Abstract
Recombinant live oral vaccines expressing pathogen-derived antigens offer a unique set of attractive properties. Among these are the simplicity of administration, the capacity to induce mucosal and systemic immunity, and the advantage of permitting genetic manipulation for optimal antigen presentation. In this study, the benefit of having a heterologous antigen expressed on the surface of a live vector rather than intracellularly was evaluated. Accordingly, the immune response of mice immunized with a Salmonella enterica serovar Typhimurium vaccine strain expressing the Escherichia coli 987P fimbrial antigen on its surface (Fas+) was compared with the expression in the periplasmic compartment (Fas−). Orally immunized BALB/c mice showed that 987P fimbriated Salmonella serovar Typhimurium CS3263 (aroA asd) with pCS151 (fas+ asd+) elicited a significantly higher level of 987P-specific systemic immunoglobulin G (IgG) and mucosal IgA than serovar Typhimurium CS3263 with pCS152 (fasD mutant, asd+) expressing 987P periplasmic antigen. Further studies were aimed at determining whether the 987P fimbriae expressed by serovar Typhimurium χ4550 (cya crp asd) could be used as carriers of foreign epitopes. For this, the vaccine strain was genetically engineered to express chimeric fimbriae carrying the transmissible gastroenteritis virus (TGEV) C (379-388) and A (521-531) epitopes of the spike protein inserted into the 987P major fimbrial subunit FasA. BALB/c mice administered orally serovar Typhimurium χ4550 expressing the chimeric fimbriae from the tet promoter in pCS154 (fas+ asd+) produced systemic antibodies against both fimbria and the TGEV C epitope but not against the TGEV A epitope. To improve the immunogenicity of the chimeric fimbriae, the in vivo inducible nirB promoter was inserted into pCS154, upstream of the fas genes, to create pCS155. In comparison with the previously used vaccine, BALB/c mice immunized orally with serovar Typhimurium χ4550/pCS155 demonstrated significantly higher levels of serum IgG and mucosal IgA against 987P fimbria. Moreover, mucosal IgA against the TGEV C epitope was only detected with serovar Typhimurium χ4550/pCS155. The induced antibodies also recognized the epitopes in the context of the full-length TGEV spike protein. Hence, immune responses to heterologous chimeric fimbriae on Salmonella vaccine vectors can be optimized by using promoters known to be activated in vivo.
Infectious diarrhea remains a major cause of mortality and morbidity in neonatal and recently weaned piglets (U.S. Department of Agriculture National Swine Survey: Morbidity/Mortality and Health Management of Swine in the United States [1992] and Swine '95 Study, Part III: 1990–1995 Changes in the U.S. Pork Industry [1997]; NAHMS_INFO@aphis.usda.gov). Transmissible gastroenteritis virus (TGEV) and enterotoxigenic Escherichia coli (ETEC) are among the leading causative agents of diarrhea in piglets (48, 58). TGEV is a coronavirus and has three major structural proteins (S, N, and M) (40). The spike (S or E2) protein, located on the surface of the virus, elicits antibodies that can neutralize virus and protect animals against infection (37, 80, 83). Four sites (A, B, C, and D) have been defined by analysis with monoclonal antibodies and sites C (positions 379 to 388) and A (positions 521 to 531) have been identified as targets for neutralization of TGEV (18, 19, 28). Both C and A are continuous epitopes and are glycosylation independent (28, 68). This feature makes them suitable for being displayed by carrier proteins as antigenic epitopes to induce anti-TGEV immunity.
Enteroadhesive fimbriae play a critical role in the pathogenesis of ETEC. The binding of fimbriae to intestinal receptors ensures optimal mucosal colonization by the bacteria and efficient enterotoxin delivery to the enterocytes. Fimbriae can serve as an effective vaccine to induce an immune response against ETEC infections. For example, piglets of dams injected with purified 987P fimbriae were protected against experimental infection with 987P-fimbriated ETEC, and this protection was correlated with the presence of specific anti-987P antibodies in the colostrum (34, 49, 51). Veterinary vaccines based on fimbrial proteins have been used successfully for many years (48), and fimbriae are considered major antigens of currently tested vaccines to protect humans from ETEC (2, 41, 62). Passive immunization of animals with anti-fimbria antibodies protects animals by blocking fimbria-mediated enteroadhesion of ETEC (33, 43). Passive immunity is also of primary importance in providing newborn piglets with immediate protection against TGEV (59, 60).
In order to take advantage of the excellent immunogenicity of fimbriae, several investigators have modified fimbriae genetically to create chimeric organelles displaying foreign epitopes (54). Recently, the CS31 and the 987P fimbriae of E. coli were engineered to present TGEV epitopes (20, 46, 56). Both purified chimeric fimbriae were shown to induce anti-TGEV and anti-fimbria specific antibodies in mice and rabbits. Protection against neonatal infectious agents such as TGEV or 987P-ETEC is currently best obtained by passive immunization of piglets after induction of colostral antibodies in the sow (48, 59, 60). Colostral antibodies can be induced by the oral delivery of protective antigens activating the gut-associated lymphoid tissues (GALT) of sows (9, 60). One method of delivering antigens to the GALT is by the use of vectors possessing tropism for Peyer's patches, such as Salmonella enterica. In the last two decades, various Salmonella vectors have been tested for their capacity to deliver antigens and induce the GALT to mount protective immune responses (14, 17). More-recent studies have been aimed at optimizing antigen expression by using promoters like the nirB or htrA promoters of S. enterica which are activated by specific environmental conditions found in the host (11, 12, 27, 57). Typically, the delivered antigens were expressed intracellularly by attenuated S. enterica serovar Typhimurium mutants (15, 32). Recently, oral administration of attenuated serovar Typhimurium expressing either human or farm animal ETEC fimbriae was shown to elicit specific immunoglobulin A (IgA) and IgG responses and even to induce protective immunity in a model using mice to study diarrhea (3, 85). Although Salmonella fimbrial proteins have also been engineered to present foreign epitopes (81, 84), the possibility that this alteration attenuated the vaccine strains to the point where they were no longer able to elicit a protective mucosal immune response was not investigated.
In this study, we found that various live attenuated strains of serovar Typhimurium can be made to express chimeric 987P fimbriae. Moreover, these fimbriated strains were shown to elicit both systemic and mucosal immune responses against both the 987P fimbriae and the foreign epitopes, namely, TGEV epitopes. Most interestingly, the best immune responses against the TGEV epitopes were obtained with a construct utilizing the nirB promoter for fimbrial expression, suggesting that inducible promoters can be used in vivo to optimize expression of chimeric fimbriae.
MATERIALS AND METHODS
Mice.
Five-week-old female BALB/cByJ mice were obtained from Jackson Laboratory and housed in filter-top cages in an air-conditioned animal facility. Water and food was provided ad libitum. Mice were adapted for one week after arrival before being used for immunization.
Bacterial strains, media, and reagents.
The E. coli and serovar Typhimurium strains used in this study are listed in Table 1. Serovar Typhimurium CS3263 strain is a ΔasdA1 derivative of SL3261 (serovar Typhimurium WRAY hisG aroA) and was constructed by generalized transduction, using phage P22HTint and χ4487 as the donor strain. E. coli 987 was grown in minimal medium E supplemented with pantothenic acid and glycerol, as described previously (26). Strains SE5000 and SL3261 were grown in L medium, whereas strains χ6212, χ4487, χ4550, and CS3263 were grown in L medium with 50 μg of diaminopimelic acid (Sigma, St. Louis, Mo.) per ml. When necessary, media were supplemented with the following antibiotics: ampicillin (200 μg/ml), tetracycline (10 μg/ml), or kanamycin (45 μg/ml). Medium components were purchased from Difco (Detroit, Mich.). Restriction and modification enzymes were from New England Biolabs, Inc. (Beverly, Mass.). Unless specified, reagents were purchased from Sigma.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| 987 | O9:K103:987P (prototype F6) | 35, 50 |
| SE5000 | MC4100 recA56 (Fim−) | 67 |
| χ6212 | ΔasdA1 derivative of DH5α | 52 |
| BL21 | lon ompT | Novagen, Madison, Wis. |
| SG396 | htpR lonR9 | 29 |
| Serovar Typhimurium | ||
| χ4487 | Δ(crp-cysG)-10 Δ(zhc-1431::Tn10) Δasd1 Δ(zhf-4::Tn10) Δcya-12 Δ(zid-62::Tn10) | R. Curtiss |
| χ4550 | gyrA1816 ΔasdA1 Δ(zhf-4::Tn10) Δcrp-1 Δcya-1 | 66 |
| SL3261 | ΔaroA | B. Stocker |
| CS3263 | ΔaroA ΔasdA1 | This study |
| Plasmids | ||
| pYA3332 | asd-containing vector | R. Curtiss |
| pLG339 | pSC101 derivative, Kmr | 70 |
| pDMS167 | pBR322 fas+ | 64 |
| pDMS203 | pDS167 fasD (Fas−) | 24, 64 |
| pRS234 | pDMS167 with TGEV C epitope in fasA (Fas+) | 56 |
| pCS101 | pRS234 with the TGEV A epitope in fasA (Fas+) | This study |
| pCS110 | pCS101 with PacI site changed to BamHI site (Fas+) | This study |
| pCS112 | pCS110 with AflII site changed to BamHI site (Fas+) | This study |
| pCS114 | pCS101 with asd gene in BamHI site (Fas+) | This study |
| pCS150 | pLG339 with the TGEV C and A epitopes inserted into fasA of pCS112 (Fas+) | This study |
| pCS151 | pDMS167 with asd in its BamHI site (Fas+) | This study |
| pCS152 | pDMS203 with asd in its BamHI site (Fas−) | This study |
| pCS154 | pCS150 asd (Fas+) | This study |
| pCS155 | pCS154 with the nirB promoter upstream fasA (Fas+) | This study |
Plasmid constructs.
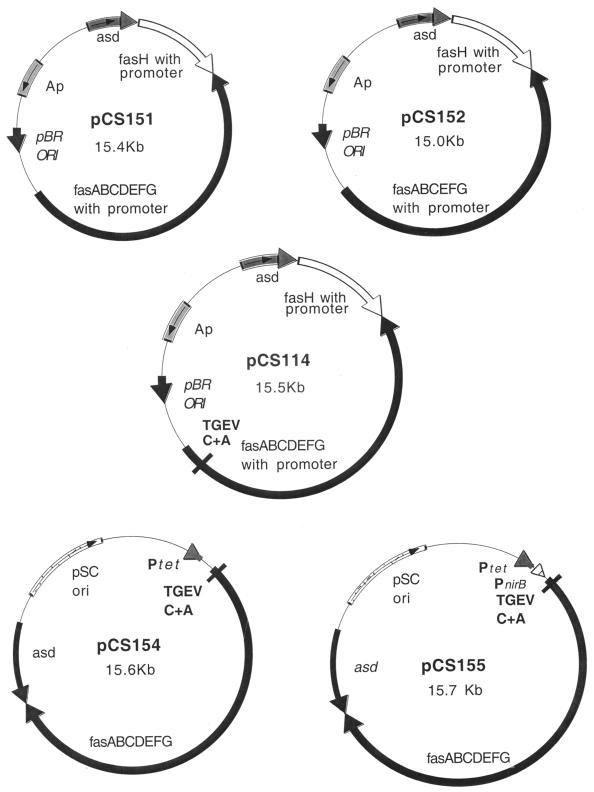
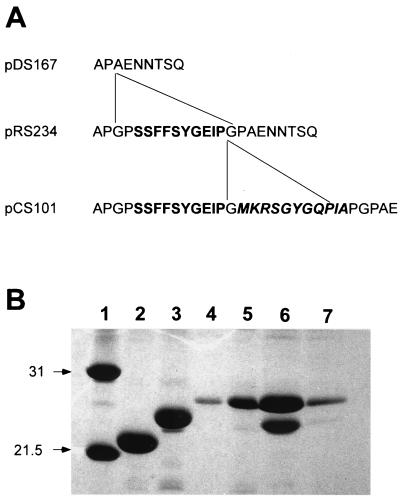
Standard procedures were used to construct the following plasmids (Fig. 1). In order to add a second epitope of TGEV to the major subunit FasA of 987P fimbriae carrying already epitope TGEV C, plasmid pRS234 was linearized with XmaI and ligated with a previously annealed 39-bp pair of oligonucleotides (5′-CCGGGTATGAAACGTTCCGGTTACGGTCAGCCGATCGCT-3′ and 5′-CCGGAGCGATCGGCTGACCGTAACCGGAACGTTTCATAC-3′) which encodes the TGEV A epitope, resulting in plasmid pCS101. Correct orientation and in-frame insertion of the TGEV A segment in pCS101 was confirmed by DNA sequencing. Plasmids pCS151, pCS152, and pCS114 were constructed by inserting a gel-purified 1.8-kb BglII fragment of pYA3332 containing the asd gene into BamHI-linearized pDMS167, pDMS203, or pCS101, respectively. A gene cluster of pCS101 encoding all the structural genes for fimbriation (fasA to fasG) but missing the 5′ end of fasH, was flanked in two steps by BamHI restriction sites. For this, a 12-mer BamHI linker d(CGCGGATCCGCG) was inserted into Klenow enzyme-treated PacI-linearized pCS101 to obtain pCS110; second, a similar linker was inserted into Klenow enzyme-treated AflII-linearized pCS110 to obtain pCS112. The 7.76-kb BamHI fragment of pCS112 encoding the fasA to fasG genes with the two TGEV epitopes in fasA was inserted into the low-copy-number plasmid pLG339 to obtain pCS150. The BglII-fragment of pYA3332 containing the asd gene was inserted into the BamHI site at the 3′ end of fasG in pCS150 to obtain pCS154. pCS155 was constructed by inserting an annealed 70-bp pair oligonucleotides (5′-GATCCAGGTAAATTTGATGTAC ATCAAATGGTACCCCTTGCTGAATCGTTAAGGTAGGCGGTAAGATC TG-3′ and 5′-GATCCAGATCTTACCGCCTACCTTAACGATTCAGCAAGGGGTACCATTTGATGTACATCAAATTTACCTG-3′), encoding the nirB promoter with its FNR binding site, into the BamHI site of pCS154, upstream fasA.
FIG. 1.
Physical maps of the expression plasmids used in the immunization experiments. Plasmids pCS151 and pCS114 are derivatives of pBR322 carrying all the 987P genes (fasABCDEFG and fasH). Plasmid pCS152 is a derivative of pDMS151 which expresses intracellular 987P fimbrial components because a portion of the gene for the usher protein FasD was deleted. In these plasmids, expression of fasA is under the control of the transcriptional activator fasH. Plasmids pCS154 and pCS155 are derivatives of pLG339 expressing the genes for the 987P export and assembly (fasABCDEFG) under the control of the tetracycline promoter (pCS154) and the nirB promoter (pCS155). Plasmid pCS151 expresses wild-type 987P fimbriae, while plasmids pCS114, pCS154, and pCS155 express chimeric 987P fimbriae with the TGEV C and A epitopes in the major subunit FasA.
Seroagglutination and antibodies.
Slide seroagglutination tests were performed with rabbit anti-987P antiserum (63) previously preadsorbed with nonfimbriated phase variants of strain 987, with 987P quaternary structure-specific monoclonal antibodies (63), with an anti-TGEV C epitope antiserum (56), and/or with an anti-TGEV A epitope antiserum. Anti-TGEV A epitope antibodies were induced in rabbits by subcutaneous injections of 200 μg of TGEV A peptide cross-linked to keyhole limpet hemocyanin (56) in complete Freund's adjuvant, followed by three booster injections of 200 μg of the same antigen in incomplete Freund's adjuvant at 2-week intervals. Seroagglutination was evaluated semi-quantitatively (+++, immediate very strong agglutination; ++, strong agglutination after 10 s; +, weak agglutination after 10 s; −, no agglutination for 1 min), as described previously (65).
Peptides and fimbriae.
The TGEV C and A peptides of the spike protein, corresponding to amino acid residues 379 to 388 and 521 to 531, respectively, were both synthesized with a cysteine added to their carboxy termini (SSFFSYGEIPC and MKRSGYGQPIAC) at the Protein Chemistry Laboratory of the University of Pennsylvania School of Medicine. Fimbriae expressed on the bacterial surface were prepared by heat extraction, as described previously (39).
SDS-PAGE and Western blotting.
Bacterial pellets, isolated fimbriae, or the baculovirus TGEV S protein lysate R2-2 and baculovirus Sf9 mock protein (kind gift from Dr. Linda Saif) were resuspended in sample buffer, boiled for 5 min, and the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blots were probed with sera of immunized mice or with rabbit anti-987P fimbriae antibodies as controls using horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (ECL) for detection (64). The relative steady-state amount of FasA protein in different constructs was evaluated by comparing proteins densitometry with NIH Image Software (Division of Computer Research and Technology, National Institutes of Health, Bethesda, Md.). Amounts of extracts were qualibrated by using the same number of bacteria (CFU).
Immunization and sampling.
For each immunization, a single colony of Salmonella serovar Typhimurium was grown in L broth without any antibiotics at 37°C on a rotary shaker at 150 rpm overnight. The bacterial cells were gently washed once and resuspended in sterile phosphate-buffered saline (PBS; 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl; pH 7.2) at a concentration of 1 × 1011 to 5 × 1011 CFU/ml. Viable counts were performed on all inocula. Before immunization, the mice were deprived of food and water for 4 h. The mice were intubated with feeding needles for intragastrical delivery of 200 μl of bacterial suspensions and fasted for an additional 30 min. The mice were immunized at days 0 and 26. For each immunized group of mice, pooled fecal pellets were collected biweekly. Approximately 500 mg of feces was added to tubes containing 2 ml of a protease inhibitor solution (PBS with 0.5% bovine serum albumin (BSA) and a cocktail of protease inhibitors [Complete; Boehringer Mannheim] using the manufacturer's recommended concentration). The fecal pellets were soaked in ice for 15 min, and the tubes were agitated vigorously for 5 min twice on a vortex mixer at maximum speed. The suspensions were centrifuged at 13,000 × g for 15 min, and the supernatants were stored at −20°C. To collect serum samples, intestinal secretions, and bile, mice were anesthetized with Metofane (methoxyflurane; Mallinckrodt Veterinary, Inc., Mundelein, Ill.) at between 6 and 8 weeks postimmunization. Blood and bile were collected by heart and gallbladder punctures, respectively. Whole small intestines, from the duodenum to the ileocecal junction, were excised, and luminal contents were carefully collected with the help of 3 ml of protease inhibitor solution introduced into intestinal lumens. Recovered intestinal contents were vortexed vigorously for 5 min. After centrifugation at 13,000 × g for 15 min at 4°C, supernatants were collected and stored at −20°C.
ELISA.
Individual mouse serum, intestinal secretion, bile, and group-pooled fecal pellet extract were tested for IgA, IgG, IgG1, and/or IgG2a antibodies against 987P fimbriae by enzyme-linked immunosorbent assay (ELISA) essentially as described earlier (63). Briefly, 96-well ELISA plates (Immulon-4; Dynatech Laboratories, Inc., Chantilly, Va.) were coated with isolated wild-type 987P fimbriae (0.2 μg in 100 μl of 0.1 M carbonate buffer, pH 9.6, per well) overnight at 4°C or with TGEV C peptide (1 μg in 100 μl of 0.1 M carbonate buffer, pH 9.6, per well) by using a household microwave oven at 145 W, twice for 10 s, followed by overnight incubation at 4°C (86). The plates were blocked with 0.5% BSA in PBS at 37°C for 2 h, washed four times with PBS, and incubated with serial dilutions of body fluid samples in PBS–0.1% BSA–0.05% Tween 20 for 2 h at 37°C. After the second washing step, plates were incubated with HRP-conjugated anti-mouse IgG or IgA antibodies at 37°C for 1 h. After the last washing step, bound antibodies were detected by using o-phenylenediamine as the chromogenic reagent and then reading the absorbance at 450 nm.
Dot blot assay.
Nitrocellulose strips were spotted with 3 μl of dilutions of TGEV C peptide (2, 0.4, and 0.1 μg) or TGEV A peptide (2 and 0.4 μg) or with isolated 987P fimbriae (0.1 μg). All dilutions were made in TNS buffer (0.01 M Tris, pH 7.3; 0.15 M NaCl; 0.1% Nonidet P-40). The strips were air dried and blocked with 3% BSA in TNT buffer (0.01 M Tris, pH 7.3; 0.9% NaCl; 0.05% Tween 20) at room temperature for 1 h. The strips were incubated with the diluted sera or pooled fecal extracts for 2 h at room temperature with shaking. The blots were developed with HRP-conjugated with goat anti-mouse IgG or IgA antibodies and then visualized by ECL.
Statistical analysis.
Antibody titers were compared by using the unpaired Student's t test, as described elsewhere (47). Statistical significance was assessed at P values of <0.05, <0.01, or <0.001.
RESULTS
Construction of Salmonella-based vaccines with stable expression of E. coli 987P fimbriae.
To stabilize antigen expression and to optimize the immunogenicity of 987P encoded by multicopy-number plasmids, we used the asd+ balanced lethal system originally developed with Salmonella crp cya strains. Accordingly, the asd gene from pYA3332 was inserted into the tetracycline-resistance gene of pDS167, which carries all the genes for 987P fimbriation (fas genes), creating pCS151. E. coli asd strain χ6212 transformed with pCS151 stably expressed 987P fimbriae on its surface in vitro in the absence of ampicillin. All 20 tested colonies of transformed bacteria grown overnight on L agar plates, and all subcultures in L broth were fimbriated in the absence of antibiotics, as shown by seroagglutination assays (++/+++), with antibodies recognizing only fully assembled fimbriae. In contrast, none of 20 tested colonies of Salmonella crp cya asd vaccine strain χ4550 transformed with pCS151 expressed these fimbriae, suggesting that catabolite repression was regulating 987P expression not only in the wild-type strain 987, as described previously (26), but also when the appropriate genes were cloned in multicopy-number plasmids. This was confirmed by constructing an asd derivative of the Salmonella aroA vaccine strain SL3261, designated CS3263, and showing that crp+ and cya+ strain CS3263/pCS151 stably expressed the 987P fimbriae in vitro.
Improved mucosal and systemic immune responses with a surface-exposed antigen.
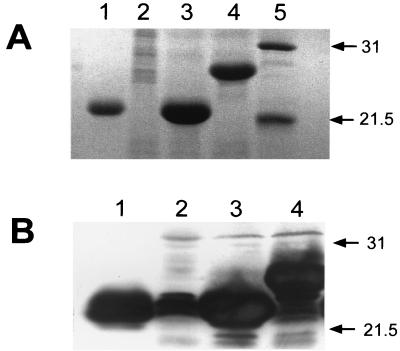
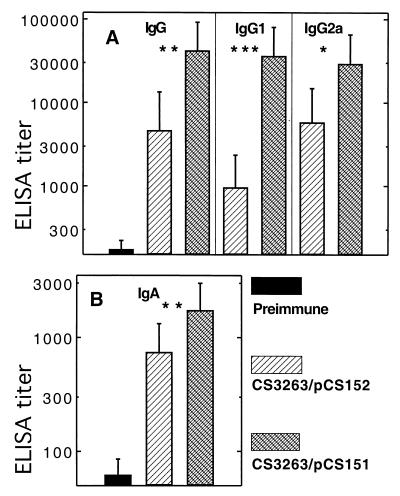
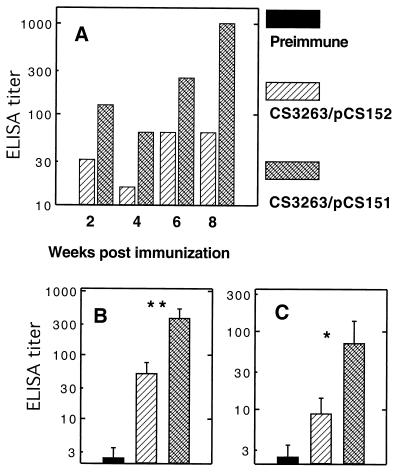
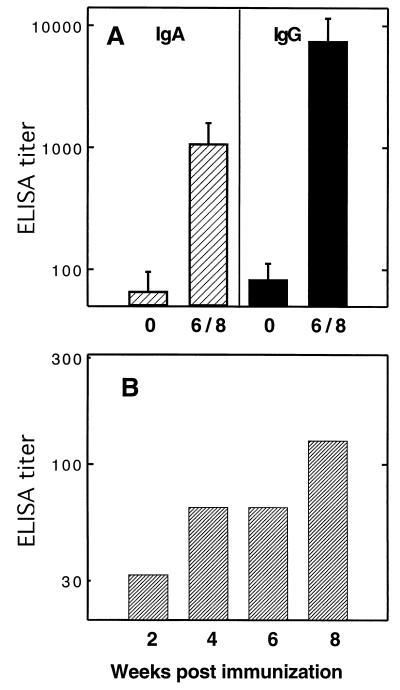
In order to determine whether an antigen is a better immunogen when expressed intracellularly or exported to the bacterial surface, the immunogenicity of the major 987P antigen FasA was compared when FasA was expressed by Salmonella vaccine strain CS3263 carrying either pCS151 (fas+ asd+) or pCS152 (fasD mutant, asd+). Use of pCS151 results in FasA export and bacterial fimbriation, as shown by seroagglutination and SDS-PAGE (Fig. 2A) of heat-extracted preparations of fimbriae. In contrast, salmonellae do not export FasA or express 987P fimbriae when the usher protein FasD is missing, as observed with strain CS3263/pCS152. Nevertheless, the latter strain still synthesized the major 987P antigen FasA, albeit at a lower level (approximately 22%) than strain CS3263/pCS151, as determined by Western blotting with rabbit anti-987P fimbriae serum (Fig. 2B). BALB/c mice (eight per group) were immunized with 3 × 1010 Salmonella sp. strains CS3263/pCS151 or CS3263/pCS152, respectively. Both strains could be isolated from the feces for at least 4 days. All tested fecal isolates (20 colonies) of strain CS3263/pCS151 (fas+) were 987P fimbriated, as determined by seroagglutination, suggesting the absence of in vivo selection of nonfimbriated plasmid-carrying salmonellae. All mice from each group were euthanized at between 6 and 8 weeks postimmunization, and samples were used for evaluating the systemic and mucosal humoral immune responses against 987P antigen. Although mice immunized with CS3263/pCS152 showed serum antibodies to 987P, the mice immunized with CS3263/pCS151 developed significantly higher titers of 987P-specific serum IgG, including both IgG1 and IgG2a (Fig. 3A) and IgA (Fig. 3B). Similarly, mucosal secretory IgA titers determined in stool (Fig. 4A), gut wash (Fig. 4B) and bile (Fig. 4C) were also significantly higher with CS3263/pCS151. Interestingly, in Fig. 3 the IgG2a titers were significantly higher than the IgG1 titers for the mice immunized with CS3263/pCS152 (P < 0.05), suggesting that this construct induced a predominant Th1 response, whereas there was a mixed response in the mice immunized with CS3263/pCS151. How much these results relate to the total amount of steady-state subunit antigen or to the export and assembly status of the subunit expressed by the respective constructs remains undetermined. However, a system capable of exporting an overexpressed antigen to the cell surface may diminish the intracellular degradation of this antigen or may prevent negative feedbacks that decrease either antigen expression or bacterial growth.
FIG. 2.
SDS-PAGE of isolated 987P fimbriae (A) and Western blot analysis of whole bacterial cells with anti-987P antibody (B) of E. coli SE5000 or serovar Typhimurium CS3263 with different plasmids. Lane 1, SE5000/pDMS167 (Fas+); lane 2, CS3263/pCS152 (fasD); lane 3, CS3263/pCS151 (Fas+); lane 4, CS3263/pCS114 (Fas+); lane 5, protein molecular mass standards in kilodaltons. Densitometric analysis suggests that in comparison to strain CS3263/pCS151 (Fas+), strain CS3263/pCS152 (fasD) expresses 22% of the amounts of FasA. Molecular masses (in kilodaltons) of standard proteins are shown on the right.
FIG. 3.
987P fimbria-specific total serum IgG, serum IgG1, and IgG2a (A) and serum IgA (B) responses. BALB/c mice were immunized orally with two doses (26-day interval) of serovar Typhimurium CS3263/pCS152(fasD) expressing fimbrial antigen in the periplasm (hatched bars) or serovar Typhimurium CS3263/pCS151 expressing 987P fimbriae on the surface (crossed bars). The sera collected before immunization (solid bars) were used as controls. Means and standard deviations of serum IgG and IgA titers of mice tested 6 to 8 weeks postimmunization are shown. Serum antibody titers elicited by serovar Typhimurium CS3263/pCS151 were significantly higher than the titers induced by serovar Typhimurium CS3263/pCS152 (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
FIG. 4.
987P fimbria-specific mucosal IgA responses in pooled stools (A), gut washes (B), and bile (C). BALB/c mice were immunized orally with two doses (26-day interval) of serovar Typhimurium CS3263/pCS152 (fasD) expressing fimbrial antigen in the periplasm (hatched bars) or serovar Typhimurium CS3263/pCS151 expressing 987P fimbriae on the surface (crossed bars). The fecal pellets were collected biweekly. The gut washes and bile were collected before immunization and at 6 to 8 weeks after immunization. The samples collected before immunization (solid bars) were used as a control. The IgA in the stools were measured from pooled fecal pellets. IgA levels in gut washes and bile were measured individually, and error bars represent standard deviations of the values for eight mice. No antibodies were detected in fecal pellets from preimmunized mice. IgA titers elicited by serovar Typhimurium CS3263/pCS151 in the gut washes and bile were significantly higher than the titers induced by serovar Typhimurium CS3263/pCS152 (∗, P < 0.05; ∗∗, P < 0.01).
Construction of chimeric 987P fimbriae carrying two epitopes of the TGEV S protein.
The 987P fimbrial subunit gene fasA was previously genetically engineered to use as a polymeric surface display system for immunogenic foreign epitopes (56). Having shown above that 987P can be expressed on salmonellae, we first showed that pRS234 (56), a plasmid that harbors a modified 987P gene cluster containing the TGEV C epitope between residues 2 and 3 of FasA, also assembles fimbriae on salmonellae, as shown by the seroagglutination (++/+++). We next showed that plasmid pCS101 containing a second epitope, TGEV A, added at the carboxy terminus of the C epitope (Fig. 5A) directed the expression of chimeric fimbriae on the surface of E. coli SE5000/pCS101, as demonstrated by seroagglutination with anti-987P, anti-TGEV C, and anti-TGEV A epitope antibodies. The TGEV C and A peptide segments were chosen for their known immunogenic properties, with both continuous epitopes being recognized by neutralizing monoclonal antibodies (19). No more than two epitopes consisting of a total of 27 residues could be added to the permissive site of FasA without interfering with fimbriation (data not shown). We also found that, consistent with a previous study (46), insertion of the TGEV A epitope into fimbriae mediated partial proteolytic cleavage of the chimeric fimbrial subunit when using K-12 E. coli SE5000 with a wild-type phenotype for protease production, as shown by SDS-PAGE (Fig. 5B). By using other E. coli host strains known to lack certain proteases, such as BL21 (71) or SG396 (29), subunit cleavage was not significant, although the total amounts of detectable subunits were also lower (Fig. 5B). Most importantly, significant amounts of only full-length subunits were detectable when pCS101 was in the Salmonella sp. strain SL3261 (Fig. 5B), indicating that most subunits in the chimeric fimbriae carried both TGEV epitopes and that this construct was suitable for immunization studies.
FIG. 5.
Expression product of the allelic FasA proteins by different plasmids. (A) Amino acid sequences of the amino terminus of the allelic FasA proteins. pDS167, pCS151, and pCS152 encode the genes for the wild-type 987P fimbriae; pRS234 encodes the genes for expressing chimeric fimbriae with the FasA-TGEV C protein (epitopes in boldface); and pCS101, pCS114, pCS154, and pCS155 encode the genes for expressing chimeric fimbriae with the FasA-TGEV C-TGEV A proteins (epitopes in boldface and italic, respectively). (B) SDS-PAGE of isolated wild-type or chimeric 987P fimbriae from different E. coli or serovar Typhimurium strains. Lane 1, protein molecular mass standards in kilodaltons; lane 2, E. coli SE5000/pDMS167; lane 3, E. coli SE5000/pRS234; lane 4, E. coli BL21/pCS101; lane 5, serovar Typhimurium SL3261/pCS101; lane 6, E. coli SE5000/pCS101; lane 7, E. coli SG396/pCS101. SE5000 (wild type for protease production) cleaved the chimeric FasA protein; BL21 (lon ompT), SG396 (htpR lonR9), and SL3261 did not cleave the chimeric FasA protein.
Immunogenicity of chimeric 987P with a Salmonella aroA asd mutant.
The asd balanced-lethal system was used to stabilize plasmid maintenance for in vivo experiments. Accordingly, an asd gene was introduced into the tetracycline-resistance gene of pCS101, generating pCS114, and Salmonella sp. strain CS3263, an asd mutant of strain SL3261, was prepared by generalized transduction. Salmonella sp. strain CS3263/pCS114 was found to stably express chimeric fimbriae without antibiotics. Expression of the chimeric fimbriae was shown to be under the control of the 987P transcriptional regulator fasH and to require CRP-Cya (data not shown), as in the original ETEC strain 987 (25, 26). The immunogenicity of the chimeric fimbriae expressed by CS3263/pCS114 was tested 6 to 8 weeks after oral administration of the salmonellae to eight BALB/c mice. Although the fimbriae elicited serum IgG and IgA (Fig. 6A) and mucosal IgA (Fig. 6B) specific for 987P epitopes, the antibody titers were generally low. Only a low titer (1:100) of anti-TGEV C epitope IgG was detected by dot blot assay in the sera of two of seven mice, and no anti-TGEV epitope IgA was detectable (data not shown).
FIG. 6.
Responses of 987P fimbria-specific serum IgG and IgA (A) and mucosal IgA in stools (B). BALB/c mice were immunized orally with two doses of serovar Typhimurium CS3263/pCS114 in a 26-day interval. Sera were collected before and at 6 to 8 weeks after the first immunization. Serum IgG (solid bars) and IgA (hatched bars) were measured individually. Error bars represent the standard deviations of the values for seven mice. The IgA levels in stools were measured from the pooled fecal pellets. No antibodies were detected in fecal pellets from preimmunized mice.
Induction of mucosal response with a Salmonella cya crp asd mutant expressing chimeric fimbriae.
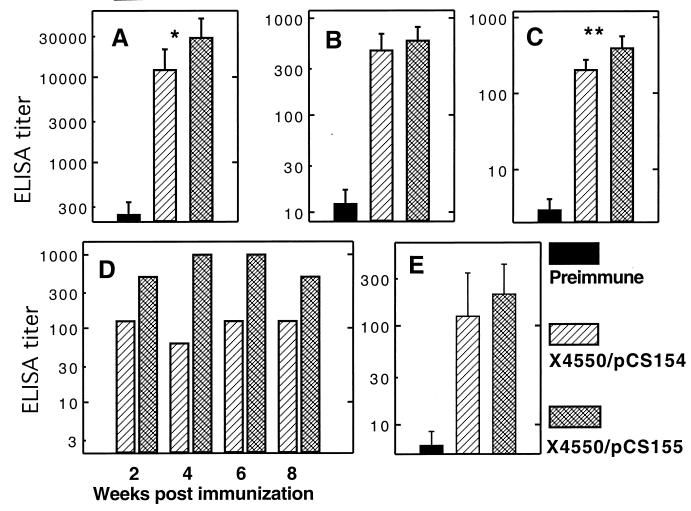
987P fimbrial expression is regulated by catabolite repression, and it has been proposed that these fimbriae are only expressed in the distal portion of the small intestine (25, 26). Thus, it is possible that the poor immune response to the TGEV epitopes may be related to suboptimal expression of the fimbrial antigen in the intestines. To circumvent this potential problem, plasmid pCS154 was constructed. This plasmid expresses the chimeric FasA protein carrying both TGEV epitopes, as well as the FasB to FasG proteins, under the control of the tetracycline promoter from the low-copy-number pLG339 vector. In vitro-grown χ4550/pCS154 produced significant amounts of chimeric 987P, as shown by seroagglutination with anti-TGEV epitope and anti-987P antibodies. The antigen specific humoral response of eight BALB/c mice was investigated 6 to 8 weeks after oral administration of χ4550/pCS154 (Fig. 7). Compared to the CS3263/pCS114-immunized animals, the χ4550/pCS154-immunized mice developed higher titers of systemic and mucosal antibodies against 987P fimbriae, but the differences were not statistically significant. Moreover, anti-TGEV antibodies were again only detected against the TGEV C epitope as serum IgG (Fig. 7E and Fig. 8A).
FIG. 7.
Responses of 987P fimbria-specific serum IgG (A), serum IgA (B), mucosal IgA in gut washes (C) and stools (D), and TGEV C peptide-specific serum IgG (E). BALB/c mice were immunized orally with two doses (26-day interval) of 987P-fimbriated serovar Typhimurium χ4550/pCS155 (crossed bars) or serovar Typhimurium χ4550/pCS154 (hatched bars). Samples collected before immunization (solid bars) were used as controls. Serum IgG, serum IgA, and IgA in gut washes were measured 6 to 8 weeks postimmunization. Error bars represent the standard deviations of the values for eight mice. Stool IgA levels were measured from pooled fecal pellets. No antibodies were detected in fecal pellets from preimmunized mice. Serum IgG and mucosal IgA titers elicited by serovar Typhimurium χ4550/pCS155 were significantly higher than the titers induced by serovar Typhimurium χ4550/pCS154 (∗, P < 0.05; ∗∗, P < 0.01).
FIG. 8.
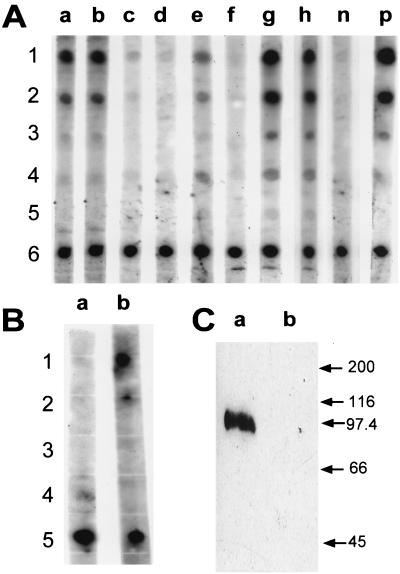
Dot blots and Western blot demonstrating systemic and mucosal antibody response to the TGEV C or A epitope. (A) TGEV C epitope-specific serum IgG were detected by dot blot assay after oral immunization of four different BALB/c mice with serovar Typhimurium χ4550/pCS154 (lanes a to d). TGEV C and A epitope-specific serum IgG were detected after oral immunization of four different BALB/c mice with serovar Typhimurium χ4550/pCS155 (lanes e to h). Rabbit anti-wild-type 987P serum was used as negative control (lane n), and rabbit anti-chimeric 987P-TGEV C serum was used as positive control (lane p). The sera from the immunized mice were diluted 1:100. The antigen in rows 1, 2, and 3 was TGEV C peptide at 2, 0.4, and 0.1 μg per spot, respectively. The antigen in rows 4 and 5 was TGEV A peptide at 2 and 0.4 μg per spot, respectively. The antigen in row 6 was purified wild-type 987P fimbriae at 0.1 μg per spot. (B) TGEV C epitope-specific IgA was detected in pooled fecal pellets (diluted 1:10) by a dot blot after oral immunization of BALB/c mice with serovar Typhimurium χ4550/pCS155 (lane b) but not after oral immunization with serovar Typhimurium χ4550/pCS154 (lane a). The antigen in rows 1 and 2 was TGEV C peptide at 2 and 0.4 μg per spot, respectively. The antigen in rows 3 and 4 was TGEV A peptide at 2 and 0.4 μg per spot, respectively. The antigen in row 5 was purified wild-type 987P fimbriae at 0.1 μg per spot. (C) Western blot with serum from one representative mouse immunized with serovar Typhimurium χ4550/pCS155. The anti-TGEV epitope antibody can recognize the epitope in the context of the TGEV S protein R2-2 expressed by recombinant baculovirus (lane a). Sf9 is the baculovirus mock protein used as negative control (lane b). Molecular masses (in kilodaltons) of standard proteins are shown on the right.
Enhanced immunogenicity with the nirB promoter directing the expression of chimeric fimbriae.
In an attempt to improve the production of chimeric fimbriae in the intestines of mice, the nirB promoter was introduced into pCS154, just upstream of the putative ribosomal binding site for the chimeric fasA gene. The resulting plasmid, pCS155, thus contains a promoter typically activated in the anaerobic intestinal or intracellular environment. Comparison of the immune response with χ4550/pCS155 versus χ4550/pCS154 yielded results that differed significantly in only some of the parameters measured (Fig. 7). χ4550/pCS155 elicited significantly higher levels of anti-987P IgG (Fig. 7A) in the serum and of IgA in gut washes (Fig. 7C) of orally immunized BALB/c mice. Addition of the nirB promoter enhanced the immune response to the TGEV epitopes, as best visualized in dot blot assays (Fig. 8A), with serum anti-TGEV A epitope IgG being induced in the mice immunized with χ4550/pCS155 (Fig. 8A). Moreover, mucosal IgA against the TGEV C epitope was only developed in the mice immunized with χ4550/pCS155 (Fig. 8B). These results indicated that use of the nirB promoter improved the immunogenicity of chimeric 987P fimbriae delivered by salmonellae. Having shown that the specific antibodies obtained reacted with the short TGEV C and TGEV A peptides, we further determined whether they were also able to recognize the full-length protein, namely, the recombinant TGEV Spike protein. Western blotting showed that the antibodies reacted with this protein (Fig. 8C), indicating that the TGEV epitopes remained accessible to the antibodies in the context of the TGEV S protein.
DISCUSSION
In addition to the panoply of better-studied attenuated S. enterica serovar Typhimurium vaccine strains, new mutants continue to be evaluated for their use and advantages as antigen delivery vectors. Construction of chimeric proteins containing heterologous sequences, the expression of these proteins by Salmonella vectors, and the evaluation of the recombinant strains as potential vaccines have been reported by many groups (11, 42, 45, 53). In most studies, the foreign antigens were expressed and maintained intracellularly (15, 32). In some cases, to facilitate direct interaction of the expressed antigen with the host's immune system, the antigens were exported to the bacterial surface by constructing fusions with genes for outer membrane proteins, flagellin, or fimbrin (1, 30, 78, 79, 81). The goal of the present study was the development of a multivalent vaccine for swine diarrhea. Thus, an attenuated Salmonella strain has been made to express antigens of two different porcine enteropathogens: the 987P fimbriae of a porcine ETEC carrying antigenic epitopes of the porcine TGEV. To our knowledge, this is the first report describing a prototype Salmonella vaccine delivering antigens as foreign chimeric fimbriae.
Several permissive sites in the major subunit of 987P were recently identified by random linker insertion mutagenesis of the subunit gene fasA (56). A site near the amino terminus of the processed FasA was characterized as the most tolerant for additional foreign epitope insertion. In the current study, two epitopes of the TGEV S protein, designated epitope C and A, were found to be tolerated by the 987P biogenesis machinery since, as shown with epitope-specific antibodies, chimeric fimbriae displayed both epitopes on the bacterial surface. This construction resulted in the insertion of 27 additional amino acid residues into FasA. A third TGEV epitope could not be added to this site (data not shown), suggesting that successful fimbria export and assembly constrain the length of an inserted segment, as proposed previously (4, 7, 73, 76). Moreover, as observed with the CS31A fimbriae (7, 46), the addition of the TGEV A epitope resulted both in decreased fimbria production and in significant cleavage of chimeric subunits. However, by using certain E. coli hosts like BL21, which does not express the OmpT protease (71), subunit cleavage was significantly decreased. Most importantly and fortuitously, this cleavage was also minimal in the Salmonella strains used in this study.
Various constructions were prepared for 987P expression by an aroA or a cya crp serovar Typhimurium mutant. 987P expression in the aroA mutant was regulated by fasH, the 987P transcriptional regulator and, therefore, by catabolite repression. In the cya crp mutant, 987P fimbriae were directly expressed from the plasmid-encoded tetracycline promoter. Although oral immunization of mice with these two strains resulted in significant systemic and mucosal humoral responses against 987P, the responses against the TGEV epitopes were weak or not detectable. Regulation by catabolite repression was previously proposed to be involved in the expression of 987P only in distal segments of the small intestine (25, 26). It was previously shown that the level of the immune response in animals was proportionally related to the amount of antigens expressed in the Salmonella vectors used (16, 82). Thus, we reasoned that it may be possible to improve the immune response by increasing the level of fimbriation expressed in vivo. For this, we used the nirB promoter, known to be activated by Fnr under anaerobic conditions as found in the intestinal environment or intracellularly (12, 27, 57). This approach led to better systemic and mucosal immune responses to the chimeric fimbriae. Moreover, antibodies were elicited against both TGEV epitopes, although mucosal antibodies were only detected for the TGEV C epitope. That this epitope is more immunogenic than the TGEV A epitope is consistent with findings by others (20, 46).
Fimbria proteins typically are highly immunogenic, and this property has been attributed mainly to their polymeric structure (54). They share their immunogenic advantage with aggregated proteins which make better antigens than soluble ones. That the particulate structure and highly repetitive nature of some antigens enhances their immunogenicity was recently demonstrated with cross-linked protein crystals which induced a higher level of antibodies than the soluble form of the same protein (69). Since each fimbria thread consists of several hundred subunits and each bacterium expresses hundreds of fimbria filaments, the abundance of these proteins makes fimbriae major cell-associated antigens of fimbriated bacterial vaccines (54). Our results with Salmonella vaccine strains CS3263/pCS151 and CS3263/pCS152 clearly show that fimbria subunits are better immunogens when exported and assembled than when retained intracellularly by salmonellae. The in vitro data may suggest that this observation results mainly from the increased amounts of subunit proteins detectable under in vitro conditions. However, it remains likely that other variables, such as the location and multimericity of the displayed subunits contributed in vivo to the results obtained. For example, a malaria antigen elicited comparable immune responses when expressed in the periplasm or on the surface of salmonellae despite a 10- to 100-times-higher expression of periplasmic antigen (30). Similarly, better protection was achieved when the p60 protein of Listeria monocytogenes was secreted in the phagocytic vacuole containing the Salmonella host vector than when the protein was kept intracellularly (31).
Some researchers claim that the key point to an active immune response is the initial amount of antigens that prime the GALT (10, 16, 55). Consistent with this, our data suggest that increased amounts of antigen delivered by salmonellae in vivo, with the use of the nirB promoter enhanced the humoral immune response. However, because the genetic background and the nature of the attenuation of different Salmonella vaccine strains has been shown to have a profound influence on immune responses (6, 23, 74, 77, 87), other researchers propose that a longer persistence of salmonellae in the mucosal immune system, especially in the Peyer's patches, is the critical issue for the induction of mucosal immune responses (23, 72). Thus, vaccine strain viability may also have influenced some of our data.
A major attribute of the 987P fimbria as a foreign epitope carrier is its enteroadhesive property. It was recently demonstrated with the F4 fimbriae of porcine ETEC strains that the fimbria-induced immunity is receptor dependent (75). This suggests that, like the mucosal adjuvant effect of the B subunit from cholera toxin, a fimbria-receptor interaction can amplify a mucosal immune response. Since BALB/c mice lack 987P receptors (22), it will be most relevant to study the immune responses of piglets immunized with attenuated bacteria expressing chimeric 987P fimbriae. Moreover, unlike many other ETEC fimbriae whose enteroadhesion is mediated by their major subunits (5, 8, 21, 36, 44, 61), enteroadhesion of the 987P fimbriae is essentially mediated by a minor subunit, the FasG protein (13, 38, 39). Therefore, an advantage of the 987P carrier over other fimbrial systems is the ability to genetically engineer the major subunit FasA as a carrier molecule without affecting the enteroadhesive property of the fimbriae.
In conclusion, we have demonstrated that the 987P antigen exposed on the surface of attenuated Salmonella strains, delivered orally to animals, elicits both systemic and mucosal immune responses. The fimbriae are capable of presenting foreign epitopes to the immune system and can induce a specific anti-foreign epitope immune response after oral administration of the 987P-expressing Salmonella strains. Furthermore, the fimbria-based polymeric display system expressed by salmonellae can be optimized by using a promoter which is known to be induced in vivo. The enhanced immunogenicity of chimeric fimbriae expressed by such strains may present significant opportunities for vaccine development.
ACKNOWLEDGMENTS
We are grateful to Roy Curtiss III for providing bacterial strains of χ4487, χ4550, and χ6212 and plasmid pYA3332; Bruce Stocker for providing SL3261 strain; Linda Saif for providing recombinant baculovirus and baculovirus proteins; Jay Farrell for useful suggestions and practical expertise; and Leonard Bello for critically reading the manuscript. Peptide synthesis was provided by the Protein Chemistry Laboratory of the Medical School of the University of Pennsylvania supported by core grants of the Diabetes and Cancer Centers (DK-19525) and (CA-16520). The oligonucleotide synthesis and DNA sequencing were provided by Veterinary School DNA Services, University of Pennsylvania.
This research was supported by USDA grant 980-2623. H.C. was the recipient of a joint Scholarship from the Ministry of Agriculture and Nanjing Agricultural University, Nanjing, China.
REFERENCES
- 1.Agterberg M, Adriaanse H, Lankhof H, Meloen R, Tommassen J. Outer membrane PhoE protein of Escherichia coli as a carrier for foreign antigenic determinants: immunogenicity of epitopes of foot-and-mouth disease virus. Vaccine. 1990;8:85–91. doi: 10.1016/0264-410x(90)90184-n. [DOI] [PubMed] [Google Scholar]
- 2.Ahren C, Jertborn M, Svennerholm A M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect Immun. 1998;66:3311–3316. doi: 10.1128/iai.66.7.3311-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascon M A, Hone D M, Walters N, Pascual D W. Oral immunization with a Salmonella typhimurium vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker D, van Zijderveld F G, van der Veen S, Oudega B, de Graaf F K. K88 fimbriae as carriers of heterologous antigenic determinants. Microb Pathog. 1990;8:343–352. doi: 10.1016/0882-4010(90)90093-6. [DOI] [PubMed] [Google Scholar]
- 5.Bakker D, Willemsen P T J, Simons L H, van Zijderveld F G, de Graaf F K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol Microbiol. 1992;6:247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 6.Benyacoub J, Hopkins S, Potts A, Kelly S, Kraehenbuhl J P, Curtiss III R, De Grandi P, Nardelli-Haefliger D. The nature of the attenuation of Salmonella typhimurium strains expressing human papillomavirus type 16 virus-like particles determines the systemic and mucosal antibody responses in nasally immunized mice. Infect Immun. 1999;67:3674–3679. doi: 10.1128/iai.67.7.3674-3679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousquet F, Martin C, Girardeau J P, Méchin M C, der Vartanian M, Laude H, Contrepois M. CS31A capsule-like antigen as an exposure vector for heterologous antigenic determinants. Infect Immun. 1994;62:2553–2561. doi: 10.1128/iai.62.6.2553-2561.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bühler T, Hoschützky H, Jann K. Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11 fimbrial morphology and location of the receptor-binding site. Infect Immun. 1991;59:3876–3882. doi: 10.1128/iai.59.11.3876-3882.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler J E. Immunoglobulins and immunocytes in animal milks. In: Ogra P, Mestecky J, Lamm M, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. New York, N.Y: Academic Press, Inc.; 1999. pp. 1531–1554. [Google Scholar]
- 10.Cardenas L, Dasgupta U, Clements J D. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine. 1994;12:833–840. doi: 10.1016/0264-410x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 11.Chabalgoity J A, Khan C M, Nash A A, Hormaeche C E. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8-23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex virus infection. Mol Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 13.Choi B-K, Schifferli D M. Lysine residue 117 of the FasG adhesin of enterotoxigenic Escherichia coli is essential for binding of 987P fimbriae to sulfatide. Infect Immun. 1999;67:5755–5761. doi: 10.1128/iai.67.11.5755-5761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements J D, Chong C, Bost K L. Use of attenuated Salmonella vectors for oral vaccines. In: Kagnoff M F, Kiyono H, editors. Mucosal immunology. New York, N.Y: Academic Press, Inc.; 1996. pp. 513–542. [Google Scholar]
- 15.Collins L V, Schödel F. Live bacterial vectors. In: Pastoret P-P, Blancou J, Vannier P, Verschueren C, editors. Veterinary vaccinology. New York, N.Y: Elsevier Science Press; 1997. pp. 293–308. [Google Scholar]
- 16.Covone M G, Brocchi M, Palla E, Dias da Silveira W, Rappuoli R, Galeotti C L. Levels of expression and immunogenicity of attenuated Salmonella enterica serovar Typhimurium strains expressing Escherichia coli mutant heat-labile enterotoxin. Infect Immun. 1998;66:224–231. doi: 10.1128/iai.66.1.224-231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtiss R, III, Doggett T, Nayak A, Srinivasan J. Strategies for the use of live recombinant avirulent bacterial vaccines for mucosal immunization. In: Kagnoff M F, Kiyono H, editors. Mucosal immunology. New York, N.Y: Academic Press, Inc.; 1996. pp. 499–511. [Google Scholar]
- 18.Delmas B, Gelfi J, Laude H. Antigenic structure of transmissible gastroenteritis virus. II. Domains of the peplomer glycoprotein. J Gen Virol. 1986;67:1405–1418. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- 19.Delmas B, Rasschaert D, Godet M, Gelfi J, Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J Gen Virol. 1990;71:1313–1323. doi: 10.1099/0022-1317-71-6-1313. [DOI] [PubMed] [Google Scholar]
- 20.Der Vartanian M, Girardeau J-P, Martin C, Rousset E, Chavarot M, Laude H, Contrepois M. An Escherichia coli CS31A fimbrillum chimera capable of inducing memory antibodies in outbred mice following booster immunization with the enteropathogenic coronavirus gastroenteritis virus. Vaccine. 1997;15:111–120. doi: 10.1016/S0264-410X(96)00172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Martino P, Girardeau J P, Der Vartanian M, Joly B, Darfeuille-Michaud A. The central variable V2 region of the CS31A major subunit is involved in the receptor-binding domain. Infect Immun. 1997;65:609–616. doi: 10.1128/iai.65.2.609-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchet-Suchaux M, Le Maitre C, Bertin A. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J Med Microbiol. 1990;31:185–190. doi: 10.1099/00222615-31-3-185. [DOI] [PubMed] [Google Scholar]
- 23.Dunstan S J, Simmons C P, Strugnell R A. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect Immun. 1998;66:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards R A, Cao J, Schifferli D M. Identification of major and minor chaperone proteins involved in the export of 987P fimbriae. J Bacteriol. 1996;178:3426–3433. doi: 10.1128/jb.178.12.3426-3433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards R A, Puente J L. Fimbrial expression in enteric bacteria: a critical step in intestinal pathogenesis. Trends Microbiol. 1998;6:282–287. doi: 10.1016/s0966-842x(98)01288-8. [DOI] [PubMed] [Google Scholar]
- 26.Edwards R A, Schifferli D M. Differential regulation of fasA and fasH expression of Escherichia coli 987P fimbriae by environmental cues. Mol Microbiol. 1997;25:797–809. doi: 10.1046/j.1365-2958.1997.5161875.x. [DOI] [PubMed] [Google Scholar]
- 27.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–102. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 28.Gebauer F, Posthumus W P, Correa I, Sune C, Smerdou C, Sanchez C M, Lenstra J A, Meloen R H, Enjuanes L. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff S A, Casson L P, Goldberg A L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci USA. 1984;81:6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad D, Liljeqvist S, Kumar S, Hansson M, Stahl S, Perlmann H, Perlmann P, Berzins K. Surface display compared to periplasmic expression of a malarial antigen in Salmonella typhimurium and its implications for immunogenicity. FEMS Immunol Med Microbiol. 1995;12:175–186. doi: 10.1111/j.1574-695X.1995.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 31.Hess J, Gentschev I, Miko D, Welzel M, Ladel C, Goebel W, Kaufmann S H. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci USA. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hormaeche C E, Khan C M A, Mastroeni P, Villarreal B, Dougan B, Roberts M, Chatfield S N. Salmonella vaccines: mechanisms of immunity and their use as carriers of recombinant antigens. In: Ala'Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 119–153. [Google Scholar]
- 33.Isaacson R E, Dean E A, Morgan R L, Moon H W. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified K99 and 987P pili: antibody production in response to vaccination. Infect Immun. 1980;29:824–826. doi: 10.1128/iai.29.2.824-826.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isaacson R E, Fusco P C, Brinton C C, Moon H W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978;21:392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacson R E, Nagy B, Moon H W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977;135:531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs A A C, Simons B H, de Graaf F K. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 1987;6:1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiménez G, Correa I, Melgosa M P, Bullido M J, Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J Virol. 1986;60:131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan A S, Johnston N H, Goldfine H, Schifferli D M. Porcine 987P glycolipid receptors on intestinal brush borders and their cognate bacterial ligands. Infect Immun. 1996;64:3688–3693. doi: 10.1128/iai.64.9.3688-3693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan A S, Schifferli D M. A minor 987P protein different from the structural fimbrial subunit is the adhesin. Infect Immun. 1994;62:4233–4243. doi: 10.1128/iai.62.10.4233-4243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laude H, Rasschaert D, Delmas B, Godet M, Gelfi J, Charley B. Molecular biology of transmissible gastroenteritis virus. Vet Microbiol. 1990;23:147–154. doi: 10.1016/0378-1135(90)90144-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine M M, Girón J O, Noriega F R. Fimbrial vaccines. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis, and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 255–270. [Google Scholar]
- 42.Londono L P, Chatfield S, Tindle R W, Herd K, Gao X M, Frazer I, Dougan G. Immunisation of mice using Salmonella typhimurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine. 1996;14:545–552. doi: 10.1016/0264-410x(95)00216-n. [DOI] [PubMed] [Google Scholar]
- 43.Marquardt R R, Jin L Z, Kim J W, Fang L, Frohlich A A, Baidoo S K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 44.Marron M B, Smyth C J. Molecular analysis of the cso operon of enterotoxigenic Escherichia coli reveals that CsoA is the adhesin of CS1 fimbriae and that the accessory genes are interchangeable with those of the cfa operon. Microbiology. 1995;141:2849–2859. doi: 10.1099/13500872-141-11-2849. [DOI] [PubMed] [Google Scholar]
- 45.Martineau P, Leclerc C, Hofnung M. Modulating the immunological properties of a linear B-cell epitope by insertion into permissive sites of the MalE protein. Mol Immunol. 1996;33:1345–1358. doi: 10.1016/s0161-5890(96)00091-0. [DOI] [PubMed] [Google Scholar]
- 46.Méchin M-C, Der Vartanian M, Martin C. The major subunit ClpG of Escherichia coli CS31A fibrillae as an expression vector for different combinations of two TGEV coronavirus epitopes. Gene. 1996;179:211–218. doi: 10.1016/S0378-1119(96)00348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittrücker H W, Köhler A, Mak T W, Kaufmann S H. Critical role of CD28 in protective immunity against Salmonella typhimurium. J Immunol. 1999;163:6769–6776. [PubMed] [Google Scholar]
- 48.Moon H W, Bunn T O. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11:200–213. doi: 10.1016/0264-410X(93)90020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan R L, Isaacson R E, Moon H W, Brinton C C, To C-C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978;227:771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagy B, Moon H W, Isaacson R E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977;16:344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagy B, Moon H W, Isaacson R E, To C-C, Brinton C C. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect Immun. 1978;21:269–274. doi: 10.1128/iai.21.1.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakayama K, Kelly S M, Curtiss R., III Construction of an Asd+ expression vector: stable maintenance and high expression of cloned genes in Salmonella vaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 53.Newton S M, Jacob C O, Stocker B A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 54.Pallesen L, Klemm P. Chimeric fimbrial vaccines. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis, and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 271–276. [Google Scholar]
- 55.Pashine A, John B, Rath S, George A, Bal V. Th1 dominance in the immune response to live Salmonella typhimurium requires bacterial invasiveness but not persistence. Int Immunol. 1999;11:481–489. doi: 10.1093/intimm/11.4.481. [DOI] [PubMed] [Google Scholar]
- 56.Rani D B R, Bayer M E, Schifferli D M. Polymeric display of immunogenic epitopes from herpes simplex virus and transmissible gastroenteritis virus surface proteins on an enteroadherent fimbria. Clin Diagn Lab Immunol. 1999;6:30–40. doi: 10.1128/cdli.6.1.30-40.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts M, Li J, Bacon A, Chatfield S. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect Immun. 1998;66:3080–3087. doi: 10.1128/iai.66.7.3080-3087.1998. . (Erratum, 67:468, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saif L J. Comparative pathogenesis of enteric viral infections of swine. Adv Exp Med Biol. 1999;473:47–59. doi: 10.1007/978-1-4615-4143-1_4. [DOI] [PubMed] [Google Scholar]
- 59.Saif L J. Mucosal immunity: an overview and studies of enteric and respiratory coronavirus infections in a swine model of enteric disease. Vet Immunol Immunopathol. 1996;54:163–169. doi: 10.1016/S0165-2427(96)05702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saif L J, Jackwood D J. Enteric virus vaccines: theoretical considerations, current status, and future approaches. In: Saif L J, Theil K W, editors. Viral diarrhea of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 313–329. [Google Scholar]
- 61.Sakellaris H, Scott J R. New tools in an old trade: CS1 pilus morphogenesis. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- 62.Savarino S J, Hall E R, Bassily S, Brown F M, Youssef F, Wierzba T F, Peruski L, El-Masry N A, Safwat M, Rao M, El Mohamady H, Abu-Elyazeed R, Naficy A, Svennerholm A M, Jertborn M, Lee Y J, Clemens J D. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. J Infect Dis. 1999;179:107–114. doi: 10.1086/314543. [DOI] [PubMed] [Google Scholar]
- 63.Schifferli D M, Abraham S N, Beachey E H. Use of monoclonal antibodies to probe subunit- and polymer specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987;55:923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schifferli D M, Alrutz M. Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli. J Bacteriol. 1994;176:1099–1110. doi: 10.1128/jb.176.4.1099-1110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schifferli D M, Beachey E H, Taylor R K. Genetic analysis of 987P adhesion and fimbriation of Escherichia coli: the fas genes link both phenotypes. J Bacteriol. 1991;173:1230–1240. doi: 10.1128/jb.173.3.1230-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schödel F, Kelly S M, Peterson D L, Milich D R, Curtiss R., III Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect Immun. 1994;62:1669–1676. doi: 10.1128/iai.62.5.1669-1676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusion. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 68.Smerdou C, Urniza A, Curtiss III R, Enjuanes L. Characterization of transmissible gastroenteritis coronavirus S protein expression products in avirulent S. typhimurium Δcya Δcrp persistence, stability and immune response in swine. Vet Microbiol. 1996;48:87–100. doi: 10.1016/0378-1135(95)00141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.St. Clair N, Shenoy B, Jacob L D, Margolin A L. Cross-linked protein crystals for vaccine delivery. Proc Natl Acad Sci USA. 1999;96:9469–9474. doi: 10.1073/pnas.96.17.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stoker N G, Fairweather N F, Spratt B G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 71.Sugimura K, Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988;170:3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tacket C O, Kelly S M, Schodel F, Losonsky G, Nataro J P, Edelman R, Levine M M, Curtiss R., III Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain expressing plasmid-encoded hepatitis B antigens stabilized by the Asd-balanced lethal vector system. Infect Immun. 1997;65:3381–3385. doi: 10.1128/iai.65.8.3381-3385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiry G, Clippe A, Scarcez T, Petre J. Cloning of DNA sequences encoding foreign peptides and their expression in the K88 pili. Appl Environ Microbiol. 1989;55:984–993. doi: 10.1128/aem.55.4.984-993.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valentine P J, Devore B P, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun. 1998;66:3378–3383. doi: 10.1128/iai.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Broeck W, Cox E, Goddeeris B M. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine. 1999;17:2020–2029. doi: 10.1016/s0264-410x(98)00406-x. [DOI] [PubMed] [Google Scholar]
- 76.van Die I, van Oosterhout J, van Megen I, Bergmans H, Hoekstra W, Enger-Valk B, Barteling S, Mooi F. Expression of foreign epitopes in P-fimbriae of Escherichia coli. Mol Gen Genet. 1990;222:297–303. doi: 10.1007/BF00633832. [DOI] [PubMed] [Google Scholar]
- 77.VanCott J L, Chatfield S N, Roberts M, Hone D M, Hohmann E L, Pascual D W, Yamamoto M, Kiyono H, McGhee J R. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 78.Verma N K, Ziegler H K, Stocker B A, Schoolnik G K. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. Vaccine. 1995;13:235–244. doi: 10.1016/0264-410x(95)93308-v. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Michel V, Leclerc C, Hofnung M, Charbit A. Immunogenicity of viral B-cell epitopes inserted into two surface loops of the Escherichia coli K12 LamB protein and expressed in an attenuated aroA strain of Salmonella typhimurium. Vaccine. 1999;17:1–12. doi: 10.1016/s0264-410x(98)00153-4. [DOI] [PubMed] [Google Scholar]
- 80.Welch S K, Saif L J. Monoclonal antibodies to a virulent strain of transmissible gastroenteritis virus: comparison of reactivity with virulent and attenuated virus. Arch Virol. 1988;101:221–235. doi: 10.1007/BF01311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White A P, Collinson S K, Burian J, Clouthier S C, Banser P A, Kay W W. High efficiency gene replacement in Salmonella enteritidis: chimeric fimbrins containing a T-cell epitope from Leishmania major. Vaccine. 1999;17:2150–2161. doi: 10.1016/s0264-410x(98)00491-5. [DOI] [PubMed] [Google Scholar]
- 82.Wick M J, Harding C V, Normark S J, Pfeifer J D. Parameters that influence the efficiency of processing antigenic epitopes expressed in Salmonella typhimurium. Infect Immun. 1994;62:4542–4548. doi: 10.1128/iai.62.10.4542-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woods R D, Wesley R D, Kapke P A. Neutralization of porcine transmissible gastroenteritis virus by complement-dependent monoclonal antibodies. Am J Vet Res. 1988;49:300–304. [PubMed] [Google Scholar]
- 84.Woodward M J, Thorns C J, Turcotte C. Fimbriae of Salmonella enteritidis: molecular analysis of SEF14 and vaccine development potential. In: Cabello F, Hormaeche C, Mastroeni P, Bonina L, editors. Biology of Salmonella. New York, N.Y: Plenum Press, Inc.; 1993. pp. 79–82. [Google Scholar]
- 85.Wu S, Pascual D W, VanCott J L, McGhee J R, Maneval D R, Levine M M, Hone D M. Immune responses to novel Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli in the absence of the CFA/I positive regulator cfaR. Infect Immun. 1995;63:4933–4938. doi: 10.1128/iai.63.12.4933-4938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L Z, Gong Y F, Fang Y, Zhang Y S, Gu F S. Use of microwaves in immunoenzyme techniques. Clin Chem. 1993;39:2021. [PubMed] [Google Scholar]
- 87.Zhang X, Kelly S M, Bollen W, Curtiss R., III Protection and immune responses induced by attenuated Salmonella typhimurium UK-1 strains. Microb Pathog. 1999;26:121–130. doi: 10.1006/mpat.1998.0245. [DOI] [PubMed] [Google Scholar]