Abstract
This study aimed to evaluate the use of equine amniotic membrane (EAM), frozen indirectly using liquid nitrogen and stored between −10° and −24°C, in the treatment of equine skin lesions. Six healthy female horses, aged 3-10 years, were included in this study. EAM was collected from previously evaluated healthy parturient mares. Wounds were surgically created at the distal ends of the forelimbs. One limb was chosen for treatment, and the contralateral limb was chosen as the control. Pain sensitivity, presence of granulation tissue, secretions, and bleeding after debridement during cleaning were evaluated. Microscopically, the following were evaluated: the integrity of the epithelium, the organization of the connective tissue, the presence of hemorrhage, fibroplasia, epithelial hyperplasia, hyperkeratosis, neovascularization, and the types of cells present. Assessments were performed on days 0, 3, 7, 14, 21, 28, and 63, and the time to complete the lesion closure. Treatment with EAM promoted faster recovery, greater neovascularization, better quality fibroplasia, and less sensitivity to pain than the control group. We concluded that the use of EAM was advantageous compared to the control group.
Keywords: biomaterials, equine amniotic membrane, graft, wound
Resumo
O objetivo deste estudo foi avaliar a utilização da membrana amniótica equina (MAE) congelada indiretamente por meio do nitrogênio líquido e conservada entre -10° e -24°C no tratamento de lesões cutâneas de equinos. Foram empregados seis equinos, fêmeas, hígidas, com idades de 3 a 10 anos. A MAE foi coletada de éguas parturientes hígidas, previamente avaliadas. As feridas foram produzidas cirurgicamente nas extremidades distais dos membros anteriores. Um dos membros foi escolhido como tratado, permanecendo o contralateral como controle. Foram avaliados a sensibilidade dolorosa; presença de tecido de granulação; secreções; e hemorragia após desbridamento durante a limpeza. Microscopicamente foram avaliados: a integridade do epitélio, a organização do tecido conjuntivo, a presença de hemorragia, fibroplasia, hiperplasia epitelial, hiperqueratose, neovascularização e os tipos celulares presentes. As avaliações foram realizadas nos dias 0, 3, 7, 14, 21, 28, e 63, e o tempo até o fechamento completo da lesão. O tratamento com MAE promoveu uma recuperação mais rápida que o grupo controle, maior neovascularização, melhor qualidade fibroplasia e menor sensibilidade à dor. Conclui-se que o emprego da MAE foi vantajoso em relação ao grupo controle.
Palavras-chave: biomateriais, enxerto, ferida, membrana amniótica equina
Introduction
Horses respond to danger through a fight and flight instinct, which predisposes them to extensive skin lesions. Most farm horses are destined for athletic life; therefore, defective tissue repair represents a significant economic burden on the industry, with 7% of animals being reported to retire due to injuries resulting from wounds (Theoret, 2004).
Conventional wound treatment approaches include surgical debridement, topical medication, and bandaging; however, characteristics such as genetics, inflammatory response, blood and oxygen supply, synthesis, growth, and fibroblast phenotype etc., make wound management very challenging, mainly in the lower extremities of the distal limbs (Stashak & Theoret, 2008).
Although skin wounds are common injuries in horses, exceptional knowledge and care are required to achieve satisfactory results (Dahlgren, 2018). Therefore, new methods of injury management are needed to assist veterinary practitioners. Theoret (2004) warns that while conservative and time-honored methods of wound care are unlikely to disappear, the development of substances that stimulate healing is imminent and equine clinicians must remain aware of these innovations to better serve their clients and patients.
In this scenario, the amniotic membrane, one of the oldest biomaterials used in tissue recovery, is given attention. Its use in human skin transplants was first documented by Davis et al. (2010). Since then, it has gained importance because of its ability to reduce scarring and inflammation, improve wound healing, and aid cell proliferation and differentiation owing to its antimicrobial properties. It is a biomaterial that can be easily obtained, processed, and transported (Niknejad et al., 2008).
The use of this material in veterinary medicine has already shown good results, as demonstrated in equine ophthalmology by Plummer et al. (2009) and in cats by Barachetti et al. (2010). Equine membranes have also been used in dogs with good results, as reported by Fahie and Shettko (2007).
The objective of this study was to evaluate the use of equine amniotic membrane (EAM) frozen using liquid nitrogen and stored between −10°C and −24°C as an auxiliary method in the treatment of skin lesions in experimental wounds. In addition, the time of complete closure of the lesions when using the membrane in the limb that received treatment was compared to the control that did not receive any treatment, and histopathological tests were conducted.
Materials and methods
Six mixed-breed healthy mares, aged 3-10 years, kept in rotated paddocks and fed exclusively on pasture, were used in this experiment. They underwent a clinical and hematological examination (complete blood count) prior to the study to qualify for inclusion. No animals showed any significant changes in the tests performed.
Amniotic membrane
The material was collected from healthy parturient mares, previously evaluated for infectious diseases and vaccinated. Samples were collected up to 4 h after delivery. Fetal appendages, including the amniotic membrane, were collected after spontaneous shedding by the mare. The placenta was then separated from the other attachments and washed to remove coarse dirt and the segment to be used was chosen.
The technique used for the conservation of the material was proposed by Kim and Tseng (1995), in which washing was performed after the separation of the other fetal annexes, first with saline solution (0.9% NaCl), and subsequently the separation between the chorionic and amniotic portions of the placenta was performed manually. The amniotic portion was immersed in a phosphate-buffered saline solution (PBS) containing 1000 UI/ml of penicillin G, 20 mcg/ml of streptomycin, and 2.5 mcg/ml of amphotericin B, with three cycles of 30 min of total immersion of the material.
The amniotic membrane was then removed from the tampon and placed on a sterile field cloth, cut into pieces with dimensions corresponding to the size of the lesions (4 × 4 cm), stored on a surgical grade paper, and placed in contact with liquid nitrogen for instant freezing. Following this contact, it was stored on another grade paper, sealed, and kept in a freezer at a temperature between −10°C and −24°C.
Wounds
Wounds were surgically created on the distal extremities of the forelimbs in the metacarpal region. Regional block of the superior palmar and palmar metacarpal nerves, known as the "high four point block," was performed with a 2% lidocaine solution, with 3-5 ml of the drug being infiltrated at each point with a 30 × 0.8-mm needle, as well as an infiltrative block around the lesion.
A 4 × 4-cm skin lesion was produced on each limb in the mid-metacarpal region. One of the limbs of each animal was chosen for the treatment randomly with the contralateral as the control, thereby making it possible to evaluate the response to the proposed therapy when compared to antisepsis alone. The treatment performed on the wounds of the control group consisted of washing with water and neutral detergent, drying with sterile gauze, and placing a cotton bandage. The treated wounds received the application of EAM to cover the wound, which was fixed owing to the pressure and placement of the cotton bandage.
The material was taken from the freezer and bathed in a saline solution (0.9% sodium chloride) at room temperature for hydration for 5 min, and later placed on the incision sites of the test limbs of the animals for future comparison with the control group.
The EAM was retained in the lesion for three days until the change of first dressing. During this period, it dried, lost its initial shape, and was no longer useful for treatment. The treated wound was then evaluated, cleaned, and managed in a manner similar to that of the untreated limb. Bandaging was done after the 17th day and only on the evaluation days. After dressing removal, from the 21st day, cleaning was done only on the assessment days, which consisted of weekly evaluations until the complete closure of the incision site was observed.
Evaluation
Lesions were evaluated and classified in terms of macroscopic and sensory parameters, such as pain sensitivity to digital palpation around the wound, presence of granulation tissue, secretions, and bleeding after debridement during cleaning.
Sensitivity of painful palpation was classified as: non-sensitive “0”; small sensitivity “1”; sensitive “2”; and very sensitive “3”. The other parameters were estimated on a scale from 0 to 3, where “0” indicated the absence of the evaluated parameters; “1” indicated occurrence in up to 30% of the lesions; “2” indicated occurrence in 30-60% of the lesions; and “3” indicated occurrence in 60-100% of the lesions.
Evaluations were performed on days 0, 3, 7, 14, 21, 28, and 63 to standardize the statistical results, as complete closure of the lesion differed for each animal, negatively affecting the results.
Fragments were collected by biopsy from the edges of the lesions and fixed in buffered formaldehyde in 10% aqueous solution. Subsequently, they were reduced to small fragments and submitted to routine techniques of embedding in paraffin, cutting in a manual microtome, and staining with hematoxylin and eosin (HE). The microscopically evaluated parameters included integrity of the epithelium, organization of the connective tissue, presence of hemorrhage, fibroplasia, epithelial hyperplasia, hyperkeratosis, neovascularization, and types of cells present.
Material collection for histological analysis was performed immediately after surgery and on 3rd, 7th, 14th, 21st, 28th, and 63rd postoperative days. Samples were collected with a disposable 4-mm punch from the edges of the lesions, covering healed and unhealed areas and the transition region between them, from the control and treated sides of different animals at different times, to prevent consecutive traumas from exacerbating the process; thereby interfering with the development of the healing process.
On the same occasions on which the lesions were evaluated, their contours were outlined using a transparent PVC plastic film and a marker pen for an overhead projector, which were later submitted for area calculation using the ImageJ program, as described by Sfair (2012). These data were used for the analysis at the 63rd day; however, they were collected weekly until complete closure.
Healing time was defined as the time between the moment of incision and the start of treatment for the treated limb, and the moment of incision in the control limb, both performed on the same day, until complete closure of the skin.
Statistical analysis
The values of the wound areas, contraction rates, and healing time were analyzed using the Student's t-test for paired data, with a significance level of 5%. Non-parametric datasets were evaluated using the Wilcoxon signed rank test, which checks for differences between ordered pairs. Data presented on a non-parametric ordinal scale were analyzed using the Friedman method with 95% reliability. The healing rate was analyzed using the ANOVA Test for repeated measures, with 95% reliability (Vieira, 2010).
Results and discussion
Cryopreservation does not change the gross physical properties of the membrane; therefore, it is an effective methodology for storage and use. After the proposed hydration time of 5 min, the membrane returned to its malleability, as observed at the time of collection. The size of the membrane was not affected by time, and all wounds were covered by the material. The evolution of healing in one animal is shown in Figure 1.
Figure 1. Photos of the cicatricial evolution showing the comparison of the lesions on the control limb (CTL) and the lesions treated with equine amniotic membrane (MBN) in one of the mares in the study.

Regarding pain, only 2 animals in the control limb group demonstrated discomfort during wound cleaning on the 3rd day. In relation to this fact, Tenenhaus (2017) suggests that covering the wound with EAM has a calming effect in addition to protecting the area, as the exposed nerve endings are covered. This pain relief was also observed in a study by Duarte and Duval-Araujo (2014). This may also indicate better modulation of local inflammation during treatment with amniotic membrane. Reduction in the inflammatory process and better modulation have also been observed in other studies (Bigbie et al., 1991; Nicodemo et al., 2017; Oliveira & Alvarenga, 1998).
In relation to the mean granulation scores in the wounds, there was no significant difference between the treated and control groups, contrary to the results reported by Bigbie et al. (1991), in which EAM-treated equine wounds had significantly less exuberant granulation tissue than controls. Oliveira and Alvarenga (1998) also reported that EAM-treated wounds developed less granulation tissue and Goodrich et al. (2000), observed the same with ponies as test subjects. This contradiction can perhaps be explained by the fact that, in this study, the bandage was maintained for 17 days, and despite being within the limits established by the literature, it may have contributed to the faster development of granulation tissue. This fact is justified by the friction that occurs between the wound and bandage during the animal's movement, which promotes granulation. The observed result indicated that this could have influenced the total healing time. Theoret (2004) explains that occlusive dressings in horses result in significantly prolonged healing times and the production of excess wound exudate and granulation tissue. This observation is also supported by Jacobsen (2017), who stated that to prevent the development of granulation tissue, occlusive dressings should only be used during the inflammatory phase of healing, and that their use should be stopped at the first sign of granulation tissue formation or when the wound bed is covered with granulation tissue.
Even though there was no significant difference between the groups regarding secretion and macroscopic hemorrhage, it was observed that the presence of both was greater in the treatment with amniotic membrane than in the control limb, which can be explained by the greater neovascularization promoted by the membrane, as described by Moyer et al. (2018), Murphy et al. (2017), and Duarte and Duval-Araujo (2014), who used the amniotic membrane as a biological dressing in the healing of infected wounds in rabbits.
In this sense, the microscopic aspects of wound healing verified in this study through histological examinations prove greater neovascularization in treatment with EAM. This justifies the shorter healing time in the treated group, as it provides the exchange of gases and nutrition of metabolically active cells (Provost, 2012) to the healing process that requires a continuous supply of oxygen and nutrients.
Despite this finding, a statistical analysis of the microscopic parameters was not carried out because the collection of samples for histology was restricted to prevent consecutive traumas caused by the collection of samples from exacerbating the inflammatory process of the wounds, which would interfere with their development.
The healing rate did not differ significantly between the groups. However, it was possible to observe that the use of EAM allowed better organization of healing compared to the control limb, as visually observed in both the macroscopic and microscopic evaluations. This better organization of healing, compared to other treatments, was also observed by Nicodemo et al. (2017), who, in their study in humans with acute tendon injury treated with AM, found better organization of collagen fibers compared to the control group. In a similar study, in horses with spontaneous unilateral superficial digital flexor tendon injuries, both acute and chronic, Muttini et al. (2015) reached the same conclusion when evaluating histological analysis, which showed an almost complete restoration of the normal tendon architecture with optimal alignment of the tendon fibers. This may be explained by the better quality of fibroplasia provided by EAM. A representative table of this rate is shown in Figure 2.
Figure 2. Mean wound healing rate, comparing the control limb (CTL) and amniotic membrane (MBN) groups, where “0” corresponds to no healing and “1” corresponds to total healing.
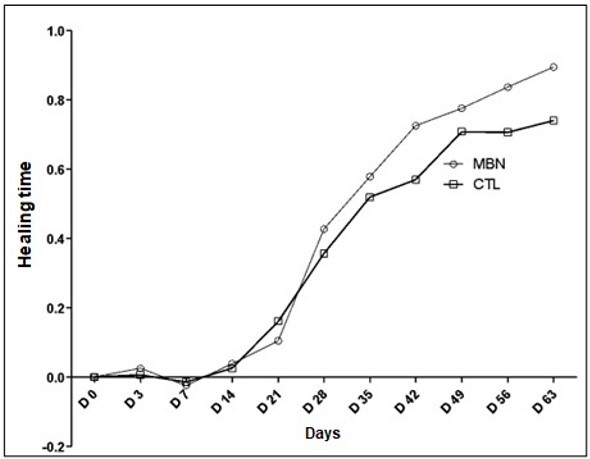
Healing time was 72.83 ± 7.3 days in the treated group and 84 ± 9.4 days in the control group, with a significant difference between the groups. This fact agrees with the results of Bigbie et al. (1991), Goodrich et al. (2000), and Oliveira and Alvarenga (1998), who reported that the application of EAM in experimental wounds in the metacarpal and metatarsal regions promoted a decrease in the number of days to complete healing compared to the control group.
Conclusion
Through this study, it was concluded that the use of EAM was effective in treatments. The preservation of EAM performed within the parameters used in this study proved to be efficient, and the fact that it could be stored in a freezer facilitates the use of this type of treatment.
This study confirms the hypothesis that the use of cryopreserved EAM as a method of aid and modulation of wounds is valid because it promotes better scarring, as suggested by greater neovascularization and fibroplasia, in addition to causing less sensitivity to pain and promoting healing in significantly less time.
Acknowledgements
This study was conducted with the support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Financing Code 001.
Footnotes
How to cite: Rosa, M. V. D., Rosa, M., & Botteon, P. T. L. (2022). Cryopreserved equine amniotic membrane and its use in cutaneous wounds of horses. Brazilian Journal of Veterinary Medicine, 44, e003122. https://doi.org/10.29374/2527-2179.bjvm003122
Ethics statement: Bioethics and Biossecurity Committee approval. All procedures were approved by the Animal Use Ethics Committee of the Veterinary Institute of the Federal Rural University of Rio de Janeiro (protocol number: 4803260617).
Financial support: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Availability of complementary results: There are no complementary information.
The study was carried out at HDM Horse Service, Cachoeiras de Macacu, Rio de Janeiro, Brasil.
References
- Barachetti L., Giudice C., Mortellaro C. M. Amniotic membrane transplantation for the treatment of feline corneal sequestrum: Pilot study. Veterinary Ophthalmology. 2010;13(5):326–330. doi: 10.1111/j.1463-5224.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- Bigbie R. B., Schumacher J., Swaim S. F., Purohit R. C., Wright J. C. Effects of amnion and live yeast cell derivative on second-intention healing in horses. American Journal of Veterinary Research. 1991;52(8):1376–1382. [PubMed] [Google Scholar]
- Dahlgren L. A. Regenerative medicine therapies for equine wound management. Veterinary Clinics of North America: Equine Practice. 2018;34(3):605–620. doi: 10.1016/j.cveq.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Davis J. W., Davis J. C. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. The Johns Hopkins Medical Journal. 2010;15:307–396. [Google Scholar]
- Duarte I. G. L., Duval-Araujo I. Amniotic membrane as a biological dressing in infected wound healing in rabbits. Acta Cirurgica Brasileira. 2014;29(5):334–339. doi: 10.1590/S0102-86502014000500008. [DOI] [PubMed] [Google Scholar]
- Fahie M. A., Shettko D. Evidence-based wound management: A systematic review of therapeutic agents to enhance granulation and epithelialization. The Veterinary Clinics of North America. Small Animal Practice. 2007;37(3):559–577. doi: 10.1016/j.cvsm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Goodrich L. R., Moll H. D., Crisman M. V., Lessard P., Bigbie R. B. Comparison of equine amnion and a nonadherent wound dressing material for bandaging pinch-grafted wounds in ponies. American Journal of Veterinary Research. 2000;61(3):326–329. doi: 10.2460/ajvr.2000.61.326. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. Update on wound dressings: Indications and best use. Veterian Key; 2017. https://veteriankey.com/6-update-on-wound-dressings-indications-and-best-use/ [Google Scholar]
- Kim J. C., Tseng S. C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473–484. doi: 10.1097/00003226-199509000-00006. [DOI] [PubMed] [Google Scholar]
- Moyer C. T., Barrett J. G., White C. Evaluation of lyophilized human amnion for equine wound management. Faculty of Virginia Polytechnic Institute and State University; 2018. Master thesis. https://www.semanticscholar.org/paper/Evaluation-of-Lyophilized-Human-Amnion-for-Equine-Moyer-Barrett/2dcb0df23c8b3cc4d8cd0af4f11d35908e4268e7 . [Google Scholar]
- Murphy S. V., Skardal A., Song L., Sutton K., Haug R., Mack D. L., Jackson J., Soker S., Atala A. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Translational Medicine. 2017;6(11):2020–2032. doi: 10.1002/sctm.17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttini A., Russo V., Rossi E., Mattioli M., Barboni B., Tosi U., Maffulli N., Valbonetti L., Abate M. Pilot experimental study on amniotic epithelial mesenchymal cell transplantation in natural occurring tendinopathy in horses. Ultrasonographic and histological comparison. Muscles, Ligaments and Tendons Journal. 2015;5(1):5–11. doi: 10.32098/mltj.01.2015.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemo M. C., Neves L. R., Aguiar J. C., Brito F. S., Ferreira I., Sant’Anna L. B., Raniero L. J., Martins R. Á. L., Barja P. R., Arisawa E. A. L. S. Amniotic membrane as an option for treatment of acute Achilles tendon injury in rats. Acta Cirurgica Brasileira. 2017;32(2):125–139. doi: 10.1590/s0102-865020170205. [DOI] [PubMed] [Google Scholar]
- Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., Seifalian A. M. Properties of the amniotic membrane for potential use in tissue engineering. European Cells & Materials. 2008;15:88–99. doi: 10.22203/eCM.v015a07. [DOI] [PubMed] [Google Scholar]
- Oliveira V. A., Alvarenga J. Membrana amniótica preservada em glicerina no reparo de feridas cutâneas de membros locomotores de eqüinos. Ciência Rural. 1998;28(4):623–628. doi: 10.1590/S0103-84781998000400014. [DOI] [Google Scholar]
- Plummer C. E., Ollivier F., Kallberg M., Brooks D., Barrie K., Utter M., Gelatt K. The use of amniotic membrane transplantation for ocular surface reconstruction: A review and series of 58 equine clinical cases (2002-2008) Veterinary Ophthalmology. 2009;12(Suppl. 1):17–24. doi: 10.1111/j.1463-5224.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- Provost P. J. In: Equine Surgery. 4th. Auer J. A., Stick J. A., editors. Elsevier; 2012. Wound healing; pp. 47–61. [DOI] [Google Scholar]
- Sfair J. Usando o ImageJ para calcular a área foliar. Universidade Federal de Pernambuco; 2012. Nov 12, http://evertontetila.ws.ufgd.edu.br/topicosVC/areaFoliarImageJ.pdf . [Google Scholar]
- Stashak T. S., Theoret C. L. Equine Wound Management. 2nd. Wiley-Blackwell; 2008. [Google Scholar]
- Tenenhaus M. The use of dehydrated human amnion/chorion membranes in the treatment of burns and complex wounds: Current and future applications. Annals of Plastic Surgery. 2017;78(2):S11–S13. doi: 10.1097/SAP.0000000000000983. [DOI] [PubMed] [Google Scholar]
- Theoret C. L. Wound repair in the horse: Problems and proposed innovative solutions. Clinical Techniques in Equine Practice. 2004;3(2):134–140. doi: 10.1053/j.ctep.2004.08.010. [DOI] [Google Scholar]
- Vieira S. Bioestatística: Tópicos Avançados. 3rd. Editora Atlas; 2010. [Google Scholar]


