Abstract
Plasmablastic lymphoma is a rare, aggressive subtype of non-Hodgkin lymphoma initially described in patients infected with human immunodeficiency virus (HIV) but recently recognized in HIV-negative individuals as well. Disease most often presents as advanced, with a median overall survival time of 14 months. We examined outcomes of patients with stage I/II disease, most of whom received combined-modality therapy. Treatment was well tolerated, and long-term survival was achieved.
Background:
Plasmablastic lymphoma (PBL) is an aggressive variant of diffuse large B-cell lymphoma. We sought to assess the treatment outcomes after combined-modality therapy for early-stage PBL.
Materials and Methods:
We retrospectively reviewed the outcomes of 10 consecutive patients diagnosed with stage I-II PBL from February 2001 to December 2013 at a single institution. The baseline clinical characteristics, treatment modalities, overall outcomes, and treatment-related toxicity were assessed.
Results:
The median age at diagnosis was 50.5 years. All patients had extranodal disease; 2 were positive for human immunodeficiency virus. Seven patients received hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone)-based chemotherapy, 2 received CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), and 1 received dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin). Radiotherapy (RT) was administered after a complete response to chemotherapy in 7 patients and a partial response in 1 patient. At a median follow-up period of 42 months, the estimated 2-year progression-free and overall survival rates were 90% and 100%, respectively.
Conclusion:
PBL can be successfully treated with aggressive chemotherapy followed by RT. The treatment was well tolerated and can result in long-term survival for patients with limited-stage disease.
Keywords: Combined modality therapy, Early stage, Non-Hodgkin lymphoma, Radiotherapy, Survival
Introduction
Plasmablastic lymphoma (PBL) is an aggressive type of non-Hodgkin lymphoma (NHL) that was initially described in the late 1990s as a new clinical entity in patients with human immunodeficiency virus (HIV) infection. In the original case description, 16 patients, of whom 15 were HIV-positive, presented with oral cavity involvement and an aggressive clinical course with poor outcomes.1 Currently, PBL is recognized by the World Health Organization as an aggressive type of NHL that is more often seen in patients with HIV infection or other types of immunodeficiency, although about 30% of cases occur in apparently immunocompetent patients.2-4
Pathologically, PBL is characterized by large lymphoma cells that often resemble B immunoblasts but have a plasmacytic immunophenotype, with loss of pan B-cell markers such as CD20, PAX-5, and CD79a, and expression of plasma cell-associated markers such as CD38 and CD138 and MUM1/IFR4.4 Genetic profiling analysis has shown that the genetic signature of PBL cells is more closely aligned with that of diffuse large B cell lymphoma.5 These tumors have a nongerminal center B-cell immunophenotype.4 An association with Epstein-Barr virus (EBV) has been documented,2 and MYC gene arrangements have been reported in up to one half of cases.6,7
Roughly 60% of patients with PBL present with advanced, Ann Arbor stage III or IV disease.2 Oral cavity involvement is typical; however, intraoral involvement was found in 1 review to be more common in HIV-positive patients than in HIV-negative patients (58% vs. 16%).2 Nodal, cutaneous, and gastrointestinal sites are also affected.8 The initial therapy has most often been cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens. Rituximab, an anti-CD20–targeted antibody, is usually not used because PBL does not express CD20. However, the outcomes from this approach have been disappointing, with a median overall survival (OS) of only about 14 months and 5-year OS rate of 31%.8 The National Comprehensive Cancer Network guidelines have advocated the use of more intensive initial therapy, such as dose-adjusted etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide (DA-EPOCH) or fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD). However, the results have been mixed regarding an improvement in outcomes.9-12
A role for consolidative radiation therapy (RT) in the treatment of patients with diffuse large B cell lymphoma has recently become apparent, with findings emerging that highlight improvements in event-free survival and potentially OS in patients who receive consolidative RT.9-11 In the ongoing UNFOLDER trial (Unfavorable Low-Risk Patients Treated with Densification of R-Chemo Regimens; ClinicalTrials.gov identifier, NCT00278408), a 2 × 2 randomized trial comparing R-CHOP on a 14- or 21-day schedule with or without consolidative RT for patients with stage I-IV diffuse large B cell lymphoma, the no-RT groups were closed early when a statistically significant difference in event-free survival was observed on interim analysis. To date, however, no series has evaluated the effect of RT for PBL, probably because of the rarity of the disease and its predilection for presenting at advanced stages, when RT is perceived to have little role. In the present analysis, we evaluated the outcomes of patients with limited-stage PBL treated at our institution with and without RT in an attempt to clarify whether RT is useful in this situation.
Materials and Methods
The appropriate institutional review board approved the present retrospective analysis. We identified 11 consecutive patients with limited-stage (stage I or II) PBL treated at our institution from February 2001 through December 2013. Of these 11 patients, 10 completed therapy at our center and were included in the present report.
Disease was staged according to the Ann Arbor staging system. The full workup consisted of core or excisional biopsy; baseline 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) or, for 1 patient treated before routine use of PET-CT, gallium scanning; baseline contrast-enhanced CT; basic laboratory studies; HIV testing; serum and urine protein electrophoresis; and bilateral bone marrow biopsy.
Chemotherapy
Hyper-CVAD consisted of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with methotrexate and cytarabine.12 CHOP chemotherapy involved cyclophosphamide, vincristine, doxorubicin, and prednisone.13 DA-EPOCH consisted of dose-adjusted etoposide, doxorubicin, and cyclophosphamide administered with vincristine and prednisone.14,15
Radiation Therapy
External beam RT was given as consolidation, with involved field or involved site targeting.16,17 Involved site RT was administered with the goal of treating prechemotherapy sites of disease involvement with a margin, according to the guidelines from the International Lymphoma Radiation Oncology Group.17 Intensity-modulated RT (IMRT) or volumetric modulated arc therapy (VMAT) was often used to spare the normal tissues in the head and neck, the salivary glands in particular.
PET-CT Evaluation of Response
Two experts in the interpretation of PET-CT scans (1 nuclear medicine physician and 1 radiologist) retrospectively and independently reviewed all the PET-CT scans. One patient had undergone PET-only imaging (and did not receive RT); all other patients had undergone PET-CT imaging. PET-CT has been used throughout to refer to either PET only or PET-CT. On completion of therapy, the response was assessed according to the International Working Group criteria for PET-CT for end-of-treatment evaluation.18 A 5-point scoring system (5-PS; also known as the Deauville criteria) was used to assess the response. In the 5-PS, based on the avidity of the mediastinal and liver blood pools, 1 denotes no uptake over background; 2, uptake less than that of the mediastinal blood pool; 3, uptake greater than that of the mediastinal blood pool but less than that of the liver; and 4 and 5, moderately or markedly greater than liver uptake, respectively. Scores of 1 to 3 are consistent with a complete response, regardless of whether a residual mass is present. A partial response is indicated by a score of 4 or 5, with reduced uptake compared with the baseline scan. Stable disease is defined as a score of 4 or 5 with no significant change in avidity. Progressive disease is defined as scores of 4 to 5 with increased uptake relative to the baseline study or evidence of new avid foci of involvement.
Toxicity
Acute and late toxicity were evaluated according to the Radiation Therapy Oncology Group radiation morbidity criteria.19
Endpoints and Statistical Analysis
OS was calculated from the start date of initial therapy until death or censored from the date of last follow-up for surviving patients. Progression-free survival (PFS) was calculated from the start of initial chemotherapy until progression, relapse, or death. Patients free of disease progression or relapse were censored on the date of the last follow-up visit or contact. Local control was defined as the absence of relapse within the radiation field. The Kaplan-Meier method was used to calculate the probabilities of OS and PFS with SPSS statistical software.20
Results
Patient Characteristics
The characteristics of the 10 patients treated are listed in Table 1. The median age at diagnosis was 50.5 years (range, 28-74 years). Nine patients were men and one was a woman. All patients presented with a primary site of extranodal disease, which involved the head and neck in 7 patients; 3 patients (30%) also had nodal involvement. The International Prognostic Index score was 0 for 8 patients and 1 (because of age) in 2 patients. Two patients were HIV-positive; the 8 HIV-negative patients had no documented history of immunosuppression. Bulky disease, defined as sites of involvement > 10 cm, was present in only 1 patient. Of the 8 patients with a complete (n = 7) or partial (n = 1) response, 7 underwent involved site targeting RT and 1 underwent involved-field RT.
Table 1.
Patient Characteristics
| Characteristic | Value or Number of Patients (%) |
|---|---|
| Age (years) | |
| Median | 50.5 |
| Mean | 48.6 |
| Range | 28-74 |
| Sex | |
| Male | 9 (90) |
| Female | 1 (10) |
| Disease stage | |
| I | 7 (70) |
| II | 3 (30) |
| HIV status | |
| Positive | 2 (20) |
| Negative | 8 (80) |
| Lactate dehydrogenase level (IU/L) | |
| Median | 463 |
| Range | 242-549 |
| IPI | |
| 0 | 8 (80) |
| 1 | 2 (20) |
| Bulky disease | |
| Yes | 1 (10) |
| No | 9 (90) |
| Median lesion size (cm) | 4.6 |
| Site of primary disease | |
| Head and neck | 7 (70) |
| Gastrointestinal | 1 (10) |
| Genitourinary | 1 (10) |
| Bone | 1 (10) |
| Ki-67 | |
| Median | 80 |
| >90% | 4 (40) |
| <90% | 5 (50) |
| Unknown | 1 (10) |
| EBV status | |
| Positive | 7 (70) |
| Negative | 3 (30) |
| CD20 status | |
| Positive | 0 |
| Dim | 1 (10) |
| Negative | 9 (90) |
Abbreviations: EBV = Epstein-Barr virus; HIV = human immunodeficiency virus; IPI = International Prognostic Index.
Biopsy specimens were obtained from 9 patients. The sites were sinonasal in 4 and the palatine tonsil, oral mucosa overlying the mandible, base of the tongue, soft tissue near the clavicle, and testis in 1 patient each. One patient underwent colectomy for primary colon involvement. In all cases, the neoplastic cells were positive for CD38 and/or CD138 and were either completely negative (n = 9) or focally and dimly positive (n = 1) for CD20. Of the 10 biopsy specimens, 7 were positive for EBV. The Ki-67 proliferation index ranged from approximately 70% to > 95%; in 4 cases, it was > 90%. Three cases were assessed for MYC overexpression using immunohistochemistry, and all 3 were positive. Of these, 2 were evaluated for MYC rearrangement by fluorescence in situ hybridization, and 1 was positive.
Systemic Therapy and Autologous Stem Cell Transplantation
All patients received upfront doxorubicin-based chemotherapy (Table 2), which was hyper-CVAD or modified hyper-CVAD in 7 patients, CHOP in 2 patients, and DA-EPOCH in 1 patient. Rituximab was given to the 1 patient with dim CD20 positivity in the initial biopsy sample. Intrathecal prophylactic methotrexate was given to patients with paranasal (n = 4), testicular (n = 1), or extensive oropharyngeal involvement (n = 1). All patients received 4 or 6 cycles of chemotherapy. Only 1 patient, with a partial response to chemotherapy, received high-dose chemotherapy with gemcitabine, melphalan, and busulfan, followed by an autologous stem cell transplantation (ASCT).
Table 2.
Treatment, Outcome, and Response
| Pt. No. | Primary Disease Site |
Chemotherapy | PET-CT Response (5-PS) |
RT | Dose (Gy) | Post-RT PET-CT Response (5-PS) |
ASCT | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Right maxillary sinus | R-CHOP × 6 cycles | Gallium | IMRT | 39.6 | Gallium | No | NED |
| 2 | Oral cavity, mandible | CHOP × 6 cycles | 1 | IMRT | 36 | 1 | No | NED |
| 3 | Left testis | Hyper-CVAD × 6 cycles | 1 | Electrons | 30.6 | 1 | No | NED |
| 4 | Right nasal cavity, maxillary sinus | Hyper-CVAD × 4 cycles | 2 | IMRT | 41.4 | 4 | No | NED |
| 5 | Colon | Modified CVAD × 6 cycles | 1 | None | NA | NA | No | NED |
| 6 | Right nasal cavity | Hyper-CVAD × 4 cycles | 1 | IMRT | 39.6 | 1 | No | NED |
| 7 | Right tonsil | Hyper-CVAD × 6 cycles | 1 | None | NA | NA | No | Dieda |
| 8 | Left base of tongue | Hyper-CVAD × 4 cycles | 1 | IMRT | 34.2 | 5 | No | NED |
| 9 | Right clavicle | Hyper-CVAD × 4 cycles | 2 | IMRT | 50 | 2 | Yes | NED |
| 10 | Left ethmoid sinus | DA-EPOCH × 6 cycles | 1 | VMAT | 41.4 | NA | No | NED |
Abbreviations: 5-PS = 5-point scale; ASCT = autologous stem cell transplant; DA-EPOCH = dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; Hyper-CVAD = cyclophosphamide, vincristine, doxorubicin, dexamethasone; IMRT = intensity modulated RT; NED = no evidence of disease; PET-CT = positron emission tomography-computed tomography; Pt. No. = patient number; R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; RT = radiation therapy; VMAT = volumetric modulated arc therapy.
Unknown cause.
Radiation Therapy
Of the 10 patients in the present study, 8 received RT (Table 2). One patient (patient 5) did not receive RT because the patient had undergone extensive prechemotherapy colectomy for primary disease. A second patient (patient 7) had stage II disease involving the tonsil and cervical neck and refused consolidative RT. Of the 8 patients who received RT, 7 received consolidative RT after a complete response (5-PS scores of 1-3), and 1 received salvage RT after a partial response (5-PS score, 4) to initial systemic therapy. Appositional electron treatment was used for 1 patient, and the other 7 underwent IMRT (6 patients) or VMAT (1 patient). The mean radiation dose was 39.1 Gy (range, 30.6-50 Gy). Patients treated with the consolidative technique received doses ranging from 30.6 to 41.4 Gy. The 1 patient who was treated to sites of refractory, gross disease received 50 Gy. The dose response could not be assessed owing to the small patient numbers.
Outcomes
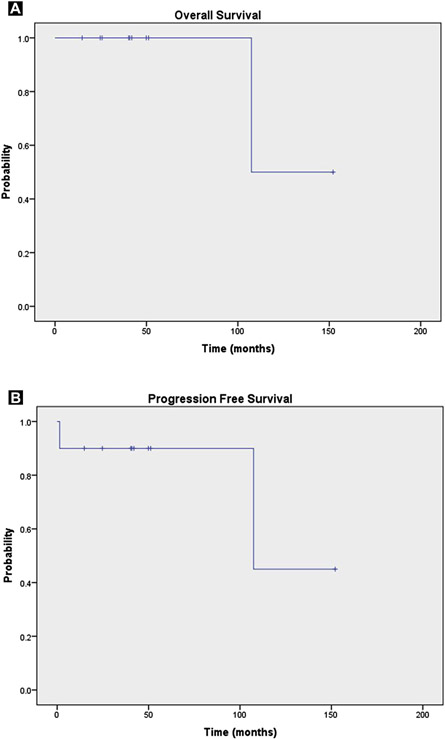
All patients had a response to initial chemotherapy; 9 complete (5-PS, 1-2) and 1 partial (patient 9). Patient 9, with a partial response, had presented with a 7.6-cm mass involving the clavicle with associated soft tissue involvement. Initial chemotherapy produced only a minimal reduction in the size of the mass and persistent low levels of fluorodeoxyglucose uptake, although the 5-PS score was only 2. He then experienced clinical disease progression and was referred for salvage RT. After receipt of salvage RT to 50 Gy with IMRT, the 5-PS score remained at 2, at which point the patient received consolidative high-dose chemotherapy and an ASCT. That patient was disease-free at the time of the present analysis. Two instances of false-positive post-RT PET-CT imaging findings occurred. The first was in patient 4, who had received H-CVAD and RT for right nasal cavity and maxillary sinus PBL. On post-RT PET-CT imaging, activity was found in the sinus (standard uptake value [SUV], 3.8), just greater than liver level (SUV, 3.1). This was suspected to be sinusitis and was found to be stable on subsequent PET-CT images during a 2-year period. The second patient (patient 10) had an excellent response to initial chemotherapy and received consolidative RT to a dose of 34.2 Gy for left base of tongue PBL. In the post-radiation period, she developed a herpes zoster outbreak affecting the left neck with small avid cervical lymph nodes on her PET-CT scan (5-PS score, 5). She sub-sequently had clinical and radiographic resolution of these lymph nodes with no clinical evidence of disease. The only deceased patient in the present series died about 9 years after single-modality chemotherapy for stage II PBL, and we were unable to confirm the cause of death. At a median follow-up period of 42 months (range, 15-149 months), the 2-year estimated OS and PFS rates were 100% and 90%, respectively (Figure 1). No patient experienced local failure.
Figure 1.
(A) Overall Survival and (B) Progression-Free Survival Estimates for 10 Patients With Stage I or II Plasmablastic Lymphoma
Treatment was generally well tolerated, with mild to moderate temporary side effects noted during RT (Table 3). The most common side effects were reversible grade 1 and 2 dermatitis and mucositis. No feeding tubes were required during or after RT. No grade 3 or greater acute toxicity was observed. Late toxicity (grade 1 or 2) was observed in 3 patients. No patient experienced grade 3 or greater late toxicity.
Table 3.
Acute and Late Radiation Toxicity
| Pt. No. | Primary Disease Site |
Acute Toxicity |
Late Toxicity |
|---|---|---|---|
| 1 | Right maxillary sinus | G1 skin, G2 MM | None |
| 2 | Oral cavity, mandible | G1 skin, G2 MM | G2 xerostomia |
| 3 | Left testis | G1 skin | None |
| 4 | Right nasal cavity/maxillary sinus | G1 skin, G1 MM | None |
| 6 | Right nasal cavity | G1 skin, G1 MM | G1 MM |
| 8 | Left base of tongue | G2 MM | NA |
| 9 | Right clavicle | G1 esophagus, G2 skin, G2 MM | None |
| 10 | Left ethmoid sinus | G2 Skin, G2 MM | G1 MM |
Abbreviations: G = grade; MM = mucous membrane; NA = not applicable (follow-up time too short to assess long-term toxicity); Pt. No. = patient number.
Discussion
Despite reports of poor outcomes for patients with advanced PBL, in the present small series of patients with stage I-II PBL, the outcomes were excellent, with an estimated 2-year OS rate of 100%. RT was administered, most often to involved sites, with IMRT or VMAT with normal-tissue sparing in most patients. Thus, acute toxicity was tolerable and temporary, and no patient experienced grade 3 or greater late toxicity.
Although the contribution of RT to the outcomes of these patients could not be determined, the results of previous retrospective studies have suggested that RT might have some positive effects. In 1 retrospective review of 112 HIV-positive patients with PBL, 60% presented with stage I-II disease and the other 40% had stage IV PBL at presentation.8 The Kaplan-Meier estimates of survival for stage I versus stage IV disease showed no significant differences in OS, and the median OS time for the entire cohort was 15 months. Of the patients with stage I disease, only 27% had received combined-modality therapy with chemotherapy and RT. Similarly, in another review of 76 HIV-negative patients with PBL, 40% had presented with stage I or II disease, and none received combined-modality therapy. The median OS time in that study was 9 months for the entire group, and the clinical stage was not associated with OS.21 These retrospective findings suggest that having limited-stage disease at presentation does not account for the favorable PFS and OS of the patients in our study.
In contrast, a retrospective study of 17 HIV-positive patients with PBL treated at the University of Brescia reported some of the most favorable outcomes to date for PBL.22 Of the 15 patients who received first-line systemic therapy, involved-field RT was also given as consolidation in the 47% of patients who presented with bulky or locally aggressive disease. At a median follow-up time of 42 months, the 3-year OS and PFS rates were 67% and 53%, respectively. High-dose chemotherapy and ASCT were also given upfront to 33% of patients. Although RT alone could not account for the improved prognosis in the present small series, given the poor outcomes typically reported when RT is underused, RT could be hypothesized to contribute to the improved outcomes for these patients.
Differences in the clinicopathologic factors could also account for the favorable outcomes in our series compared with previous reports.8,21 EBV tumor positivity is associated with improved survival outcomes among patients with PBL.23-25 In our series, 70% of patients had EBV-positive tumors, which could also have affected the positive outcomes in the present study. Furthermore, HIV status influences the prognosis in patients with PBL. Castillo et al2 demonstrated that HIV-positive patients were more likely to respond to chemotherapy and had significantly prolonged OS compared with HIV-negative patients in a review of 228 cases of PBL. Only 20% of the patients in our series were HIV-positive, suggesting that HIV infection does not explain the prolonged survival of the patients in our review.
The role of upfront high-dose therapy and ASCT for PBL is controversial and often debated.26 Further complicating this issue is the concern for patients with active HIV infection to tolerate aggressive therapy with ASCT. In our series of 10 patients with stage I or II disease, 9 did not receive consolidative ASCT and maintained excellent disease control. Possibly, when patients with limited-stage PBL achieve a complete response to initial chemotherapy, consolidation with RT could be sufficient, and ASCT can be reserved for salvage therapy.
In addition to the retrospective nature of our small series, our findings were limited by our lack of knowledge of MYC rearrangement status. Others have reported that MYC translocations, partnered with either the immunoglobulin heavy chain or, less commonly, other genes, has been reported to be a marker of poor prognosis in patients with PBL.6,7 Two patients were tested for MYC rearrangement in the present study (patients 3 and 9). Only patient 3 had a MYC rearrangement and was negative for EBV. In patient 9, who responded poorly to frontline chemotherapy; MYC rearrangement was not present and was positive for EBV. In the largest series to evaluate MYC rearrangement in PBL, MYC translocations were observed in 49% of 42 patients and were more common in the EBV-positive patients (P < .05).6 The favorable outcomes in that study might reflect tumor biology, and perhaps the patients in our series had infrequent MYC rearrangements. Although we could not exclude this possibility, some data have suggested that among patients with diffuse large B cell lymphoma and MYC rearrangement, RT diminishes the negative prognostic effects of the MYC rearrangement.27
The rarity of PBL precludes conducting prospective randomized trials to evaluate the true contribution of RT to the outcomes in this disease. However, given the low morbidity of RT in the era of normal tissue sparing from modern RT techniques, such as IMRT and VMAT, coupled with restricting RT to involved sites, it is difficult to justify the omission of RT to patients with a disease with a known poor prognosis.
Conclusion
Our findings have shown that PBL can be successfully treated with aggressive upfront combined-modality therapy. RT was well tolerated acutely and was associated with a low risk of late morbidity.
Clinical Practice Points.
PBL is a rare, aggressive variant of diffuse large B cell lymphoma that is often CD20 negative and associated with EBV positivity.
When patients present with early-stage disease, involvement of the head and neck is common.
With intensive doxorubicin-based chemotherapy, followed by consolidative RT to moderate doses, long-term survival can be achieved.
Acknowledgments
This work was supported in part by the National Cancer Institute, National Institutes of Health [Cancer Center Support (Core) Grant CA016672] to The University of Texas MD Anderson Cancer Center.
Footnotes
Disclosure
The authors declare that they have no competing interests.
References
- 1.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 1997; 89:1413–20. [PubMed] [Google Scholar]
- 2.Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma 2010; 51:2047–53. [DOI] [PubMed] [Google Scholar]
- 3.Rafaniello Raviele P, Pruned G, Maiorano E. Plasmablastic lymphoma: a review. Oral Dis 2009; 15:38–45. [DOI] [PubMed] [Google Scholar]
- 4.Stein H, Harris NL, Campo E. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008:256–7. [Google Scholar]
- 5.Chang CC, Zhou X, Taylor JJ, et al. Genomic profiling of plasmablastic lymphoma using array comparative genomic hybridization (aCGH): revealing significant overlapping genomic lesions with diffuse large B-cell lymphoma. J Hematol Oncol 2009; 2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valera A, Balague O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol 2010; 34:1686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogusz AM, Seegmiller AC, Garcia R, Shang P, Ashfaq R, Chen W. Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature. Am J Clin Pathol 2009; 132:597–605. [DOI] [PubMed] [Google Scholar]
- 8.Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol 2008; 83:804–9. [DOI] [PubMed] [Google Scholar]
- 9.Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol 2010; 28:4170–6. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BA. The role of radiation therapy in the treatment of stage I-II diffuse large B-cell lymphoma. Curr Hematol Malig Rep 2013; 8:236–42. [DOI] [PubMed] [Google Scholar]
- 11.Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol 2013; 31:4115–22. [DOI] [PubMed] [Google Scholar]
- 12.Oki Y, Westin JR, Vega F, et al. Prospective phase II study of rituximab with alternating cycles of hyper-CVAD and high-dose methotrexate with cytarabine for young patients with high-risk diffuse large B-cell lymphoma. Br J Haematol 2013; 163:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993; 328:1002–6. [DOI] [PubMed] [Google Scholar]
- 14.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood 2002; 99:2685–93. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol 2008; 26:2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yahalom J, Mauch P. The involved field is back: issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol 2002; 13(Suppl 1):79–83. [DOI] [PubMed] [Google Scholar]
- 17.Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2014; 89:49–58. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31:1341–6. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:471–81. [Google Scholar]
- 21.Castillo JJ, Winer ES, Stachurski D, et al. HIV-negative plasmablastic lymphoma: not in the mouth. Clin Lymphoma Myeloma Leuk 2011; 11:185–9. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center’s experience. Leuk Lymphoma 2015; 56:267–9. [DOI] [PubMed] [Google Scholar]
- 23.Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol 2014; 38:875–86. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann H, Oschlies I, Fink S, et al. Plasmablastic posttransplant lymphoma: cytogenetic aberrations and lack of Epstein-Barr virus association linked with poor outcome in the prospective German Posttransplant Lymphoproliferative Disorder Registry. Transplantation 2012; 93:543–50. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep 2015; 33:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Malki MM, Castillo JJ, Sloan JM, Re A. Hematopoietic cell transplantation for plasmablastic lymphoma: a review. Biol Blood Marrow Transplant 2014; 20:1877–84. [DOI] [PubMed] [Google Scholar]
- 27.Tzankov A, Xu-Monette ZY, Gerhard M, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol 2014; 27:958–71. [DOI] [PubMed] [Google Scholar]