitomi M, Andino R. Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in Drosophila. Cell. 2017;169:314–325.e13. doi: 10.1016/j.cell.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futo M, Sell MP, Kutzer MAM, Kurtz J. Specificity of oral immune priming in the red flour beetle Tribolium castaneum. Biol Lett. 2017;13. doi: 10.1098/rsbl.2017.0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G, Li M, Liu Y, Ding Y, Yi Y. The specificity of immune priming in silkworm, Bombyx mori, is mediated by the phagocytic ability of granular cells. J Insect Physiol. 2015;81:60–68. doi: 10.1016/j.jinsphys.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 19.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum Proc Biol Sci. 2009;276:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, Zhao Z, Liu C, Qiu L. Priming Galleria mellonella (Lepidoptera: Pyralidae) larvae with heat-killed bacterial cells induced an enhanced immune protection against Photorhabdus luminescens TT01 and the role of innate immunity in the process. J Econ Entomol. 2014;107:559–569. doi: 10.1603/ec13455 [DOI] [PubMed] [Google Scholar]
- 21.Eleftherianos I, Marokhazi J, Millichap PJ, Hodgkinson AJ, Sriboonlert A, ffrench-Constant RH, et al. Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem Mol Biol. 2006;36:517–525. doi: 10.1016/j.ibmb.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Visweswariah SS. Intramacrophage ROS primes the innate immune system via JAK/STAT and Toll activation. Cell Rep. 2020;33:108368. doi: 10.1016/j.celrep.2020.108368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao T, Lu J, Bai C, Xie Z, Ye L. The enhanced immune protection in small abalone Haliotis diversicolor against a secondary infection with Vibrio harveyi. Front Immunol. 2021;12:685896. doi: 10.3389/fimmu.2021.685896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno-García M, Vargas V, Ramírez-Bello I, Hernández-Martínez G, Lanz-Mendoza H. Bacterial exposure at the larval stage induced sexual immune dimorphism and priming in adult Aedes aegypti mosquitoes. PLoS ONE. 2015;10:e0133240. doi: 10.1371/journal.pone.0133240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacot A, Scheuber H, Kurtz J, Brinkhof MWG. Juvenile immune system activation induces a costly upregulation of adult immunity in field crickets Gryllus campestris. Proc Biol Sci. 2005;272:63–69. doi: 10.1098/rspb.2004.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers JC, Turangan R, Joosse BA, Hillyer JF. Adult mosquitoes infected with bacteria early in life have stronger antimicrobial responses and more hemocytes after reinfection later in life. Insects. 2020;11:331. doi: 10.3390/insects11060331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas V, Cime-Castillo J, Lanz-Mendoza H. Immune priming with inactive dengue virus during the larval stage of Aedes aegypti protects against the infection in adult mosquitoes. Sci Rep. 2020;10:6723. doi: 10.1038/s41598-020-63402-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little TJ, O’Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- 29.Zanchi C, Troussard J-P, Martinaud G, Moreau J, Moret Y. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J Anim Ecol. 2011;80:1174–1183. doi: 10.1111/j.1365-2656.2011.01872.x [DOI] [PubMed] [Google Scholar]
- 30.Tidbury HJ, Pedersen AB, Boots M. Within and transgenerational immune priming in an insect to a DNA virus. Proc Biol Sci. 2011;278:871–876. doi: 10.1098/rspb.2010.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández López J, Schuehly W, Crailsheim K, Riessberger-Gallé U. Trans-generational immune priming in honeybees. Proc Biol Sci. 2014;281:20140454. doi: 10.1098/rspb.2014.0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondotte JA, Gausson V, Frangeul L, Suzuki Y, Vazeille M, Mongelli V, et al. Evidence for long-lasting transgenerational antiviral immunity in insects. Cell Rep. 2020;33:108506. doi: 10.1016/j.celrep.2020.108506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc Biol Sci. 2008;275:463–471. doi: 10.1098/rspb.2007.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linenberg I, Christophides GK, Gendrin M. Larval diet affects mosquito development and permissiveness to Plasmodium infection. Sci Rep. 2016;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondotte JA, Gausson V, Frangeul L, Blanc H, Lambrechts L, Saleh M-C. Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat Microbiol. 2018;3:1394–1403. doi: 10.1038/s41564-018-0265-9 [DOI] [PubMed] [Google Scholar]
- 36.Ramirez JL, Barletta ABF, Barillas-Mury CV. Molecular mechanisms mediating immune priming in anopheles gambiae mosquitoes. Arthropod Vector: Controller of Disease Transmission, Volume 1. Elsevier; 2017. p. 91–100. [Google Scholar]
- 37.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barletta ABF, Trisnadi N, Ramirez JL, Barillas-Mury C. Mosquito midgut prostaglandin release establishes systemic immune priming. iScience. 2019;19:54–62. doi: 10.1016/j.isci.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramirez JL, de Almeida Oliveira G, Calvo E, Dalli J, Colas RA, Serhan CN, et al. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat Commun. 2015;6. doi: 10.1038/ncomms8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes FM, Tyner MDW, Barletta ABF, Saha B, Yenkoidiok-Douti L, Canepa GE, et al. Double peroxidase and histone acetyltransferase AgTip60 maintain innate immune memory in primed mosquitoes. Proc Natl Acad Sci U S A. 2021;118:e2114242118. doi: 10.1073/pnas.2114242118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira GDA, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo JC, Ferreira ABB, Trisnadi N, Barillas-Mury C. Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci Immunol. 2017;2. doi: 10.1126/sciimmunol.aal1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raddi G, Barletta ABF, Efremova M, Ramirez JL, Cantera R, Teichmann SA, et al. Mosquito cellular immunity at single-cell resolution. Science. 2020;369:1128–1132. doi: 10.1126/science.abc0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto JA, Gálvez NMS, Andrade CA, Ramírez MA, Riedel CA, Kalergis AM, et al. BCG vaccination induces cross-protective immunity against pathogenic microorganisms. Trends Immunol. 2022. doi: 10.1016/j.it.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 49.Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172:135–146.e9. doi: 10.1016/j.cell.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 51.Krejčová G, Danielová A, Nedbalová P, Kazek M, Strych L, Chawla G, et al. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife. 2019;8:e50414. doi: 10.7554/eLife.50414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliveira JH, Bahia AC, Vale PF. How are arbovirus vectors able to tolerate infection? Dev Comp Immunol. 2020;103:103514. doi: 10.1016/j.dci.2019.103514 [DOI] [PubMed] [Google Scholar]
- 53.Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: A review. Infect Genet Evol. 2019;67:191–209. doi: 10.1016/j.meegid.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duneau D, Ferdy J-B, Revah J, Kondolf H, Ortiz GA, Lazzaro BP, et al. Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. Elife. 2017;6. doi: 10.7554/eLife.28298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riehle MM, Markianos K, Niaré O, Xu J, Li J, Touré AM, et al. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. doi: 10.1126/science.1124153 [DOI] [PubMed] [Google Scholar]
- 56.Blandin SA, Wang-Sattler R, Lamacchia M, Gagneur J, Lycett G, Ning Y, et al. Dissecting the genetic basis of resistance to malaria parasites in Anopheles gambiae. Science. 2009;326:147–150. doi: 10.1126/science.1175241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayton EH, Tramonte AR, Wearing HJ, Christofferson RC. Age-structured vectorial capacity reveals timing, not magnitude of within-mosquito dynamics is critical for arbovirus fitness assessment. Parasit Vectors. 2020;13:310. doi: 10.1186/s13071-020-04181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennison NJ, Jupatanakul N, Dimopoulos G. The mosquito microbiota influences vector competence for human pathogens. Curr Opin Insect Sci. 2014;3:6–13. doi: 10.1016/j.cois.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaidyanathan R, Fleisher AE, Minnick SL, Simmons KA, Scott TW. Nutritional stress affects mosquito survival and vector competence for West Nile virus. Vector Borne Zoonotic Dis. 2008;8:727–732. doi: 10.1089/vbz.2007.0189 [DOI] [PubMed] [Google Scholar]
- 60.Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol Lett. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x [DOI] [PubMed] [Google Scholar]
- 61.Bekkering S, Domínguez-Andrés J, Joosten LAB, Riksen NP, Netea MG. Trained immunity: Reprogramming innate immunity in health and disease. Annu Rev Immunol. 2021;39:667–693. doi: 10.1146/annurev-immunol-102119-073855 [DOI] [PubMed] [Google Scholar]
- 62.Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. Potential role of gut Microbiota in induction and regulation of innate immune memory. Front Immunol. 2019;10:2441. doi: 10.3389/fimmu.2019.02441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabrieli P, Caccia S, Varotto-Boccazzi I, Arnoldi I, Barbieri G, Comandatore F, et al. Mosquito trilogy: Microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front Microbiol. 2021;12:630438. doi: 10.3389/fmicb.2021.630438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weger-Lucarelli J, Auerswald H, Vignuzzi M, Dussart P, Karlsson EA. Taking a bite out of nutrition and arbovirus infection. PLoS Negl Trop Dis. 2018;12:e0006247. doi: 10.1371/journal.pntd.0006247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;CD008923. doi: 10.1002/14651858.CD008923.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO. Larval Source Management. 2013 [cited 2022 Mar 6]. Available from: https://www.who.int/malaria/publications/atoz/larval_source_management_2-pager_eng.pdf?ua=1.
- 67.Imbahale SS, Mweresa CK, Takken W, Mukabana WR. Development of environmental tools for anopheline larval control. Parasit Vectors. 2011;4:130. doi: 10.1186/1756-3305-4-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruiz JL, Yerbanga RS, Lefèvre T, Ouedraogo JB, Corces VG, Gómez-Díaz E. Chromatin changes in Anopheles gambiae induced by Plasmodium falciparum infection. Epigenetics Chromatin. 2019;12:5. doi: 10.1186/s13072-018-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee K, Dobrindt U. The emerging role of epigenetic mechanisms in insect defense against pathogens. Curr Opin Insect Sci. 2022;49:8–14. doi: 10.1016/j.cois.2021.10.004 [DOI] [PubMed] [Google Scholar]
- 70.Srygley RB. Ontogenetic changes in immunity and susceptibility to fungal infection in Mormon crickets Anabrus simplex. J Insect Physiol. 2012;58:342–347. doi: 10.1016/j.jinsphys.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 71.Maya-Maldonado K, Cime-Castillo J, Maya-Lucas O, Argotte-Ramos R, Rodríguez MC, Lanz-Mendoza H. Transcriptome analysis uncover differential regulation in cell cycle, immunity, and metabolism in Anopheles albimanus during immune priming with Plasmodium berghei. Dev Comp Immunol. 2021;120:104046. doi: 10.1016/j.dci.2021.104046 [DOI] [PubMed] [Google Scholar]
- 72.Brown LD, Shapiro LLM, Thompson GA, Estévez-Lao TY, Hillyer JF. Transstadial immune activation in a mosquito: Adults that emerge from infected larvae have stronger antibacterial activity in their hemocoel yet increased susceptibility to malaria infection. Ecol Evol. 2019:6082–6095. doi: 10.1002/ece3.5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan J, Zhao N, Yang Z, Li Y, Bai H, Zou W, et al. A trade-off switch of two immunological memories in Caenorhabditis elegans reinfected by bacterial pathogens. J Biol Chem. 2020:17323–17336. doi: 10.1074/jbc.RA120.013923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu C-H, Chen J-C, Lin Y-C, Chen Y-Y, Liu P-C, Lin B-W, et al. White shrimp Litopenaeus vannamei that have received mixtures of heat-killed and formalin-inactivated Vibrio alginolyticus and V. harveyi exhibit recall memory and show increased phagocytosis and resistance to Vibrio infection. Fish Shellfish Immunol. 2021;112:151–158. doi: 10.1016/j.fsi.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 76.Valdez A, Yepiz-Plascencia G, Ricca E, Olmos J. First Litopenaeus vannamei WSSV 100% oral vaccination protection using CotC::Vp26 fusion protein displayed on Bacillus subtilis spores surface. J Appl Microbiol. 2014;117:347–357. doi: 10.1111/jam.12550 [DOI] [PubMed] [Google Scholar]
- 77.Trauer U, Hilker M. Parental legacy in insects: variation of transgenerational immune priming during offspring development. PLoS ONE. 2013;8:e63392. doi: 10.1371/journal.pone.0063392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christofi T, Apidianakis Y. Drosophila immune priming against Pseudomonas aeruginosa is short-lasting and depends on cellular and humoral immunity. F1000Res. 2013;2:76. doi: 10.12688/f1000research.2-76.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhinaut J, Chogne M, Moret Y. Immune priming specificity within and across generations reveals the range of pathogens affecting evolution of immunity in an insect. J Anim Ecol. 2018:448–463. doi: 10.1111/1365-2656.12661 [DOI] [PubMed] [Google Scholar]
- 80.Moret Y. “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor Proc Biol Sci. 2006;273:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dubuffet A, Zanchi C, Boutet G, Moreau J, Teixeira M, Moret Y. Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle. PLoS Pathog. 2015;11:e1005178. doi: 10.1371/journal.ppat.1005178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, et al. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum J Anim Ecol. 2010;79:403–413. [DOI] [PubMed] [Google Scholar]
- 83.Khan I, Prakash A, Agashe D. Divergent immune priming responses across flour beetle life stages and populations. Ecol Evol. 2016;6:7847–7855. doi: 10.1002/ece3.2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milutinović B, Fritzlar S, Kurtz J. Increased survival in the red flour beetle after oral priming with bacteria-conditioned media. J Innate Immun. 2014;6:306–314. doi: 10.1159/000355211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas AM, Rudolf VHW. Challenges of metamorphosis in invertebrate hosts: maintaining parasite resistance across life-history stages. Ecological Entomology. 2010:200–205. doi: 10.1111/j.1365-2311.2009.01169.x [DOI] [Google Scholar]