Abstract
Colchicine is useful for the prevention and treatment of gout and a variety of other disorders. It is a substrate for CYP3A4 and P-glycoprotein (P-gp), and concomitant administration with CYP3A4/P-gp inhibitors can cause life-threatening drug–drug interactions (DDIs) such as pancytopenia, multiorgan failure, and cardiac arrhythmias. Colchicine can also cause myotoxicity, and coadministration with other myotoxic drugs may increase the risk of myopathy and rhabdomyolysis. Many sources of DDI information including journal publications, product labels, and online sources have errors or misleading statements regarding which drugs interact with colchicine, as well as suboptimal recommendations for managing the DDIs to minimize patient harm. Furthermore, assessment of the clinical importance of specific colchicine DDIs can vary dramatically from one source to another. In this paper we provide an evidence-based evaluation of which drugs can be expected to interact with colchicine, and which drugs have been stated to interact with colchicine but are unlikely to do so. Based on these evaluations we suggest management options for reducing the risk of potentially severe adverse outcomes from colchicine DDIs. The common recommendation to reduce the dose of colchicine when given with CYP3A4/P-gp inhibitors is likely to result in colchicine toxicity in some patients and therapeutic failure in others. A comprehensive evaluation of the almost 100 reported cases of colchicine DDIs is included in table form in the electronic supplementary material. Colchicine is a valuable drug, but improvements in the information about colchicine DDIs are needed in order to minimize the risk of serious adverse outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-022-01265-1.
Key Points
| Colchicine is a valuable drug for many indications, but it has a number of potentially serious drug interactions. |
| Most current sources of colchicine drug interaction information have errors of omission or commission regarding which drugs interact clinically with colchicine. |
| Adverse colchicine drug interactions can almost always be prevented through proper management and patient education. |
Introduction
Colchicine has been a valuable drug in the prevention and treatment of gout for many centuries, and it is also approved for use in familial Mediterranean fever. Colchicine has a variety of off-label uses, including pericarditis, various dermatologic disorders, Behçet’s disease, and more recently it has been used to reduce cardiovascular events in patients with coronary artery disease [1–4]. Colchicine has also been tested in patients with COVID-19 in numerous clinical trials, but the results to date have been disappointing [5, 6]. Colchicine is still under investigation for treatment of long COVID [7], and it can be used to treat the pericarditis that can occur in COVID patients [8]. As the indications for colchicine increase beyond gout, it is logical to expect to see more adverse drug–drug interactions (DDIs) because more people will receive colchicine, and the prescribers may have little previous experience using the drug. In clinical trials of low-dose colchicine (0.5 mg/day) following myocardial infarction, few DDIs have been observed [2]. This lack of DDIs in low-dose colchicine trials is consistent with the case reports detailed in the electronic supplementary material (ESM), in which the vast majority of adverse colchicine DDIs involved colchicine doses > 0.5 mg. Nonetheless, it is likely that the risk of colchicine DDIs is lower in these clinical trials as opposed to the general clinical setting in which patients may receive higher colchicine doses and where patients are not as carefully screened for risk factors, concomitant therapy, and may not be carefully monitored for adverse outcomes [9]. Given that colchicine DDIs can result in serious or fatal outcomes, it is important that health care professionals (i) know which drugs can interact with colchicine, and (ii) understand how to manage such DDIs to minimize the risk of patient harm. The purpose of this paper is to provide that information, based on the evidence provided in the literature and the general principles of drug interaction management.
Colchicine Toxicity
Colchicine has a narrow therapeutic index and must be dosed carefully to minimize the risk of toxicity. Toxicity tends to occur when the serum colchicine concentration exceeds 3.0 μg/L [10]. While colchicine has been used safely in many patients for many centuries, colchicine toxicity can occur when patients develop excessive serum concentrations, often resulting from some combination of excessive dosing, drug interactions, and impaired renal or hepatic function [11]. Once serious colchicine toxicity occurs it can be very difficult to treat, especially if the toxicity damages the kidneys and liver, which further impairs the ability of the person to eliminate colchicine. Even when the patient survives, elevated colchicine concentrations may persist for weeks after it is discontinued [12–14] resulting in continuing damage to tissues and organs. Colchicine is not dialysable, so dialysis does not shorten the process. Indeed, patients on maintenance hemodialysis appear to be at greater risk from colchicine DDIs [15–17]. Colchicine toxicity usually manifests as gastrointestinal, musculoskeletal, hematologic, and—with severe toxicity—cardiovascular. These are summarized in Table 1. Over 100 DDI cases of colchicine-related toxicity, many of them life-threatening or fatal, have been published in case reports, case series, and adverse reaction reporting systems [18, 19]. A detailed analysis of colchicine DDI cases published in the medical literature is provided in the online Supplemental Table (see ESM).
Table 1.
Clinical findings of colchicine toxicity
| Severity | Signs and symptoms |
|---|---|
| Mild |
Gastrointestinal: diarrhea, nausea, vomiting, abdominal pain Miscellaneous: fatigue, lethargy, malaise, insomnia |
| Moderate |
Neuromuscular: muscle pain, muscle weakness, paresthesias Hematologic: moderate neutropenia and/or moderate thrombocytopenia Respiratory: shortness of breath, cough Dermatologic: alopecia (usually late) |
| Severe/fatal |
Neuromuscular: rhabdomyolysis, atonia, dark brown urine Hematologic: pancytopenia (infections, fever, bleeding) Multiorgan failure: renal failure, liver failure Cardiovascular: cardiac arrhythmias, cardiac failure, hypotension, cardiac arrest |
Colchicine Pharmacokinetics
Despite colchicine being used for many centuries, its pharmacokinetics have only been studied in the past few decades after suitable assay techniques were developed. Research is still being conducted, particularly on the effect of transporters on colchicine disposition.
Absorption
Colchicine is a lipid-soluble alkaloid and undergoes extensive first-pass metabolism resulting in an absolute bioavailability of approximately 30–50% [11, 20, 21]. Bioavailability can vary substantially from one patient to another, probably due to the numerous enzymes and transporters involved during absorption. In one study of healthy subjects, mean bioavailability was 45%, but it varied among subjects from 28 to 88% [22]. Colchicine is a substrate for CYP3A4 and P-gp in the intestine, and P-gp appears to transport colchicine back into the intestinal lumen. It has been proposed that colchicine may be a substrate for transporters such as MRP2 and OATP2B1 [23, 24] but their role remains to be established.
Distribution
Colchicine is rapidly distributed, and the apparent volume of distribution of colchicine is large; it is usually about 5–10 L/kg but can range from 2 to 12 L/kg. The wide distribution and low (nanogram per milliliter) concentrations in the plasma are consistent with the failure of dialysis to effectively treat colchicine toxicity. Binding to serum protein is about 40%, again with high variability. Colchicine crosses the placenta and is distributed into breast milk [11, 21]. There are no reported DDIs based on changes in colchicine distribution.
Metabolism
Colchicine metabolism takes place primarily through demethylation by CYP3A4 in the intestine and liver. Other CYP isozymes and phase II metabolism do not appear to play a significant role in colchicine metabolism.
Elimination
The kidneys are important in the elimination of colchicine, but estimates of the percentage of total colchicine clearance by the kidneys have varied [21]. In 12 healthy subjects given colchicine 1 mg, 40–65% was recovered unchanged in the urine [25], but others have found lower percentages for renal elimination [21]. Biliary excretion also appears to be an important pathway of colchicine elimination, and P-gp appears to be involved in both the renal tubular secretion and the biliary excretion of colchicine [21, 26]. The half-life in healthy subjects is generally about 15–30 h [21, 22]. In one small study, total body clearance was about twice as high and area under the concentration–time curve (AUC) was about four times smaller for healthy subjects compared with elderly patients [22].
Mechanisms of Colchicine Drug Interactions
Colchicine can interact with other drugs by a variety of different mechanisms (see Table 2). For some colchicine DDIs, more than one mechanism is involved. For example, several drugs that cause myopathy also inhibit CYP3A4 and/or P-gp (e.g., amiodarone, cyclosporine, statins). Colchicine is rarely the perpetrator in DDIs with other drugs, and in this paper we address only colchicine as the victim drug in DDIs.
Table 2.
Sites of potential interaction with colchicine
| Process | Mediated by | Drugs that may interact |
|---|---|---|
| Absorption | CYP3A4 and P-gp in wall of small intestinea | Inhibitors or inducers of CYP3A4 and P-gp in wall of small intestine |
| Hepatic metabolism | CYP3A4 and P-gp in liver |
Inhibitors or inducers of CYP3A4 and P-gp in liver Hepatotoxic drugs (theoretically) |
| Biliary excretion | P-gp in liver | Inhibitors or inducers of P-gp in liver |
| Renal excretion |
Glomerular filtration P-gp in kidney |
Nephrotoxic drugs (theoretically) Inhibitors or inducers of P-gp in kidney |
| Myotoxicity | Possible additive effect with myotoxic effect of colchicine | Drugs that cause myopathy |
aColchicine undergoes enterohepatic circulation, it may be exposed to CYP3A4 and P-gp more than once
Inhibition of CYP3A4 and/or P-gp
Colchicine is a substrate for CYP3A4 in the intestine and liver, and undergoes considerable first-pass metabolism resulting in bioavailability of 30–50% [11, 21]. It is also a substrate for P-gp in the small intestine, liver, and kidneys [11, 23]. Colchicine undergoes enterohepatic circulation as parent drug and metabolites. Many drugs that produce combined CYP3A4/P-gp inhibition have been shown to increase colchicine serum concentrations and cause colchicine toxicity as we describe elsewhere in this paper.
Because it is well documented that combined CYP3A4/P-gp inhibitors increase colchicine AUC, drugs that inhibit either CYP3A4 or P-gp alone are also often assumed to be capable of increasing colchicine AUC. The vast majority of drugs that inhibit CYP3A4 also inhibit P-gp, so this is not an issue for most colchicine DDIs. It is not established, however, that inhibition of either CYP3A4 or P-gp alone is enough to cause clinically significant DDIs with colchicine. In fact, the available evidence, although limited, suggests otherwise. Drugs that primarily inhibit one or the other—such as voriconazole (CYP3A4 inhibitor) or propafenone (P-gp inhibitor)—have not had much effect on colchicine pharmacokinetics.
Voriconazole is classified by the US FDA as a ‘strong’ CYP3A4 inhibitor, and it can substantially increase the AUC of CYP3A4 substrates [27, 28]. Voriconazole may not inhibit P-gp, however, suggested by a lack of voriconazole on digoxin pharmacokinetics in healthy subjects [29]. A pharmaceutical company applying to the US Food and Drug Administration (FDA) for approval of their colchicine product performed a non-randomized, open-label, crossover study in 12 healthy subjects given colchicine 0.6 mg before and after voriconazole 200 mg twice daily for 5 days. Voriconazole did not affect colchicine pharmacokinetics [30].
On the other hand, propafenone inhibits P-gp but is not known to inhibit CYP3A4. A pharmaceutical company applying to the US FDA for approval of their colchicine product conducted a non-randomized, open label, crossover study in nine healthy male subjects who were given a single dose of colchicine 0.6 mg before and after propafenone 225 mg twice daily for 5 days. Propafenone did not appear to affect colchicine pharmacokinetics [30]. However, one cannot rule out that a larger daily dose of propafenone given for a longer period might interact with colchicine. It is also possible that P-gp inhibition without CYP3A4 inhibition has little effect on colchicine pharmacokinetics. A tentative conclusion from the studies of voriconazole and propafenone is that inhibition of both CYP3A4 and P-gp is needed in order to manifest clinically important effects on colchicine pharmacokinetics. Nonetheless, one cannot rule out that inhibition of either CYP3A4 or P-gp alone may be sufficient to interact with colchicine, especially in patients predisposed to colchicine toxicity such as those with renal or hepatic toxicity, or those with reduced activity of P-gp or CYP3A4 due to genetics.
Enzyme Inducers
Enzyme inducers such as rifampin, barbiturates, carbamazepine, efavirenz, lumacaftor, phenytoin, primidone, and St. John’s wort increase CYP3A4/P-gp activity, and would be expected to reduce colchicine AUC. Case reports suggest that both rifampin and carbamazepine can substantially reduce colchicine concentrations [31, 32]. (See case report details in the online Supplemental Table in the ESM). Lesinurad appears to be a modest CYP3A4 inducer, and it produced modest reductions in colchicine AUC in healthy subjects [33]. Although the evidence for the effect of CYP3A43 inducers on colchicine is limited, it seems very likely that patients on enzyme inducers are at increased risk of subtherapeutic colchicine concentrations.
Additive Myotoxic Effects
Colchicine therapy alone has been associated with myopathy and rhabdomyolysis [34–40], and it is possible that additive myotoxic effects with other drugs can occur. Myotoxicity has been reported in numerous patients receiving colchicine along with drugs that can independently lead to myotoxicity such as certain statins and cyclosporine. (See case report details in the online Supplemental Table, ESM.) Although it is thought that these reactions are due to additive myotoxic effects, it cannot yet be ruled out that pharmacokinetic DDIs may also occur, involving CYP3A4, P-gp, OATP [24] or MRP2 [23, 41]. Evidence to date suggests that not all statins pose the same risk when combined with colchicine, as discussed in Table 3. The signs and symptoms of myotoxicity in these cases often included muscle weakness and/or muscle pain, and sometimes darkened urine; myopathy is also often accompanied by nonspecific symptoms such as fatigue and malaise, and shortness of breath.
Table 3.
Management options for colchicine drug interactions
| Drugs | Evidence for drug interaction (DDI) | ORCA classa and management options | Management if concurrent use needed |
|---|---|---|---|
| Antiarrhythmics | |||
| Amiodarone | Amiodarone inhibits CYP3A4/P-gp and would be expected to ↑ colchicine AUC; [61] several case reports suggest that a DDI may occurb |
ORCA Class 2: Avoid if possiblec Use alternative: other than amiodarone and dronedarone, most other antiarrhythmics are not known to inhibit CYP3A4 and P-gp Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Dronedarone | Dronedarone inhibits CYP3A4/P-gp and theoretically would be expected to ↑ colchicine AUC [62, 63] |
ORCA Class 2: Avoid if possiblec Use alternative: other than amiodarone and dronedarone, most other antiarrhythmics are not known to inhibit CYP3A4 and P-gp Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Propafenone | Propafenone inhibits P-gp but has little effect on CYP3A4; no colchicine DDI was found in healthy subjects with propafenone 450 mg/d × 5 days [30, 56] |
ORCA Class 4: Low risk Concurrent use need not be avoided, but some patients might have increased colchicine levels especially if they have low CYP3A4 activity due to other drugs or genetics |
Normal monitoring for colchicine toxicity |
| Quinidine | Quinidine is a potent inhibitor of P-gp, but not CYP3A4 [64]; it is not known if P-gp inhibition alone affects colchicine pharmacokinetics, no clinical data are available |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but increased colchicine levels possible, especially in predisposed patients (e.g., on CYP3A4 inhibitors, severe renal or hepatic impairment)c |
Monitor for altered colchicine effect if quinidine is started, stopped or changed in dosage Advise patient about colchicine toxicitye |
| Ranolazine | Ranolazine appears to be a modest inhibitor of CYP3A4 and P-gp [65–67]; it is not known if inhibition of CYP3A4 and P-gp is large enough for interaction |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but increased colchicine levels possible, especially in predisposed patients (e.g., on CYP3A4 inhibitors, severe renal or hepatic impairment)c |
Monitor for altered colchicine effect if ranolazine is started, stopped or changed in dosage Advise patient about colchicine toxicitye |
| Azole antifungals | |||
| Fluconazole | Fluconazole is a dose-dependent inhibitor of CYP3A4 that probably has little effect on P-gp [27, 68–70]; fluconazole 400 mg on day 1 and 200 mg on days 2–5 ↑ colchicine AUC by 40% in healthy subjects [56]; one case of colchicine toxicity with fluconazole in patient with renal failureb |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but increased risk with renal impairmentc,f or if patient has low P-gp activityg Or consider alternative to fluconazole: voriconazole may be less likely to interact with colchicine (see below); terbinafine does not inhibit CYP3A4 |
If no renal impairment or ↓ P-pg activity: Monitor for colchicine toxicity particularly during start of fluconazole therapy Advise patient about colchicine toxicitye With renal impairment or ↓ P-gp activity: Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Itraconazole | Itraconazole inhibits CYP3A4/P-gp [27] and would be expected to ↑ colchicine AUC as does ketoconazole (see below) |
ORCA Class 2: Avoid if possiblec Consider stopping colchicine during short-term itraconazole Or use alternative. Fluconazole (see above) and voriconazole (see below) are less likely to interact; terbinafine does not inhibit CYP3A4 Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Ketoconazole | Ketoconazole inhibits CYP3A4/P-gp. A study in 24 healthy subjects found a mean 212% ↑ in colchicine AUC after ketoconazole 200 mg BID for 5 days; one subject had a 420% ↑ in AUC [25] |
ORCA Class 2: Avoid if possiblec Consider stopping colchicine during short-term ketoconazole Or use alternative. Fluconazole (see above) and voriconazole (see below) are less likely to interact; terbinafine does not inhibit CYP3A4 Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Voriconazole | Voriconazole is a strong CYP3A4 inhibitor, but appears to have little effect on P-gp [27–29]; a study in healthy subjects found no effect of voriconazole 200 mg BID for 5 days on colchicine AUC [30] |
ORCA Class 4: Low risk Concurrent use need not be avoided, but some patients might have increased colchicine levels, especially if P-gp activity is lowg |
Normal monitoring for colchicine toxicity |
| Antivirals—protease inhibitors and adjuvants | |||
|
Atazanavir Cobicistat Darunavirh Fosamprenavir Indinavir Nelfinavir Ritonavir Saquinavir Telaprevir |
These agents inhibit CYP3A4 and P-gp and would be expected to ↑ colchicine AUC; a study in 18 healthy subjects found a mean 296% ↑ in colchicine AUC after ritonavir 100 mg/day for 5 days; one subject had a 924% ↑ in colchicine AUCi [25, 45] |
ORCA Class 2: Avoid if possiblec Use alternative: For most patients it may be easier to avoid colchicine than the antiviral agentd. Stop colchicine before antiviral is started, and for 3–4 days after antiviral is stopped. If concurrent use is deemed necessary (which should very rarely be the case), see column to the right. Ritonavir + nirmatrelvir (Paxlovid) is normally given for 5 days, so stopping colchicine during Paxlovid therapy would usually be the best management |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Tipranavir | Unlike many other protease inhibitors, tipranavir tends to ↑ P-gp activity, which might reduce colchicine AUCj [71] |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but some patients may have reduced colchicine concentrations; more data are needed |
Monitor for altered colchicine effect if tipranavir is started, stopped, or changed in dosage; adjust colchicine dose as needed; note that the onset and offset of enzyme induction may occur slowly over 1–2 weeks |
| Antivirals—miscellaneous | |||
| Boceprevir | Boceprevir inhibits CYP3A4 [72], but its effect on P-pg is not known. Effect on colchicine requires further study |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but some patients might have increased colchicine levels, especially if P-gp activity is lowg |
Monitor for altered colchicine effect if boceprevir is started, stopped or changed in dosage Advise patient about colchicine toxicitye |
| Letermovir | Letermovir inhibits CYP3A4 [73], but may not affect P-pg [74]. Effect on colchicine requires further study |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but some patients might have increased colchicine levels, especially if P-gp activity is lowg |
Monitor for altered colchicine effect if letermovir is started, stopped or changed in dosage Advise patient about colchicine toxicitye |
|
Efavirenz Etravirine Nevirapine |
These enzyme inducers are known to induce CYP3A4, and are likely to reduce colchicine serum concentrations |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but some patients may have reduced colchicine concentrations; more data are needed |
Monitor for altered colchicine effect if these agents are started, stopped, or changed in dosage; adjust colchicine dose as needed; note that the onset and offset of enzyme induction may occur slowly over 1–2 weeks |
| Calcineurin inhibitors | |||
| Cyclosporine | Cyclosporine inhibits CYP3A4 and P-gp; a study in 23 healthy subjects found a mean 259% ↑ in colchicine AUC after a single 100-mg dose of cyclosporine; one subject had a 512% ↑ [25, 45, 75]; possible additive myopathyk; over 30 cases of colchicine–cyclosporine DDI reportedb |
ORCA Class 2: Avoid if possiblec The risk would almost always outweigh the benefit Use alternative: If appropriate, tacrolimus appears less likely to interact with colchicine (see below); many other immunosuppressants are not known to inhibit CYP3A4 and P-gp Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity, especially for signs of myopathy Advise patient about colchicine toxicitye |
| Tacrolimus | Tacrolimus inhibits P-gp somewhat, but may be only weak inhibitor of CYP3A4; Two small studies found increased colchicine AUC with tacrolimus, but less than with cyclosporine [16, 75] |
ORCA Class 3. Assess risk; take action if needed Colchicine levels are likely to increase somewhat, especially in predisposed patients (e.g., on CYP3A4 inhibitors, or with severe renal or hepatic impairment)c Consider alternative to tacrolimus or colchicined |
Monitor for altered colchicine effect if tacrolimus started, stopped, or changed in dosage Advise patient about colchicine toxicitye |
| Calcium-channel blockers (CCBs) | |||
| Diltiazem | Diltiazem inhibits CYP3A4 and P-gp; in 20 healthy subjects, diltiazem 240 mg BID for 7 days ↑ colchicine AUC by 93%; one subject had a 339% increase [25, 45]; a patient on diltiazem developed fatal colchicine toxicity, but causality was not establishedb |
ORCA Class 2: Avoid if possiblec Use alternative. If appropriate, use an alternative to diltiazem or verapamil; most other CCBs such as dihydropyridines appear to have little effect on CYP3A4 or P-gp Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity, especially for signs of myopathy Advise patient about colchicine toxicitye |
| Verapamil | Verapamil inhibits CYP3A4 and P-gp; in 24 healthy subjects, verapamil 240 mg BID for 5 days ↑ colchicine AUC by 103%; one subject had a 217% increase [25, 45]; a patient on verapamil developed serious colchicine toxicityb |
ORCA Class 2: Avoid if possiblec Use alternative. If appropriate, use an alternative to verapamil or diltiazem; most other CCBs such as dihydropyridines appear to have little effect on CYP3A4 or P-gp Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity, especially for signs of myopathy Advise patient about colchicine toxicitye |
| Enzyme inducers | |||
| Carbamazepine (CBZ) | Carbamazepine induces CYP3A4 and P-gp, and would be expected to reduce colchicine AUC; case report of very low colchicine levels in patient on CBZb |
ORCA Class 2: Avoid if possible It may be difficult to achieve therapeutic colchicine levels in presence of CBZ Use alternative. If appropriate, use an alternative to the CBZ or colchicined |
Monitor for altered colchicine effect if CBZ is started, stopped, or changed in dosage; adjust colchicine dose as needed; note that the onset and offset of enzyme induction may occur slowly over 1–2 weeks |
| Rifampin | Rifampin induces CYP3A4 and P-gp, and would be expected to reduce colchicine AUC; case report of low colchicine levels in patient on rifampinb |
ORCA Class 2: Avoid if possible It may be difficult to achieve therapeutic colchicine levels in presence of rifampin Use alternative. If appropriate, use an alternative to rifampin or colchicined |
Monitor for altered colchicine effect if CBZ is started, stopped, or changed in dosage; adjust colchicine dose as needed; note that the onset and offset of enzyme induction may occur slowly over 1–2 weeks |
|
Barbiturates Bosentan Dabrafenib Lumacaftor Phenytoin Primidone Rifabutin Rifapentine St. John’s wort Topiramate |
These enzyme inducers are known to induce CYP3A4, and are likely to reduce colchicine serum concentrations |
ORCA Class 2: Avoid if possible It may be difficult to achieve therapeutic colchicine levels in presence of these enzyme inducers Use alternative. If appropriate, use an alternative to the enzyme inducer or colchicined |
Monitor for altered colchicine effect if inducer is started, stopped, or changed in dosage; adjust colchicine dose as needed; note that the onset and offset of enzyme induction may occur slowly over 1–2 weeks |
| Foods | |||
| Grapefruit | Grapefruit inhibits CYP3A4, but appears to be a weak P-gp inhibitor; a single case of life-threatening colchicine toxicity was reported in an 8-year-old girlb and in vitro evidence [76] suggest a DDI; but study in 21 healthy subjects given 240 mL grapefruit juice BID for 4 days found no effect on colchicine AUCl [24] |
ORCA Class 3. Assess risk; take action if needed Although evidence for an interaction is weak, it might occur in patients with low P-gp activityg It would be prudent to advise patents to drink juices other than grapefruit |
If the patient drinks grapefruit juice, advise them to avoid ingesting more than 240 mL/day |
| Seville oranges | A study in 23 healthy subjects given 240 mL Seville orange juice BID for 4 days found a small decrease in colchicine AUC [24] |
ORCA Class 4. Low risk Concurrent use need not be avoided, but a slight reduction in colchicine AUC may occur |
Normal monitoring for colchicine toxicity |
| HMG-CoA reductase inhibitors | |||
| Atorvastatin | A study in 24 healthy subjects found a mean 24% ↑ in colchicine AUC after atorvastatin 40 mg/day for 14 days [77]; due to weak P-gp inhibition by atorvastatin? 7 case reports of myopathy with combinationb; additive myotoxicity? [58, 59] |
ORCA Class 3. Assess risk; take action if needed Concurrent use need not be avoided, especially if the colchicine dose is 0.6 mg/day or less If colchicine dose is > 0.6 mg/day, myopathy risk may be increased in predisposed patients, (i.e. impaired renal function, elevated statin levels due to dose, DDIs, etc.)c,m Consider alternative: fluvastatin, pravastatin, and rosuvastatin may be less likely to interact |
Monitor for colchicine toxicity, especially for signs of myopathy Advise patient to report evidence of myopathy (muscle weakness, myalgia, dark urine) |
| Fluvastatin | Not expected to affect colchicine AUC; isolated cases of rhabdomyolysis reported with combination, but causality was not establishedb; additive myotoxicity? [58, 59] |
ORCA Class 4: Low risk Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are neededn |
Normal monitoring for colchicine toxicity, with emphasis on signs of myopathy (muscle weakness, myalgia, dark urine) |
| Lovastatin | Possible P-gp inhibitory effect of lovastatin? [78] Possible additive myotoxicity? [58, 59]. Isolated cases of myopathyb; lovastatin has similar pharmacokinetic properties as simvastatin (see below) |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, especially if the colchicine dose is 0.6 mg/day or less If colchicine dose is > 0.6 mg/day, myopathy risk may be increased with renal impairment or elevated statin levels due to dose, DDIs, etc.)c,m Consider alternative: fluvastatin, pravastatin, and rosuvastatin may be less likely to interact |
Monitor for colchicine toxicity, especially for signs of myopathy Advise patient to report evidence of myopathy (muscle weakness, myalgia, dark urine) |
| Pravastatin | Not expected to affect colchicine AUC; isolated cases of myopathy reported with combination, but causality was not establishedb; additive myotoxicity? [58, 59] |
ORCA Class 4: Low risk Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are neededn |
Normal monitoring for colchicine toxicity, with emphasis on signs of myopathy (muscle weakness, myalgia, dark urine) |
| Rosuvastatin | Not expected to affect colchicine AUC; isolated cases of myopathy reported, but causality was not establishedb; additive myotoxicity? [58, 59] Combination has been used to treat COVID [79] |
ORCA Class 4: Low risk Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are neededn |
Normal monitoring for colchicine toxicity, with emphasis on signs of myopathy (muscle weakness, myalgia, dark urine) |
| Simvastatin | Possible P-gp inhibitory effect of simvastatin? [80] Numerous case reports of myopathy reported with combination, usually in patients with preexisting renal impairmentb; possible additive myotoxicity [58, 59] |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, especially if the colchicine dose is 0.6 mg/day or less If colchicine dose is > 0.6 mg/day, myopathy risk may be increased with renal impairment or elevated statin levels due to dose, DDIs, etc.c,m Consider alternative: fluvastatin , pravastatin, and rosuvastatin may be less likely to interact |
Monitor for colchicine toxicity, especially for signs of myopathy Advise patient to report evidence of myopathy (muscle weakness, myalgia, dark urine) |
| Macrolide antibiotics | |||
| Azithromycin | A study in 21 healthy subjects found a mean 57% ↑ in colchicine AUC after azithromycin 500 mg on day 1, then 250 mg/day for 4 days; one subject had a 241% increase [25, 45]; azithromycin is a modest P-gp inhibitor |
ORCA Class 3. Assess risk; take action if needed Colchicine levels are likely to increase somewhat, especially in predisposed patients (e.g., on CYP3A4 inhibitors, or with severe renal or hepatic impairment)c Consider alternative to antibiotic or colchicined |
Monitor for altered colchicine effect if azithromycin is started, stopped or changed in dosage Advise patient about colchicine toxicitye |
| Clarithromycin | A study in 23 healthy subjects found a mean 282% ↑ in colchicine AUC after clarithromycin 500 mg/day for 7 days; one subject had a 852% ↑; clarithromycin inhibits both CYP3A4 and P-gp; fatalities reported in case series [19], among >2 dozen case reports,b and ADR reporting systems [18, 81] |
ORCA Class 1. Avoid combination The risk would almost always outweigh the benefit Consider stopping colchicine during clarithromycin therapy Or use alternative to clarithromycin; azithromycin has a smaller effect on colchicine (see above); many other antibiotics have little effect on CYP3A4 or P-gp |
It should not be necessary to use colchicine and clarithromycin together. If it is absolutely necessary: Reduce colchicine dose by 75% Monitor for colchicine toxicity Advise patient about colchicine toxicitye |
| Erythromycin | Erythromycin inhibits CYP3A4 but may inhibit P-gp less than clarithromycin; isolated DDI case reportsb |
ORCA Class 2: Avoid if possiblec Consider stopping colchicine during erythromycin therapy Or use alternative. Consider an alternative to erythromycin; azithromycin has a smaller effect on colchicine (see above); many other antibiotics have little effect on CYP3A4 or P-gp |
Reduce colchicine dose 50–75% Monitor for colchicine toxicity, especially for signs of myopathy Advise patient about colchicine toxicitye |
| Miscellaneous CYP3A4 inhibitors | |||
|
Aprepitant Ceritinib Conivaptan Crizotinib Fedratinib Idelalisib Imatinib Lapatinib Lomitapide Lefamulin Mifapristone Nefazodone Quinupristin Ribociclib Telithromycin |
All of these drugs inhibit CYP3A4 and many are also known to inhibit P-gp; no information is available on the effect of these drugs on colchicine AUC, but interactions should be considered possible until proven otherwise |
ORCA Class 2: Avoid if possiblec Use alternative. If appropriate, us an alternative to the CYP3A4 inhibitor that does not inhibit CYP3A4 Or consider alternative to colchicined |
Reduce colchicine dose 50–75% Monitor for altered colchicine response if CYP3A4 inhibitor started, stopped, or changed in dosage Advise patient about colchicine toxicityd |
| Quinolines | |||
| Chloroquine | Chloroquine alone can cause myopathy [82], so additive myotoxicity with colchicine is possible; no supporting clinical data |
ORCA Class 4. Low risk Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are needed |
Normal monitoring for colchicine toxicity, with emphasis on signs of myopathy |
| Hydroxychloroquine (HQ) | HQ alone can cause myopathy; additive myotoxicity possible and single case of myopathy reportedb; HQ might inhibit P-gp, but no PK studies with colchicine; HQ has been used with colchicine to treat various disorders [83–85] |
ORCA Class 4. Low risk Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are needed |
Normal monitoring for colchicine toxicity, with emphasis on signs of myopathy |
| Other drugs | |||
| Digoxin | Digoxin is purported to cause myopathy when combined with colchicine [25, 55] but supporting evidence is lacking; the fact that digoxin is a P-gp substrate is not proof that it inhibits P-gp |
ORCA Class 4. Low risk Concurrent use need not be avoided |
Normal monitoring for colchicine toxicity |
| Disulfiram | Evidence that disulfiram inhibits P-gp or CYP3A4 is weak [86, 87]; single case report of DDI with colchicine, but causal relationship doubtfulb |
ORCA Class 4. Low risk Concurrent use need not be avoided |
Normal monitoring for colchicine toxicity |
| Fibrates | Fenofibrate and gemfibrozil known to cause myopathy, with or without statins [88]; additive myotoxic effect with colchicine? Evidence that fenofibrate inhibits P-gp is weak [89]; one case of myopathy with gemfibrozil + colchicineb |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided; it is possible that the myopathy risk is increased in predisposed patients, but more data are needed. Risk may be increased if patient is also taking a statin |
Monitor for colchicine toxicity, especially for signs of myopathy Advise patient to report evidence of myopathy (muscle weakness, myalgia, dark urine) |
| Lesinurad | Lesinurad may be a modest CYP3A4 inducer [90]; PK study in healthy subjects found modest ↓ in colchicine AUC, but lesinurad dose was high (400–600 mg/day) [33] |
ORCA Class 3: Assess risk; take action if needed Concurrent use need not be avoided, but some patients may have reduced colchicine concentrations; more data are needed |
Monitor for altered colchicine effect if lesinurad is started, stopped, or changed in dosage. Adjust colchicine dose as needed |
| Nivolumab | Thrombocytopenia reported in a patient on nivolumab and colchicineb; nivolumab may inhibit CYP3A4 by upregulating cytokines [91] but an effect on colchicine is speculative |
ORCA Class 3: Assess risk; take action if needed This DDI is largely theoretical, and concurrent use need not be avoided, some patients might have increased colchicine AUC, but more data are needed to establish a causal effect |
Monitor for altered colchicine effect if nivolumab is started, stopped, or changed in dosage. Adjust colchicine dose as needed |
| Sunitinib | Colchicine toxicity reported in a patient on sunitinibb, but the colchicine toxicity was more likely due to the excessive dose of colchicine, and the effect of concurrent diltiazem (see above) |
ORCA Class 4: Low risk Concurrent use need not be avoided |
Normal monitoring for colchicine toxicity |
ADRs adverse drug reactions, AUC area under the concentration–time curve, BID twice daily, DDIs drug–drug interactions, P-gp P-glycoprotein, PK pharmacokinetics, ↑ indicates increase, ↓ indicates decrease
aORCA: OpeRational ClassificAtion of drug interactions [92]: Class 1 = Avoid combination; Class 2 = Avoid combination unless benefit outweighs risk; Class 3 = Assess risk and take action if needed; Class 4 = Low risk: no special precautions; Class 5 = No interaction
bCase report details, including evaluation of causality, are presented in Online Resource (see ESM)
cDrug interactions that result in elevated colchicine plasma concentrations tend to be more dangerous in patients with renal impairment. Consider patients with significant renal impairment (e.g., creatinine clearance [CrCl] < 60 mL/min) to be at somewhat higher risk, and patients with severe renal disease (e.g., CrCl < 30 mL/min) at much higher risk of colchicine toxicity from drug interactions. For all drug interactions listed as ‘Class 2’, consider them contraindicated in patients with severe renal disease. The effect of liver disease is more theoretical, but assume ORCA class 2 DDIs are contraindicated if patient has a Child-Pugh score of C
dIf colchicine is being used for acute gout, consider using alternative gout therapy. If colchicine is being used chronically for gout prophylaxis, consider the risk of colchicine toxicity vs the expected benefit of gout prevention. If colchicine is being used for familial Mediterranean fever, cardiovascular disease, dermatologic disorders, Covid-19, or other disorders, it may be more difficult to find alternatives for colchicine. For some disorders such as pericarditis, colchicine may be deemed necessary
ePatient should report colchicine toxicity such as severe diarrhea, protracted vomiting, muscle weakness, muscle pain, dark urine, fever or other signs of infection, unusual bleeding or bruising, severe fatigue
fFluconazole and colchicine are both eliminated renally, so patients with severe renal impairment will have increased serum concentrations of both drugs. And since the ability of fluconazole to inhibit CYP3A4 is related to serum concentrations, there will be increased CYP3A4 inhibition
gBased on available evidence, drugs that inhibit CYP3A4 but not P-gp have little effect on colchicine AUC. But the risk of a DDI with colchicine would theoretically be increased if CYP3A4 inhibitors are given to patients with low P-gp activity due to other drugs or genetics
hDarunavir inhibits CYP3A4, but may induce P-gp somewhat. Theoretically, this might mitigate any increase in colchicine serum concentrations
iLong-term ritonavir therapy can result in enzyme induction [93], so it is not clear if these results apply to patients on chronic ritonavir
jTipranavir is generally given with ritonavir, but the result (CYP3A4 inhibition with P-gp induction) does not seem likely to increase colchicine serum concentrations based on study of tipranavir/ritonavir with other CYP3A4/P-gp substrates [71]
kBoth colchicine and cyclosporine can cause myopathy, and all case reports of colchicine–cyclosporine DDI had evidence of myopathy (e.g., some combination of muscle pain, muscle weakness, dark urine)
lThe lack of effect of grapefruit juice in the healthy subject study is consistent with the theory that inhibition of both CYP3A4 and P-gp is necessary for a DDI with colchicine. It is possible that the huge amount of grapefruit juice ingested by the 8-year-old girl (1 L/day for 2 months) was enough to inhibit P-gp. It is also possible that the grapefruit juice used in the healthy subject study did not contain sufficient quantities of the CYP3A4-inhibiting phytochemicals to affect colchicine pharmacokinetics. Different types of grapefruit are known to contain differing amounts of CYP3A4-inhibiting compounds [94, 95]
mAtorvastatin, lovastatin, and simvastatin are all metabolized by CYP3A4, so patients on CYP3A4 inhibitors are likely to be at increased risk
nPitavastatin, pravastatin, and rosuvastatin are all substrates for OATP transporters, so patients on OATP inhibitors may be at increased risk
Risk Factors for Colchicine DDIs
The risk of adverse outcomes from colchicine DDIs can be influenced substantially by the dose and duration of colchicine therapy as well as kidney and liver function
Colchicine Dose and Duration
Epidemiological studies using low-dose colchicine have generally found little evidence of adverse consequences with colchicine and interacting medications [1–4]. This is consistent with the case reports described in the online Supplemental Table in which the majority of adverse DDIs reported involved colchicine doses >0.5 mg/day (see ESM). Nonetheless, the epidemiological studies were not designed to detect adverse DDIs and we cannot rule out that some at-risk patients had DDIs. At colchicine doses > 0.5 mg/day, as one would expect, the risk of colchicine adverse DDIs appears to increase as the colchicine dose is increased. The duration of colchicine use is also important. It is unusual to see significant colchicine toxicity until at least 3 or 4 days of concurrent use of colchicine and the interacting drug, although in rare cases it can occur after only a day or two of concurrent therapy [18]. The case reports in the online Supplemental Table also provide onset of adverse reaction information. A more typical onset of adverse effects from the colchicine DDI would be after 5 or 10 days of concurrent therapy, and in some cases (especially DDIs causing myopathy) it may take weeks or even months.
Renal Disease/Age
Renal impairment predisposes to colchicine toxicity [42]. In 21 patients with varying levels of kidney impairment, the three patients with low estimated glomerular filtration rate (eGFR) (mean eGFR = 25) had 51% higher colchicine AUC compared with six patients with normal GFR (mean eGFR = 110). In patients on hemodialysis three times a week with eGFR = 0, colchicine AUC was more than 5-fold higher than those with normal GFR [16]. Another study found a colchicine half-life to be four times higher in patients with renal insufficiency compared with those with normal kidney function [26]. A study of colchicine pharmacokinetics found total body clearance of colchicine about twice as high in younger subjects as in elderly patients with a mean creatinine clearance (CrCl) of 46 mL/min [22]. Elderly patients may also be at higher risk of colchicine toxicity due to polypharmacy. Renal impairment may also increase the risk of colchicine DDIs by increasing the serum concentration of the drug interacting with colchicine. For example, fluconazole displays dose-dependent inhibition of CYP3A4, and undergoes renal elimination, so patients with severe renal dysfunction would tend to have higher concentrations of fluconazole [43]. This would lead to increased CYP3A4 inhibition, as well as compromised renal elimination of colchicine, a combination that may have led to colchicine toxicity [44].
In many case reports of colchicine toxicity due to DDIs described in the online Supplemental Table (see ESM), renal impairment appeared to be a predisposing factor. It is not possible to precisely determine the degree of increased risk of colchicine DDIs at given levels of renal impairment, but a consensus statement on the use of colchicine used an eGFR of < 30 mL/min as the point where colchicine concentrations can start to increase substantially [10]. Based on the available data, we might estimate that the renal elimination of colchicine starts to be compromised somewhere around an eGFR of 60 mL/min and becomes a serious problem as one approaches an eGFR of 30 mL/min.
Liver Disease
As with many drugs, the effect of liver disease on colchicine elimination is not well characterized. There are many types of liver disease with different etiologies, and varying effects on drug metabolism and elimination. Moreover, there is no agreed upon measurement of liver function, unlike eGFR for kidney function, to quantify the likely reduction in colchicine elimination in patients with liver disease. One consensus statement on the use of colchicine in liver disease used a Child-Pugh score of C as the point where the half-life of colchicine starts to increase substantially [10], and, in the absence of definitive data, reduction in colchicine dose at this level of hepatic impairment appears to be a reasonable guideline. Nonetheless, patients with lesser degrees of hepatic malfunction may have some reduction in colchicine clearance, thus increasing the risk of colchicine DDIs to some degree.
Transporter Polymorphisms
Given that colchicine is a substrate for P-gp, it would be expected that those with polymorphisms resulting in low P-gp activity may be at increased risk for adverse colchicine DDIs. For example, drugs that primarily inhibit CYP3A4 and appear to have little effect on colchicine (e.g., voriconazole) might have a greater effect in patients with low P-gp activity due to genomics or other drugs. Little is currently known regarding the effect of transporters other than P-gp on colchicine pharmacokinetics, so it may be useful to study the effect of polymorphisms in MRP2 or OATPs on colchicine disposition.
Dose/Duration of Perpetrator Drug
The magnitude of perpetrator drug inhibition or induction on drug metabolizing enzymes and transporters can be affected by the dose of the drug and the duration of perpetrator drug therapy.
Management Options
Given the potentially life-threatening outcomes of colchicine drug interactions and the difficulty of treating severe colchicine toxicity once it occurs, it is imperative to avoid putting patients at risk. There are essentially three ways to reduce the risk of adverse outcomes from colchicine DDIs: (i) avoid using the interacting drug while the patient is on colchicine, (ii) avoid using colchicine during administration of the interacting drug, or (iii) give colchicine and the interacting drug, but reduce the colchicine dose. We now consider these three approaches.
Avoid Using the Interacting Drug
If the patient on colchicine has severe renal or hepatic impairment, it is particularly important to avoid the use of CYP3A4/P-gp inhibitors. But even when the patient does not have significant renal or hepatic disease, avoiding the interacting drug is likely to be the preferred option when it is feasible. For example, it is difficult to imagine a scenario in which it would be necessary to give clarithromycin to a patient on colchicine, a combination that has produced numerous fatalities [18]. If another antibiotic is suitable, it would almost always be preferable to use it in place of the clarithromycin (most antibiotics do not inhibit CYP3A4 and P-gp). A similar argument could be made for calcium channel blockers, where diltiazem and verapamil inhibit CYP3A4 and P-gp, but most of the other calcium-channel blockers do not. Other calcium-channel blockers may or may not be appropriate to use in place of diltiazem or verapamil in any given patient, but if they are, an interaction with colchicine could be easily avoided. Table 3 presents non-interacting alternatives for many of the drugs that can interact with colchicine.
Stopping Colchicine During Use of the Interacting Drug
If the interacting drug is clinically necessary, stopping colchicine during the use of the interacting drug may be appropriate, especially if the interacting drug is used for a limited time (such as an antibiotic). Whether this is a viable option would also depend on the indication for colchicine. If colchicine is being used for gout, short-term alternatives to colchicine may be available. If colchicine is used for familial Mediterranean fever or for cardiovascular disorders such as pericarditis or post-myocardial infarction, stopping colchicine may or may not be the best option.
Reduce Colchicine Dose When Precipitant Drug is Started
If concurrent use of colchicine with the interacting drug cannot be avoided—which should rarely be the case—one could consider prophylactic colchicine dosage reductions. Theoretically, reducing the colchicine dose when the precipitant drug is added would offset the pharmacokinetic interaction and maintain colchicine serum concentrations in the therapeutic range, as has been recommended in the labeling for some colchicine products [25] and in published articles [45, 46]. In practice, however, because the magnitude of these DDIs is so variable from one person to another, a ‘one size fits all’ a priori colchicine dosage reduction is likely to be excessive for some people (resulting in subtherapeutic colchicine concentrations) and insufficient for others (leading to colchicine toxicity). Accordingly, colchicine dosage reduction is best considered a last resort, and only when both colchicine and the interacting drug must be used together. Consider the interaction with ritonavir, where the mean increase in colchicine AUC following ritonavir was 296%, but the range was 54–924% as shown in Fig. 1 [25, 45]. Despite this marked variability, the colchicine labeling for Colcrys states that if colchicine and ritonavir are used together, the colchicine dose should be reduced by 50% [25]. (One possible source of ritonavir’s highly variable effect on colchicine is that ritonavir affects transporters in addition to P-gp, and affects CYP isozymes in addition to CYP3A4, but this hypothesis requires confirmation.)
Fig. 1.
The high variability of changes in colchicine AUC in 18–24 healthy subjects given a single dose of colchicine 0.6 mg with and without pretreatment with various drugs. The bars represent the subject with the smallest increase and the subject with the largest increase for each interacting drug. Adapted from data presented in references [25, 45, 47]
The marked variability seen in healthy subjects is likely to be even greater in patients, who may be taking various other medications, may have varying degrees of renal or hepatic impairment, and may have other disorders that affect colchicine pharmacokinetics. Moreover, the much larger numbers in the patient population will virtually guarantee that some patients will have pharmacogenomic differences that will predispose them to colchicine toxicity (e.g., low P-gp activity). Finally, the pharmacokinetic studies conducted in healthy subjects (shown in Fig. 1) involved single, small doses of colchicine, unlike what is usually the case for patients receiving colchicine therapeutically. In patients who develop serious colchicine toxicity from a DDI, multiorgan failure can occur with marked reduction in kidney function; this in turn can worsen the colchicine toxicity. None of these problems would occur with single-dose studies in healthy subjects. For all of these reasons, one cannot use the single-dose studies in healthy subjects to predict whether a small or marked increase in colchicine plasma concentrations will occur in any given patient.
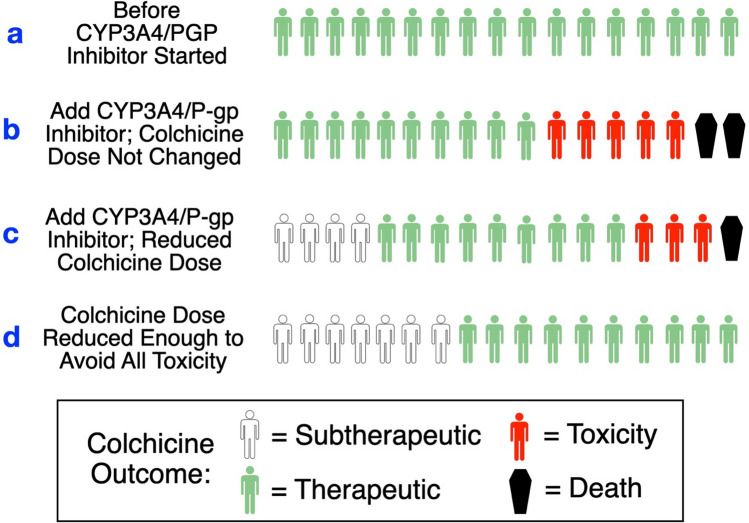
Figure 2 represents a hypothetical estimate of what might be expected to happen if we follow the rule of thumb recommendations for avoiding colchicine toxicity when patients receive CYP3A4/P-gp inhibitors with colchicine. These are hypothetical scenarios but they are entirely plausible given the evidence of high intersubject variability in the magnitude of colchicine DDIs as shown in Fig. 1. In Fig. 2, ‘a’ represents the starting conditions in a group of patients, and to simplify the discussion let us assume that they all have therapeutic colchicine serum concentrations. Next is ‘b’, which represents what may happen if a CYP3A4/PGP inhibitor is added, but the DDI is ignored; here some patients are likely to develop colchicine toxicity and some may die. Next is ‘c’, in which we adopt the colchicine dosage reduction recommendations, but—due to the large variability—some patients become subtherapeutic, and others develop colchicine toxicity. Finally, ‘d’ represents what may happen if we lowered the colchicine dose sufficiently to avoid all colchicine toxicity, resulting in an unacceptable number of patients with subtherapeutic colchicine levels.
Fig. 2.
Hypothetical predicted outcomes for various ways of managing the addition of CYP3A4/P-gp inhibitors to colchicine. a Before interacting drug added, b interacting drug added with no change in colchicine dose, c colchicine dosage reduction method, d reducing colchicine dosage enough to avoid all colchicine toxicity
Whether or not it is appropriate to prophylactically adjust the dose of an object drug when starting a precipitant drug depends on the drugs involved in the interaction. For some drugs, such as warfarin, if one adjusts the warfarin dose when adding a CYP2C9-inhibiting drug, at least one can monitor the INR to determine if the warfarin dosage adjustment was too large or too small. For colchicine, however, serum concentrations are not routinely available at this time, and life-threatening colchicine toxicity (e.g., pancytopenia, multi-organ failure, rhabdomyolysis) can begin after only a few days of administration of the interacting drug (see case reports in the online Supplemental Table, ESM). There is no specific remedy, and once the kidney and liver start to fail, colchicine may persist for weeks until the patient dies or (usually very slowly) recovers [48–50]. Dialysis is not effective in colchicine poisoning, but in one case the patient was successfully treated with kidney replacement therapy and plasmapheresis [51]. It is not known if giving an enzyme inducer such as rifampin would help eliminate colchicine, but it is an intriguing possibility given that rifampin probably increases colchicine elimination [31]. Rifampin has been used in poisonings with other drugs to increase elimination [52], but the efficacy and safety of using rifampin for colchicine toxicity is not established. The use of activated charcoal (and possibly other binding agents) may be worth trying, since colchicine undergoes enterohepatic circulation, although this has not been studied [11].
A summary of suggested management options for colchicine DDIs with CYP3A4/P-gp inhibtors is presented in Fig. 3.
Fig. 3.
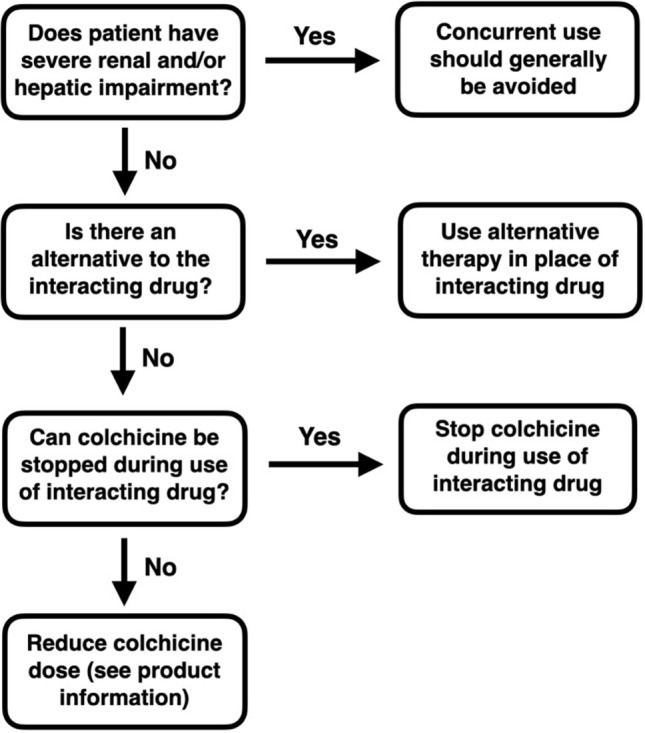
Management option algorithm for minimizing the risk of colchicine drug–drug interactions
Patient Education
If concomitant use of colchicine with interacting drugs is necessary, it is crucial to advise the patient to be alert for evidence of colchicine toxicity. There are three primary types of toxicity for which the patient should be vigilant:
Gastrointestinal toxicity: severe diarrhea (often with some combination of nausea, vomiting, or abdominal pain)
Neuromuscular toxicity: muscle weakness, myalgia, dark or brown urine
Hematologic toxicity (pancytopenia): fever, other signs of infection, excessive or unusual bleeding
Mild to moderate diarrhea is common when colchicine serum concentrations are elevated, but if the diarrhea is severe or prolonged, the patient should contact their prescriber. Regarding signs and symptoms of myopathy, some have proposed a ‘classic triad’ of myalgia, muscle weakness, and ‘tea-colored urine’ [53]. However, muscle weakness often occurs without myalgia, and sometimes the reverse is true (see cases in the online Supplemental Table, ESM). Darkened urine from myoglobinuria is usually a late sign of myotoxicity, and often occurs after the muscle weakness and/or myalgia has become so severe that the patient has already sought medical care. Moreover, using the term ‘tea-colored urine’ is problematic given that tea can have many different colors from dark brown to very light, so it would be better to say ‘dark’ or ‘brown’ urine. Darkened urine is a particularly ominous sign, because the excessive myoglobinuria may lead to acute kidney injury, thus prolonging the colchicine toxicity.
Colchicine toxicity can produce many other signs and symptoms, but they are either relatively nonspecific (fatigue, lethargy, malaise, insomnia), or they occur relatively infrequently (shortness of breath, cough, cardiac arrhythmias), or they tend to occur late in the course of colchicine toxicity (alopecia). Accordingly, it is probably best to concentrate the patient education on the more specific and common signs and symptoms as described above.
Drugs That May Interact with Colchicine
Colchicine DDIs have been extensively studied, and include pharmacokinetic studies, case reports, and case series. See Table 3 for a summary of these DDIs as well as management recommendations. Details of all case reports mentioned in Table 3, including supporting references, can be found in the online Supplemental Table (see ESM).
Misleading Colchicine Drug Interaction Information
Colchicine DDI information for health professionals is available from a variety of sources. The medical literature provides numerous pharmacokinetic studies, case reports, epidemiological studies, and reviews on colchicine DDIs, resulting in many conflicting recommendations. There are also numerous inconsistencies in the prescribing information for the various colchicine products, as well as in online DDI checking software and in clinical decision support systems [3, 10, 25, 30, 45, 46, 54–57]. The result is a jumble of confusion, with errors of omission, errors of commission, conflicting statements, and vague pronouncements as summarized in Table 4.
Table 4.
Misleading statements in published literature, product information, online sources [3, 10, 25, 30, 45, 46, 54–57]
| Type of problem | Examples |
|---|---|
| Recommending colchicine dosage adjustments instead of avoiding combinations | For the reasons detailed in the previous sections, if the drug interaction with colchicine is important enough to warrant a dose reduction, it is usually safer to avoid the combination entirely if possible |
| Errors of omission | Virtually all sources have incomplete lists of drugs likely to interact with colchicine (e.g., CYP3A4/P-gp inhibitors, enzyme inducers) |
| Errors of commission |
Many sources list drugs as interacting with colchicine that are unlikely to affect colchicine AUC or toxicity (for explanations, see Table 3). Errors include: Voriconazole as increasing colchicine AUC Tipranavir as increasing colchicine AUC Digoxin as a “potentially significant drug interaction,” a ‘serious’ DDI, and as contraindicated |
| Errors regarding effects of drugs on enzymes and transportersa |
Voriconazole as a P-gp inhibitor Clarithromycin as a ‘moderate’ 3A4 inhibitor Fluconazole as a strong P-gp inhibitor Cobicistat as a ‘mild’ CYP3A4 inhibitor Erythromycin as a ‘mild’ CYP3A4 inhibitor Amiodarone as a ‘mild’ P-gp inhibitor; no mention of 3A4 Diltiazem and verapamil as ‘mild’ P-gp inhibitors, with no mention of 3A4 Ritonavir as a ‘moderate’ inhibitor of CYP3A4 and P-gp Quinidine as a ‘mild’ P-gp inhibitor Cyclosporine as a ‘moderate’ CYP3A4 inhibitor, but no mention of P-gp |
| Excessive reliance on purported magnitude of inhibitory potency on CYP3A4 and P-gp (strong, moderate, mild) | The desire to have set guidelines is understandable, but these categories of inhibitors provide general recommendations. Clinical consequences may vary based on a host of other factors, only some of which have been characterized in clinical studies. There is a large overlap in the magnitude of effect of ‘moderate’ and ‘strong’ inhibitors in PK studiesb |
| Failure to consider dose of inhibitor | Fluconazole and grapefruit both inhibit CYP3A4, but the magnitude of inhibition is dose-related. Small doses usually have little effect, while large doses may have a strong inhibitory effect on CYP3A4c |
| Vague statements | Some sources list HIV medications (ritonavir) under moderate P-gp inhibitors. This is misleading because: (1) Some HIV medications are enzyme inducers and would be expected to reduce colchicine concentrations (e.g., tipranavir, efavirenz, etravirine, nevirapine), and (2) Many HIV medications other than ritonavir also inhibit CYP3A4/P-gp, but they are not listed as interacting with colchicine |
| Excessive reliance on data from epidemiological studies that were not designed to assess colchicine DDIs |
Epidemiological studies of colchicine for coronary artery disease Used low doses of colchicine (usually 0.5 mg/day) Patients were screened for risk factors (renal disease, drugs, etc.) Often relied on self-reporting of ADRs by patient Laboratory confirmation of DDIs may or may not have been done Epidemiologic studies for colchicine DDIs (e.g., Kwon statin study) Unlikely to detect rare DDIs due to inadequate power Statins were lumped together to obtain results |
ADRs adverse drug reactions, AUC area under the concentration–time curve, DDIs drug–drug interactions, P-gp P-glycoprotein, PK pharmacokinetics
aErrors partly result from relying on in vitro or animal studies without human in vivo data
bFor example, as seen in Fig. 1, the ‘moderate’ CYP3A4 inhibitor diltiazem produced a 339% increase in colchicine AUC in one healthy subject, while the ‘strong’ CYP3A4 inhibitor ritonavir produced a 54% increase in colchicine AUC in another subject
cOther factors may also contribute to the variability in fluconazole effect on colchicine, such as low P-gp activity (due to genetics or drugs) or serious renal impairment (increasing concentrations of both fluconazole and colchicine)
A striking example is the variability in recommendations for using concurrent colchicine and statins. Some have claimed that combining colchicine and statins does not cause adverse DDIs [46, 54] while others urge that the combinations be avoided [55]. A thorough assessment of the data by Wiggins et al. fell between these extremes and provided nuanced and useful guidelines [58], and a systematic review of the colchicine-statin DDI thoroughly assessed the data and concluded that the DDIs can be serious, and mitigation strategies are necessary to avoid patient harm [59]. While the data suggest that most people using concurrent statins and low-dose colchicine do not develop myopathy, reasonable precautions are warranted as discussed in Table 3.
In addition to improving the information on colchicine DDIs, it may be necessary to raise awareness in health care professionals on how serious these DDIs can be. In one study, over 90% of the alerts for colchicine with strong CYP3A4 inhibitors flagged in clinical decision support systems were overridden by prescribers [60]. It seems likely that more than 10% of these patients were at risk, and these results suggest a lack of awareness regarding the potentially life-threatening nature of colchicine DDIs.
Conclusion
Colchicine is a useful drug for a wide range of disorders and is usually safe when used in appropriate doses given the patient’s renal function. Numerous drugs are capable of causing colchicine toxicity, however, and given that colchicine toxicity is potentially fatal and difficult to treat, it is imperative that every effort be made to avoid placing patients at risk from these drug interactions. Currently available information on colchicine DDIs can be confusing and inconsistent, and in this paper we have tried to present recommendations based on the empirical evidence. Enough is known about colchicine drug interactions so that virtually every case of colchicine toxicity could have been prevented had the appropriate precautions been taken.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This project was supported by grant R01HS025984 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Conflict of interest
Philip Hansten and John Horn receive royalties from books published on drug–drug interactions. There was no support from any organization for the submitted work. None of the authors have a financial relationship with any organizations that might have an interest in the submitted work in the previous 3 years, and have no other relationships or activities that could appear to have influenced the submitted work.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
This paper arose out of the discussions of a research team working on an AHRQ grant. We are studying risk factors for drug interactions, and we discussed colchicine drug interactions frequently over many months at our bi-weekly meetings. PDH drafted the manuscript. All authors contributed to critical revision of the manuscript, and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Ethical approval
Not required.
References
- 1.Chen K, Schenone AL, Borges N, Militello M, Menon V. Teaching an old dog new tricks: colchicine in cardiovascular medicine. Am J Cardiovasc Drugs. 2017;17:347–360. doi: 10.1007/s40256-017-0226-3. [DOI] [PubMed] [Google Scholar]
- 2.Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. New Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 3.Imazio M, Nidorf M. Colchicine and the heart. Eur Heart J. 2021;42(28):2745–2760. doi: 10.1093/eurheartj/ehab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nidorf SM, Layland J, Robinson PC, Patel S, Psaltis PJ, Thompson PL. Emerging evidence for the use of colchicine for secondary prevention of coronary heart disease. Med J Austral. 2022;216(8):385–387. doi: 10.5694/mja2.51488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toro-Huamanchumo CJ, Benites-Meza JK, Mamini-García CS, Bustamante-Paytan D, Gracia-Ramos AE, Diaz-Vélez C, et al. Efficacy of colchicine in the treatment of COVID-19 Patients: a systemic review and meta-analysis. J Clin Med. 2022;11(9):2615. doi: 10.3390/jcm11092615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta KG, Patel T, Chavda PD, Patel P. Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomized controlled trials. RMD Open. 2021;7:e001746. doi: 10.1136/rmdopen-2021-001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledford H. The long wait for a long-COVID therapy. Nature. 2022;608:258–260. doi: 10.1038/d41586-022-02140-w. [DOI] [PubMed] [Google Scholar]
- 8.Tobler DL, Pruzansky AJ, Naderi S, Ambrosy AP, Slade JJ. Long-term cardiovascular effects of COVID-19: emerging data relevant to the cardiovascular clinician. Curr Atheroscl Rep. 2022;24:563–570. doi: 10.1007/s11883-022-01032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart S, et al. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22(1):28. doi: 10.1186/s13075-020-2120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson PC, Terkeltaub R, Pillinger MH, Shah B, Karalis V, Karatza E, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135:32–38. doi: 10.1016/j.amjmed.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein Y, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48:407–414. doi: 10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]
- 12.Garrouste C, Philipponnet C, Kaysi S, Enache I, Tiple A, Heng AE. Severe colchicine intoxication in a renal transplant recipient on cyclosporine. Transplant Proc. 2012;44:2851–2852. doi: 10.1016/j.transproceed.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Tröger U, Lins H, Scherrmann J-M, Wallesch C-W, Bode-Böger SM. Tetraparesis associated with colchicine is probably due to inhibition by verapamil of the P-glycoprotein efflux pump in the blood-brain barrier. BMJ. 2005;331:613. doi: 10.1136/bmj.38568.639688.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraco Y, Putterman C, Rahamimov R, Ben-Chetrit E. Acute colchicine intoxication—possible role of erythromycin administration. J Rheumatol. 1992;19:494–496. [PubMed] [Google Scholar]
- 15.Çelebi ZK, Akturk S, Oktay EI, Duman N, Keven K. Colchicine-induced rhabdomyolysis following concomitant use of clarithromycin in a hemodialysis patient with familial Mediterranean fever. Clin Kidney J. 2013;6:665–666. doi: 10.1093/ckj/sft129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amanova A, Celebi ZK, Bakar F, Caglayan MG, Keven K. Colchicine levels in chronic kidney diseases and kidney transplant recipients using tacrolimus. Clin Transplant. 2014;28:1177–1183. doi: 10.1111/ctr.12448. [DOI] [PubMed] [Google Scholar]
- 17.Dogukan A, Oymak FS, Taskapan H, Güven M, Tokgoz B, Utas C. Acute fatal colchicine intoxication in a patient on continuous ambulatory peritoneal dialysis (CAPD). Possible role of clarithromycin administration. Clin Nephrol. 2001;55:181–182. [PubMed] [Google Scholar]
- 18.Villa Zapata L, Hansten PD, Horn JR, Boyce RD, Gephart S, Subbian V, et al. Evidence of clinically meaningful drug–drug interaction with concomitant use of colchicine and clarithromycin. Drug Saf. 2020;43:661–668. doi: 10.1007/s40264-020-00930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung IFN, Wu AKL, Cheng VCC, Tang BSF, To KW, Yeung CK, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective study. Clin Infect Dis. 2005;41:291–300. doi: 10.1086/431592. [DOI] [PubMed] [Google Scholar]
- 20.Tateishi T, Soucek P, Caraco Y, Guengerich FP, Wood AJ. Colchicine biotransformation by human liver microsomes. Identification of CYP3A4 as the major isoform responsible for colchicine demethylation. Biochem Pharmacol. 1997;53:111–116. doi: 10.1016/s0006-2952(96)00693-4. [DOI] [PubMed] [Google Scholar]
- 21.Niel E, Scherrmann J-M. Colchicine today. Jt Bone Spine. 2006;73:672–678. doi: 10.1016/j.jbspin.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Rochdi M, Sabouraud A, Girre C, Venet R, Scherrmann JM. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. Eur J Clin Pharmacol. 1994;46:351–354. doi: 10.1007/BF00194404. [DOI] [PubMed] [Google Scholar]
- 23.Dahan A, Sabit H, Amidon GL. Multiple efflux pumps are involved in the transepithelial transport of colchicine: combined effect of P-glycoprotein and multidrug resistance-associated protein 2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metab Disposit. 2009;37:2028–2036. doi: 10.1124/dmd.109.028282. [DOI] [PubMed] [Google Scholar]
- 24.Wason S, DiGiacinto JL, Davis MW. Effects of grapefruit and Seville oranges on the pharmacokinetic properties of colchicine in healthy subjects. Clin Ther. 2012;34:2161–2173. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 25.Colcrys (colchicine) tablet [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America, 2020.
- 26.Ben Chetrit E, Scherrmann J-M, Zylber-Katz E, Levy M. Colchicine disposition in patients with familial Mediterranean fever with renal impairment. J Rheumatol. 1994;21:710–713. [PubMed] [Google Scholar]
- 27.Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–58. 10.1086/598327 [DOI] [PubMed]
- 28.Fihlman M, Hemmilä T, Hagelberg NM, Backman JT, Laitila J, Laine K, et al. Voriconazole greatly increases the exposure to oral buprenorphine. Eur J Clin Pharmacol. 2018;74:1615–1622. doi: 10.1007/s00228-018-2548-8. [DOI] [PubMed] [Google Scholar]
- 29.Purkins L, Wood N, Kleinermans D, Nichols D. Voriconazole does not affect the steady-state pharmacokinetics of digoxin. Br J Clin Pharmacol. 2003;56(Suppl 1):45–50. doi: 10.1046/j.1365-2125.2003.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CEDR: Center for Drug Evaluation and Research, Application No. 204820Orig1s000, November 11, 2013, Hikma Pharmaceuticals.
- 31.Koo HS, Kim S, Chin HJ. A case of high dose colchicine with no efficacy in a patient with chronic kidney disease taking rifampicin. J Rheum Dis. 2014;21(6):2014. doi: 10.4078/jrd.2014.21.6.314. [DOI] [Google Scholar]
- 32.Kazmi H, Cauchi M. Sub-therapeutic colchicine levels in a patient treated for pericarditis in the setting of carbamazepine use: a case report. J Clin Pharm Ther. 2021;00:1–3. doi: 10.1111/jcpt.13549. [DOI] [PubMed] [Google Scholar]
- 33.Shen Z, Tieu K, Wilson D, Bucci G, Gillen M, Lee C, et al. Evaluation of pharmacokinetic interactions between lesinurad, a new selective urate reabsorption inhibitor, and commonly used drugs for gout treatment. Clin Pharmacol Drug Dev. 2017;6(4):377–387. doi: 10.1002/cpdd.323. [DOI] [PubMed] [Google Scholar]
- 34.Pirzada NA, Medell M, Ali II. Colchicine induced neuromyopathy in a patient with normal renal function. J Clin Rheumatol. 2001;7:374–376. doi: 10.1097/00124743-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Chattopadhyay I, Shetty HGM, Routledge PA, Jeffery J. Colchicine induced rhabdomyolysis. Postgrad Med J. 2001;77:191–192. doi: 10.1136/pmj.77.905.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caglar K, Odabasi Z, Safali M, Yenicesu M, Vural A. Colchicine-induced myopathy with myotonia in a patient with chronic renal failure. Clin Neurol Neurosurg. 2003;105:274–276. doi: 10.1016/s0303-8467(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 37.Sayarlioglu M, Sayarlioglu H, Ozen S, Erkoc R, Gul A. Colchicine-induced myopathy in a teenager with familial Mediterranean fever. Ann Pharmacother. 2003;37:1821–1824. doi: 10.1345/aph.1D188. [DOI] [PubMed] [Google Scholar]
- 38.Wilbur K, Makowsky M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy. 2004;24:1784–1792. doi: 10.1592/phco.24.17.1784.52334. [DOI] [PubMed] [Google Scholar]
- 39.Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84:377–385. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 40.Altman A, Szyper-Kravitz M, Shoenfeld Y. Colchicine-induced rhabdomyolysis. Clin Rhematol. 2007;26:2197–2199. doi: 10.1007/s10067-007-0682-2. [DOI] [PubMed] [Google Scholar]
- 41.Gilibili RR, Chatterjee S, Bagul P, Mosure KW, Murali BV, Mariappan TT, et al. Coproporphyrin-I: a fluorescent, endogenous optimal probe substrate for ABCC2 (MRP2) suitable for vesicle-based MRPT inhibition assay. Drug Metab Dospos. 2017;45:604–611. doi: 10.1124/dmd.116.074740. [DOI] [PubMed] [Google Scholar]
- 42.Kuncl RW, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. N Engl J Med. 1987;316:1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 43.Berl T, Wilner KD, Gardner M, Hansen RA, Farmer B, Baris BA, et al. Pharmacokinetics of fluconazole in renal failure. J Am Soc Nephrol. 1995;6:242–247. doi: 10.1681/ASN.V62242. [DOI] [PubMed] [Google Scholar]
- 44.Su Y-C, Wu C-C. Colchicine-induced acute neuromyopathy in a patient using concomitant fluconazole: case report and literature review. Drug Saf Case Rep. 2015;2:16. doi: 10.1007/s40800-015-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63:2226–2237. doi: 10.1002/art.30389. [DOI] [PubMed] [Google Scholar]
- 46.Vrachatis DA, Papathanasiou KA, Giotaki SG, Iliodromitis KE, Papaionnaou TG, et al. Repurposing colchicine’s journey in view of drug-to-drug interactions. A review. Toxicol Rep. 2021;8:1389–1393. doi: 10.1016/j.toxrep.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wason S, DiGiacinto JL, Davis MW. Effect of cyclosporine on the pharmacokinetics of colchicine in healthy subjects. Postgrad Med. 2012;124(4):189–196. doi: 10.3810/pgm.2012.07.2579. [DOI] [PubMed] [Google Scholar]
- 48.Eleftheriou G, Bacis G, Fiocchi R, Sebastiano R. Colchicine-induced toxicity in a heart transplant patient with chronic renal failure. Clin Toxicol. 2008;46:827–830. doi: 10.1080/15563650701779703. [DOI] [PubMed] [Google Scholar]
- 49.Bouquié R, Deslandes G, Renaud C, Dailly E, Haloun A, Jolliet P. Colchicine-induced rhabdomyolysis in a heart/lung transplant patient with concurrent use of cyclosporine, pravastatin, and azithromycin. J Clin Rheumatol. 2011;17:28–30. doi: 10.1097/RHU.0b013e3182056042. [DOI] [PubMed] [Google Scholar]
- 50.Mullins M, Cannarozzi AA, Bailey TC, Ranganathan P. Unrecognized fatalities related to colchicine in hospitalized patients. Clin Toxicol. 2011;49:648–652. doi: 10.3109/15563650.2011.589844. [DOI] [PubMed] [Google Scholar]
- 51.Schaffer DH, Overbeek DL, Erckson TB, Boyer EW, Goldfine C, Muhsin SA, et al. Severe colchicine poisoning treated successfully with kidney replacement therapy and plasmapheresis: a case report. Toxicol Commun. 2022;6(1):47–51. doi: 10.1080/24734306.2022.2055817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unal S, Bayrakci B, Yasar U, Karagoz T. Successful treatment of propafenone, digoxin, and warfarin overdoseage with plasma exchange therapy and rifampicin. Clin Drug Investig. 2007;27(7):505–508. doi: 10.2165/00044011-200727070-00008. [DOI] [PubMed] [Google Scholar]
- 53.Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58–69. [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon OC, Hong S, Ghang B, Kim YG, Lee CK, Yoo B. Risk of colchicine-associated myopathy in gout: influence of concomitant use of statin. Am J Med. 2017;130:583–587. doi: 10.1016/j.amjmed.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Şen S, Karahan E, Büyükulaş C, Polat YO, Üresin AY. Colchicine for cardiovascular therapy: A drug interaction perspective and a safety meta-analysis. Anatol J Cardiol. 2021;25:753–761. doi: 10.5152/AnatolJCardiol.2021.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitigare (colchicine) capsule [prescribing information]. Memphis, TN: Hikma Americas, Inc.; June 2020.
- 57.Medscape Drug Interaction Checker, August, 2022.
- 58.Wiggins BS, Saseen JJ, Page RL, II, Reed BN, Sneed K, Kostis JB, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease. A scientific statement from the American Heart Association. Circulation. 2016;134:e468–e495. doi: 10.1161/CIR.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 59.Schwier NC, Cornelio CK, Boylan PM. A systematic review of the drug interaction between statins and colchicine: patient characteristics, etiologies, and clinical management strategies. Pharmacotherapy. 2022;42:320–333. doi: 10.1002/phar.2674. [DOI] [PubMed] [Google Scholar]
- 60.Van de Sijpe G, Quintens C, Walgraeve K, van Laer E, Penny J, de Vlieger G, et al. Overall performance of a drug-drug interaction clinical decision support system: quantitative evaluation and end-user survery. BMC Med Inform Decis Mak. 2022;22(1):48. doi: 10.1186/s12911-022-01783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald MG, Au NT, Rettie AE. P450-based drug–drug interactions of amiodarone and its metabolites: diversity of inhibitory mechanisms. Drug Metab Dispos. 2015;43:1661–1669. doi: 10.1124/dmd.115.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan MH, Rochlani Y, Aronow WS. Efficacy and safety of dronedarone in the treatment of patients with atrial fibrillation. Expert Opin Drug Saf. 2017;16:1407–1412. doi: 10.1080/14740338.2017.1387246. [DOI] [PubMed] [Google Scholar]
- 63.Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61:2495–2502. doi: 10.1016/j.jacc.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 64.Bhardwaj R, Collins JL, Stingfellow J, Madonia J, Anderson MS, Finley J, et al. P-glycoprotein and breast cancer resistance protein transporter inhibition by cyclosporine and quinidine on the pharmacokinetics of oral rimegepant in healthy subjects. Clin Pharmacol Drug Dev. 2022;11:889–897. doi: 10.1002/cpdd.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jerling M, Huan B-L, Leung K, Chu N, Abdallah H, Hussein Z. Studies to investigate the pharmacokinetic interactions between ranolazine and ketoconazole, diltiazem, or simvastatin during combined administration in healthy subjects. J Clin Pharmacol. 2005;45:422–433. doi: 10.1177/0091270004273992. [DOI] [PubMed] [Google Scholar]
- 66.Hylton AC, Ezekiel TO. Rhabdomyolysis in a patient receiving ranolazine and simvastatin. Am J Health Syst Pharm. 2010;67:1829–1831. doi: 10.2146/ajhp090299. [DOI] [PubMed] [Google Scholar]
- 67.Gumbrevičius G, Damulevičienė G, Galaunė V, Gumbrevičiūtė M. Possible interaction between dabigatran and ranolazine in patients with renal failure. Medicina (Kaunas) 2019;56:13. doi: 10.3390/medicina56010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brings A, Lehmann M-L, Foerster KI, Burhenne J, Weiss J, Haefeli WE, et al. Perpetrator effect of ciclosporin (P-glycoprotein inhibitor) and its combination with fluconazole (CYP3A4 inhibitor) on the pharmacokinetics of rivaroxaban in healthy volunteers. Br J Clin Pharmacol. 2019;85:1528–1537. doi: 10.1111/bcp.13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niwa T, Imagawa Y, Yamazaki H. Drug interactions between nine antifungal agents and drugs metabolized by human cytochromes P450. Curr Drug Metab. 2014;15:651–679. doi: 10.2174/1389200215666141125121511. [DOI] [PubMed] [Google Scholar]
- 70.Nivoix Y, Levêque D, Herbrecht R, Koffel J-C, Beretz L, Ubeaud-Sequier G. The enzymatic basis of drug- drug interactions with systemic triazole antifungals. Clin Pharmacokinet. 2008;47:779–792. doi: 10.2165/0003088-200847120-00003. [DOI] [PubMed] [Google Scholar]
- 71.Mukwaya G, et al. Interaction of ritonavir-boosted tipranavir with loperamide does not result in loperamide-associated neurologic side effects in healthy volunteers. Antimicrob Agents Chemother. 2005;49:4903–4910. doi: 10.1128/AAC.49.12.4903-4910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mora-Peris B, Elso L, Goldmeier D, Mears A, Weston R, Cooke G, et al. A phase I study to assess the safety, tolerability and pharmacokinetic profile of boceprevir and sildenafil when dosed separately and together, in healthy male volunteers. J Antimicrob Chemother. 2015;70:1812–1815. doi: 10.1093/jac/dkv035. [DOI] [PubMed] [Google Scholar]
- 73.Marciano KA, Seago K, Dillaman M, Ross KG, Veltri L, Cumpston A. Evaluation of the pharmacokinetic interaction between letermovir and tacrolimus in allogeneic hematopoietic cell transplant recipients. Transplant Cell Ther. 2022;S2666–6367(22):01194. doi: 10.1016/j.jtct.2022.03.028. [DOI] [PubMed] [Google Scholar]
- 74.Marshall WL, McCrea JB, Macha S, Menzel K, Liu F, van Schanke A, et al. Pharmacokinetics and tolerability of letermovir coadministered with azole antifungals (posaconazole or voriconazole) in healthy subjects. J Clin Pharmacol. 2018;58(7):897–904. doi: 10.1002/jcph.1094. [DOI] [PubMed] [Google Scholar]
- 75.Beaudreuil S, Schermann JM, Verstuyft C, Barrail-Tran A, Smirnova M, Durrbach A, et al. Differential pharmacokinetic interaction of cyclosporine and tacrolimus with colchicine in renal allograft recipients. Clin Transplant. 2018;32:e13405. doi: 10.1111/ctr.13405. [DOI] [PubMed] [Google Scholar]
- 76.Dahan A, Amidon GL. Grapefruit juice and its constituents augment colchicine intestinal absorption: potential hazardous interaction and the role of P-glycoprotein. Pharm Res. 2009;26:883–892. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 77.Davis MW, Wason S. Effect of steady-state atorvastatin on the pharmacokinetics of a single dose of colchicine in healthy adults under fasted conditions. Clin Drug Investig. 2014;34:259–267. doi: 10.1007/s40261-013-0168-8. [DOI] [PubMed] [Google Scholar]
- 78.Choi D-H, Chung J-H, Choi J-S. Pharmacokinetic interaction between oral lovastatin and verapamil in healthy subjects: role of P-glycoprotein inhibition by lovastatin. Eur J Clin Pharmacol. 2010;66:285–290. doi: 10.1007/s00228-009-0757-x. [DOI] [PubMed] [Google Scholar]
- 79.Shah T, McCarthy M, Nasir I, Archer H, Ragheb E, Kluger J, et al. Design and rationale of the colchicine/statin for the prevention of COVID-19 complications (C OLSTAT) trial. Contemp Clin Trials. 2021;110:106547. doi: 10.1016/j.cct.2021.106547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holtzman CW, Wiggins BS. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26:1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 81.Topliss D, Gold M, Kotsirilos V, Lander C, McNeil J, Pillans P, et al. Fatal interactions and reactions with colchicine: beware CYP3A4 inhibitors. Aust Adverse Drug React Bull. 2008;27(5):18. [Google Scholar]
- 82.Posada C, García-Cruz A, García-Doval I, San Millán B, Teijeira S. Chloroquine-induced myopathy. Lupus. 2011;20:773–774. doi: 10.1177/0961203310385553. [DOI] [PubMed] [Google Scholar]
- 83.Aisen PS, Marin DB, Brickman AM, Santoro J, Fusco M. Pilot tolerability studies of hydroxychloroquine and colchicine in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15:96–101. doi: 10.1097/00002093-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Basaran O, Aydin F, Celikel BA, Uncu N, Cakar N. Coexistence of systemic lupus erythematosus and familial Mediterranean fever in a pediatric patient. Lupus. 2016;25:1062–1063. doi: 10.1177/0961203315627872. [DOI] [PubMed] [Google Scholar]
- 85.Dabbagh MF, Aurora L, D’Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2:1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rolle F, Bincoletto V, Gazzano E, et al. Coencapsulation of disulfiram and doxorubicin in liposomes strongly reverses multidrug resistance in breast cancer cells. Int J Pharm. 2020;580:119191. doi: 10.1016/j.ijpharm.2020.119191. [DOI] [PubMed] [Google Scholar]
- 87.Damkier P, Hansen LL, Brøsen K. Effect of diclofenac, disulfiram, itraconazole, grapefruit juice and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol. 1999;48:829–838. doi: 10.1046/j.1365-2125.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J, Song Y, Li H, Chen J. Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report. Eur J Clin Pharmacol. 2009;65:1169–1174. doi: 10.1007/s00228-009-0723-7. [DOI] [PubMed] [Google Scholar]
- 89.Poruba M, Matuskova Z, Hüttl M, et al. Fenofibrate decreases hepatic P-glycoprotein in a rat model of hereditary hypertriglyceridemia. Front Parmacol. 2019;7(10):56. doi: 10.3389/fphar.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillen M, et al. Evaluation of pharmacokinetic interactions between lesinurad, a new selective urate reabsorption inhibitor, and CYP enzyme substrates sildenafil, amlodipine, tolbutamide, and repaglinide. Clin Pharmacol Drug Dev. 2017;6:363–376. doi: 10.1002/cpdd.324. [DOI] [PubMed] [Google Scholar]
- 91.Harvey RD, Morgan ET. Cancer, inflammation, and therapy: effects on cytochrome P450-mediated drug metabolism and implications for novel immunotherapeutic agents. Clin Pharmacol Ther. 2014;96:449–457. doi: 10.1038/clpt.2014.143. [DOI] [PubMed] [Google Scholar]
- 92.Hansten PD, Horn JR, Hazlet TK. ORCA: OpeRational ClassificAtion of drug interactions. J Am Pharm Assoc. 2001;41(2):161–165. doi: 10.1016/s1086-5802(16)31244-x. [DOI] [PubMed] [Google Scholar]
- 93.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–1059. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 94.Uesawa Y, Mohri K. Drug interaction potentials among different brands of grapefruit juice. Pharmazie. 2008;63:1440146. [PubMed] [Google Scholar]
- 95.Guttman Y, Yedida I, Nudel A, Zhmykhova Y, Kerem Z, Carmi N. New grapefruit cultivars exhibit low cytochrome P4503A4-inhibition activity. Food Chem Toxicol. 2020;137:111135. doi: 10.1016/j.fct.2020.111135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.