Abstract
Background:
Comprehensive assessments of the frequency and associated doses from radiologic and nuclear medicine procedures are rarely conducted. The use of these procedures and the population-based radiation dose increased remarkably from 1980 to 2006.
Purpose:
To determine the change in per capita radiation exposure in the United States from 2006 to 2016.
Materials and Methods:
The U.S. National Council on Radiation Protection and Measurements conducted a retrospective assessment for 2016 and compared the results to previously published data for the year 2006. Effective dose values for procedures were obtained from the literature, and frequency data were obtained from commercial, governmental, and professional society data.
Results:
In the United States in 2006, an estimated 377 million diagnostic and interventional radiologic examinations were performed. This value remained essentially the same for 2016 even though the U.S. population had increased by about 24 million people. The number of CT scans performed increased from 67 million to 84 million, but the number of other procedures (eg, diagnostic fluoroscopy) and nuclear medicine procedures decreased from 17 million to 13.5 million. The number of dental radiographic and dental CT examinations performed was estimated to be about 320 million in 2016. Using the tissue-weighting factors from Publication 60 of the International Commission on Radiological Protection, the U.S. annual individual (per capita) effective dose from diagnostic and interventional medical procedures was estimated to have been 2.9 mSv in 2006 and 2.3 mSv in 2016, with the collective doses being 885 000 and 755 000 person-sievert, respectively.
Conclusion:
The trend from 1980 to 2006 of increasing dose from medical radiation has reversed. Estimated 2016 total collective effective dose and radiation dose per capita dose are lower than in 2006.
Summary
In contrast to the sixfold rise in medical radiation exposure that occurred from 1980 to 2006 in the United States, per capita radiation exposure to the population decreased by 20% between 2006 and 2016.
Sources of radiation exposure to the U.S. population are derived from five broad categories: (a) ubiquitous background radiation (including radon); (b) medical procedures in patients; (c) consumer products or activities involving radiation sources; (d) industrial, security, medical, educational, and research radiation sources; and (e) occupational sources in specific categories of workers. Comprehensive assessments of the frequency and associated doses from radiologic and nuclear medicine procedures are conducted only rarely. In the United States, assessments of diagnostic radiologic procedures were conducted in 1964 (1), 1970 (2), and 1980 (3) by the U.S. Food and Drug Administration and Mettler et al (4). Beginning about 1980, the Center for Devices and Radiological Health of the Food and Drug Administration conducted more focused surveys, which included dosimetry data for selected radiologic procedures.
The last comprehensive estimates of uses of medical radiation in the United States were performed more than 10 years ago and were published in 2009 by Mettler et al (5) and the National Council on Radiation Protection and Measurements (NCRP) in its Report 160 (6) using data from 1980 to 1982 and 2006. These publications concluded that a more than sixfold increase occurred in medical radiation dose to the U.S. population compared with that in the early 1980s, and at a level equal to that from natural background radiation, predominantly as a result of increases in CT and cardiac nuclear medicine procedures.
In 2017, NCRP convened a committee to (a) reassess medical exposure; (b) determine the changes that occurred in trends, frequency, and doses as well as the associated uncertainties resulting from radiologic, dental, and nuclear medicine exposure of patients; and (c) produce a comprehensive report on the subject (7). The report included effects of changes in International Commission on Radiological Protection (ICRP) tissue-weighting factors (8,9) on the estimation of effective doses, updating effective dose per procedure, and data collection on procedure frequency. Imaging procedures used for radiation therapy and an analysis of pediatric exposures were examined.
The goal of this article was to summarize and provide highlights from the 2019 NCRP Report 184 on medical radiation exposure of patients in the United States (7). The information has many potential uses, including following and possibly predicting trends, observing the effects of health planning policies, and comparing radiation doses from various practices. Specifically excluded from the report were discussions about the appropriate use of effective dose, occupational doses, and estimation of potential benefits or risks associated with medical exposure.
Materials and Methods
For this report, several metrics were estimated. These included the (a) number and type of procedures involving patient diagnostic and interventional medical radiation procedures; (b) effective dose per procedure, which is a calculated dose based on the type of radiation and the detriment (primarily cancer risk) to tissues exposed; (c) collective effective dose, which is the number of procedures multiplied by the effective dose per procedure; and (d) annual average individual effective dose, which is the collective effective dose divided by the U.S. population, whether the persons were exposed or not. This quantity allows a comparison of the magnitude of medical radiation exposure to that from various nonmedical radiation sources.
The Committee process began with a manual and computer search and collection of relevant literature for the period from 2007 through 2018. Data on the type and number of procedures were obtained from many sources, including commercial surveys, Medicare Part B claims from 2001 through 2016, U.S. Department of Veterans Affairs procedure counts from 2013 through 2016, U.S. Food and Drug Administration Mammography Quality Standards Act and Nationwide Evaluation of X-ray Trends survey reports, reports from agencies of the U.S. Department of Health and Human Services, professional societies (including the American College of Radiology Dose Index Registry and the American College of Cardiology National Cardiovascular Data Registry), and peer-reviewed published literature.
The data were inherently fragmentary in nature and not collected by the sources on a uniform basis. Thus, estimates for various modalities were made from different sources, which typically required additional assumptions or extrapolations.
NCRP Report 184 used the term “procedure” rather than “study,” and the term “scans” was used when CT procedures had more than one acquisition. The report mostly used general category procedure counts from a commercial source (IMV Medical Information Division, Des Plaines, Ill [www.imvinfo.com]) (10–21). IMV indicates that the 90% confidence intervals for its survey results are approximately ±6%. Supplemental estimates of the distribution of procedures across body parts or organ systems were made using other databases if the IMV data failed to match the distribution in the other data sources or the IMV data were not sufficiently granular or were nonexistent. In some cases, such as with radiography, the IMV reports did not categorize procedures in a format that would allow the determination of effective dose. In those cases, this report based the calculations on the 2016 Medicare physician supplier administrative claims data. The relationship between the number of Medicare procedures and total U.S. numbers varies depending on modality. For radiography and CT, Medicare data multiplied by a factor of four compared well with IMV data. Other factors were used for some procedures owing to the difference in age and health status of the populations. For example, Medicare data represent about half of all invasive cardiac procedures, but the data represent a much smaller portion of procedures typically performed in younger patients (eg, nuclear medicine thyroid scanning). Data on procedure counts were available for some but not all modalities for 2016; for those modalities, 2014 and 2015 data were used with projected annual growth in procedures to estimate use in 2016.
The IMV data are derived from all 50 states. They do not include data from U.S. territories. All states were sampled, and the data extended to the known universe of hospitals and other facilities by size and equipment. Just under 4 million U.S. citizens live in U.S. territories, and 320 million U.S. citizens live in the 50 states. Except in limited circumstances, Medicare data include information from U.S. territories but not about U.S. citizens receiving care in other countries.
Effective dose is a quantity developed by the ICRP to be able to compare risk or detriment from different radiation sources and from partial and whole-body exposure. The absorbed dose to a specific tissue is modified by tissue-weighting factors that are primarily based on the carcinogenic potential of that tissue to ionizing radiation and then summed for the entire body. Tissue-weighting factors defined in ICRP Publication 60 (8) were used in NCRP Report 160 (6). ICRP changed these values in 2007 in Publication 103 (nine due to newer epidemiologic evidence, introduction of tissue-weighting factors for additional specific organs and tissues, and principally the reduction in tissue-weighting factors for the gonads because hereditary effects are not as likely as once thought). This resulted in challenges in comparing 2006 estimates with 2016 estimates. For this report, the effective doses per procedure are designated as E60 and E103, respectively, depending on which set of tissue-weighting factors was used. E60 was computed for both the 2006 data and the 2016 data, and E103 was computed for the 2016 data. The corresponding values of collective effective dose are referred to as S60 and S103. Values of effective dose are also affected to some extent by choice of the dosimetric method and phantom used for calculations. Data on effective dose per procedure were obtained from the prior NCRP report (7), the American College of Radiology (22–24), state and federal surveys, and peer-reviewed and other published literature (24–35).
Uncertainties are the result of estimation of procedure numbers and effective dose per procedure. Factors leading to uncertainties include, but are not limited to, survey design, data collection methods, extrapolations, dosimetry, and systemic or random errors. However, most of the source data sets and published effective dose values were insufficient to allow a precise mathematic approach to derivation of confidence intervals. As a result, uncertainties were presented in the report as subjective uncertainty intervals and characterized as low (<30%), medium (30%–90%), or high (≥90%).
Results
Overall results are shown in Table 1 and Figure 1. In estimating the total collective effective dose, the effect of using the different ICRP tissue-weighting factors is small (approximately 5%), although for some specific modalities (eg, mammography and dental radiography), the use of newer tissue-weighting factors increases the effective dose per procedure by more than two-fold. The percentages of 2016 collective doses for various modalities are shown in Figure 2.
Table 1:
Estimated Values for Number of Procedures, Collective Effective Doses, and Average Individual Effective Doses according to Modality for 2006 and 2016
| Analysis Metrics | CT* | Radiography | Noncardiac Interventional Fluoroscopy | Cardiac Interventional Fluoroscopy | Dental | Nuclear Medicine | Total |
|---|---|---|---|---|---|---|---|
| Summary 2016 | |||||||
| No. of procedures (in millions) | 74 | 275 | 4 | 4.1 | 320 | 13.5 | 691 |
| Collective effective dose (person-Sv) | |||||||
| With ICRP 2007 tissue-weighting factors (SUS 103) | 444 000 | 71 000 | 40 000 | 42 000 | 14 000 | 106 000 | 717 000 |
| With ICRP 1990 tissue-weighting factors (SUS 60) | 469 000 | 71 000 | 40 000 | 42 000 | NA | 133 000 | 755 000 |
| Average individual effective dose (mSv) | |||||||
| With tissue-weighting factors from ICRP 2007 (EUS 103) | 1.37 | 0.22 | 0.12 | 0.13 | 0.04 | 0.32 | 2.2 |
| With tissue-weighting factors from ICRP 1990 (EUS 60) | 1.45 | 0.22 | 0.12 | 0.13 | NA | 0.41 | 2.3 |
| Summary 2006 | |||||||
| No. of procedures (in millions) | 62 | 281 | 12 | 4.6 | 500† | 17‡ | |
| Collective effective dose with tissue-weighting factors from ICRP 1990 (SUS 60) (person-Sv) | 438 000 | 97 000 | 60 200 | 68 000 | 2530 | 220 000‡ | 885 000 |
| Average individual effective dose with tissue-weighting factors from ICRP 1990 (EUS 60) (mSv) | 1.46 | 0.30 | 0.20 | 0.23 | 0.73 | 2.9 |
Note.—The effective dose values were estimated using tissue-weighting factors from International Commission on Radiological Protection (ICRP) 1990 (8) and ICRP 2007 (9). Values have been rounded. Adapted with permission of the National Council on Radiation Protection and Measurements (NCRP). EUS 60 = average effective dose per person in the United States using tissue-weighting factors from reference 8, EUS 103 = average effective dose per person in the United States using tissue-weighting factors from reference 9, NA = not available, SUS 60 = collective effective dose for the United States using tissue-weighting factors from reference 8, SUS 103 = collective effective dose for the United States using tissue-weighting factors from reference 9.
For CT, the values are for procedures. The estimated number of scans in 2006 and 2016 were 67 million and 84 million, respectively.
Numbers of images.
Value adjusted from NCRP Report 160 due to an extrapolation that was likely an overestimate.
Figure 1:
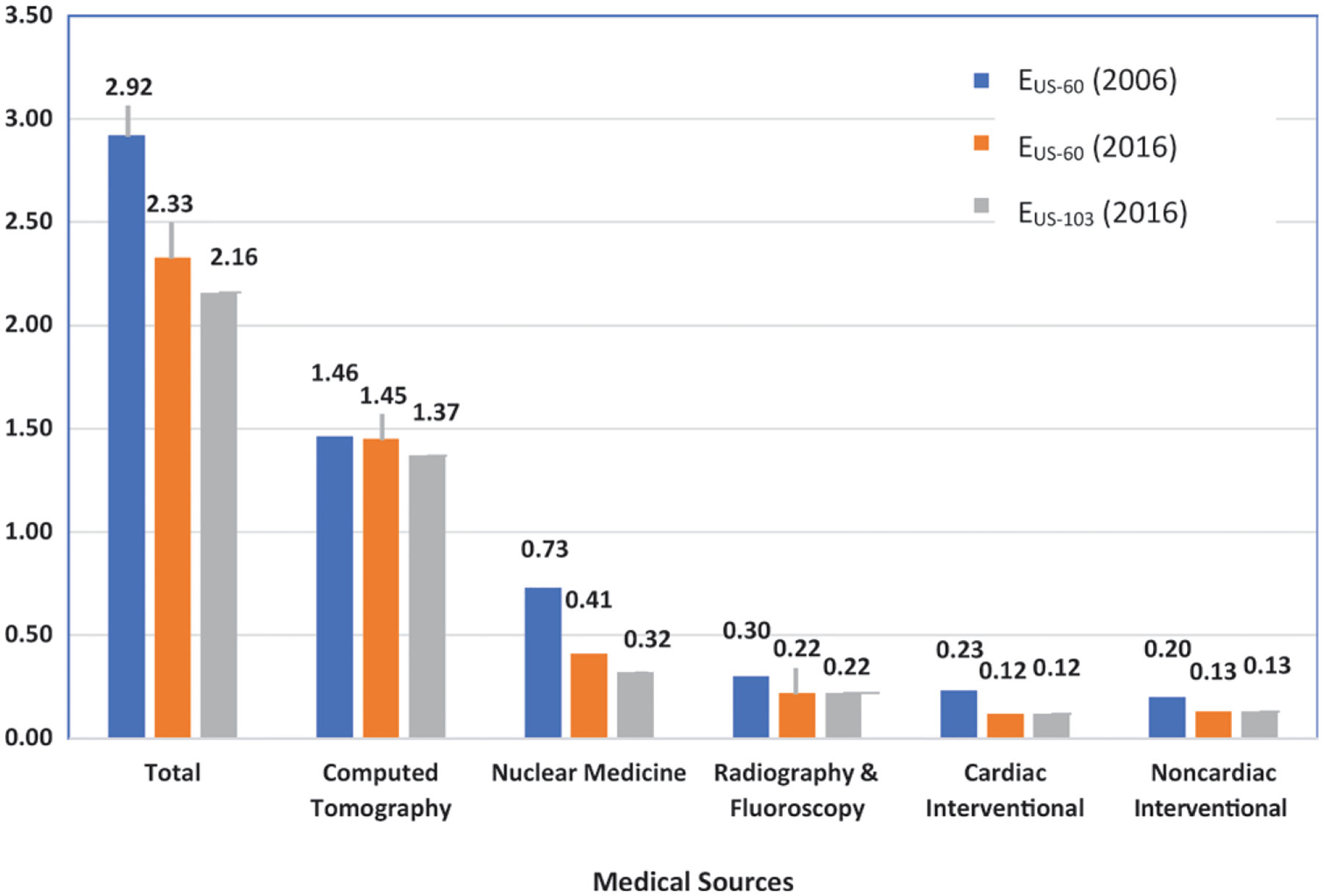
Bar graph shows estimated average annual individual effective dose in the United States from diagnostic and interventional patient radiation exposures (in millisieverts). Comparison between 2006 and 2016 has been computed by using weighting factors from International Commission on Radiological Protection (ICRP) Publications 60 and 103. EUS-60 = effective dose to a person in the United States using tissue-weighting factors from ICRP Publication 60 (8), EUS-103 = effective dose to a person in the United States using tissue-weighting factors from ICRP Publication 103 (9). Adapted with permission of the National Council on Radiation Protection and Measurements.
Figure 2:
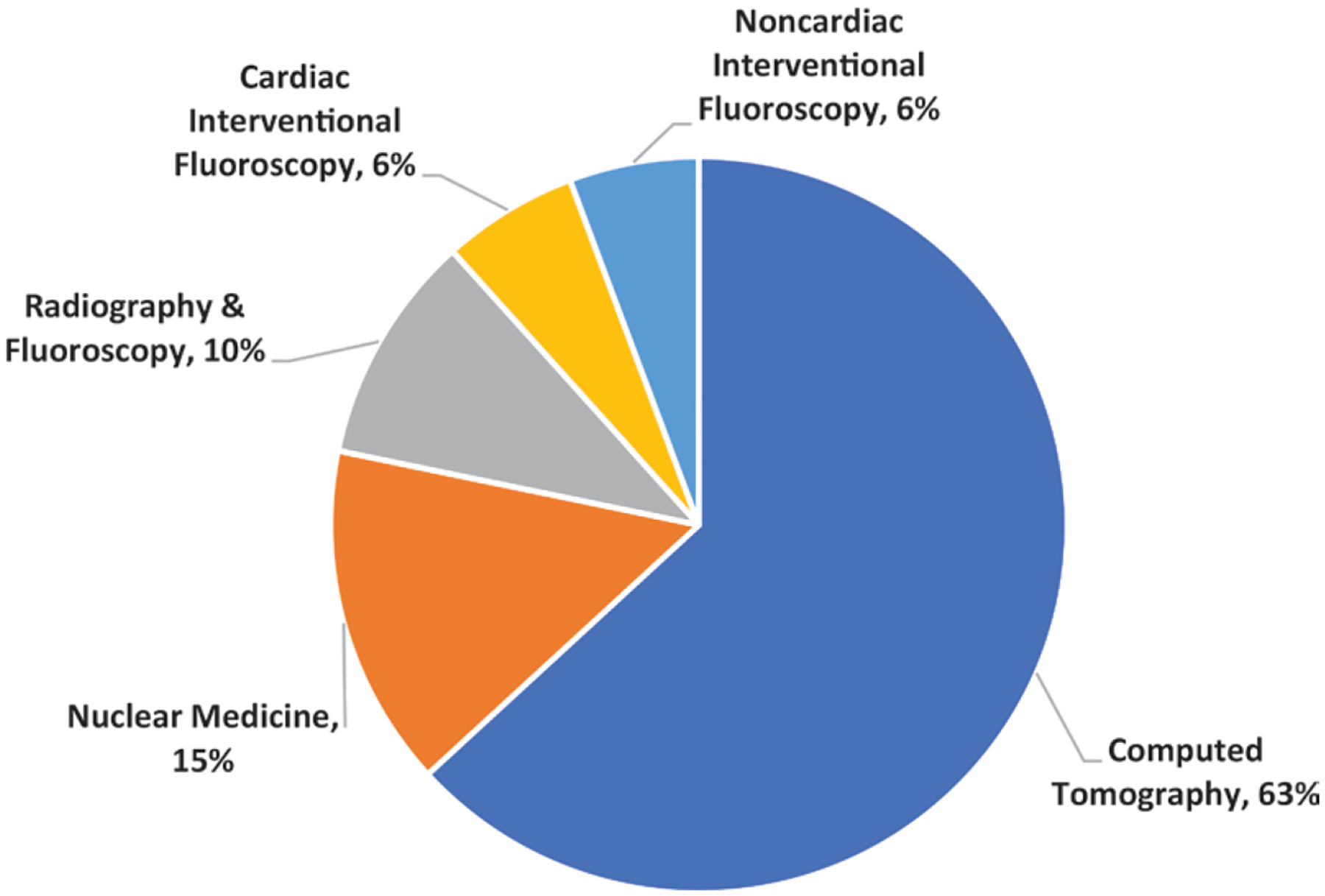
Chart shows percentage of collective effective dose (717 000 person-sievert in the United States from different modalities for 2016. Collective effective doses were obtained by using tissue-weighting factors from International Commission on Radiological Protection Publication 103 (9). Adapted with permission of the National Council on Radiation Protection and Measurements.
CT Examinations
CT procedures increased steadily in number from 1993 to 2011 and then stabilized or decreased slightly (Fig 3). For certain types of CT procedures, more than one imaging sequence may be performed over the same anatomy (eg, imaging of the liver without and with intravenous contrast material). This results in more acquisitions or scans than procedures, and it must be accounted for in estimating the radiation dose. In 2016, there were an estimated 74 million CT procedures and 84 million CT scans. Details are shown in Table 2.
Figure 3:
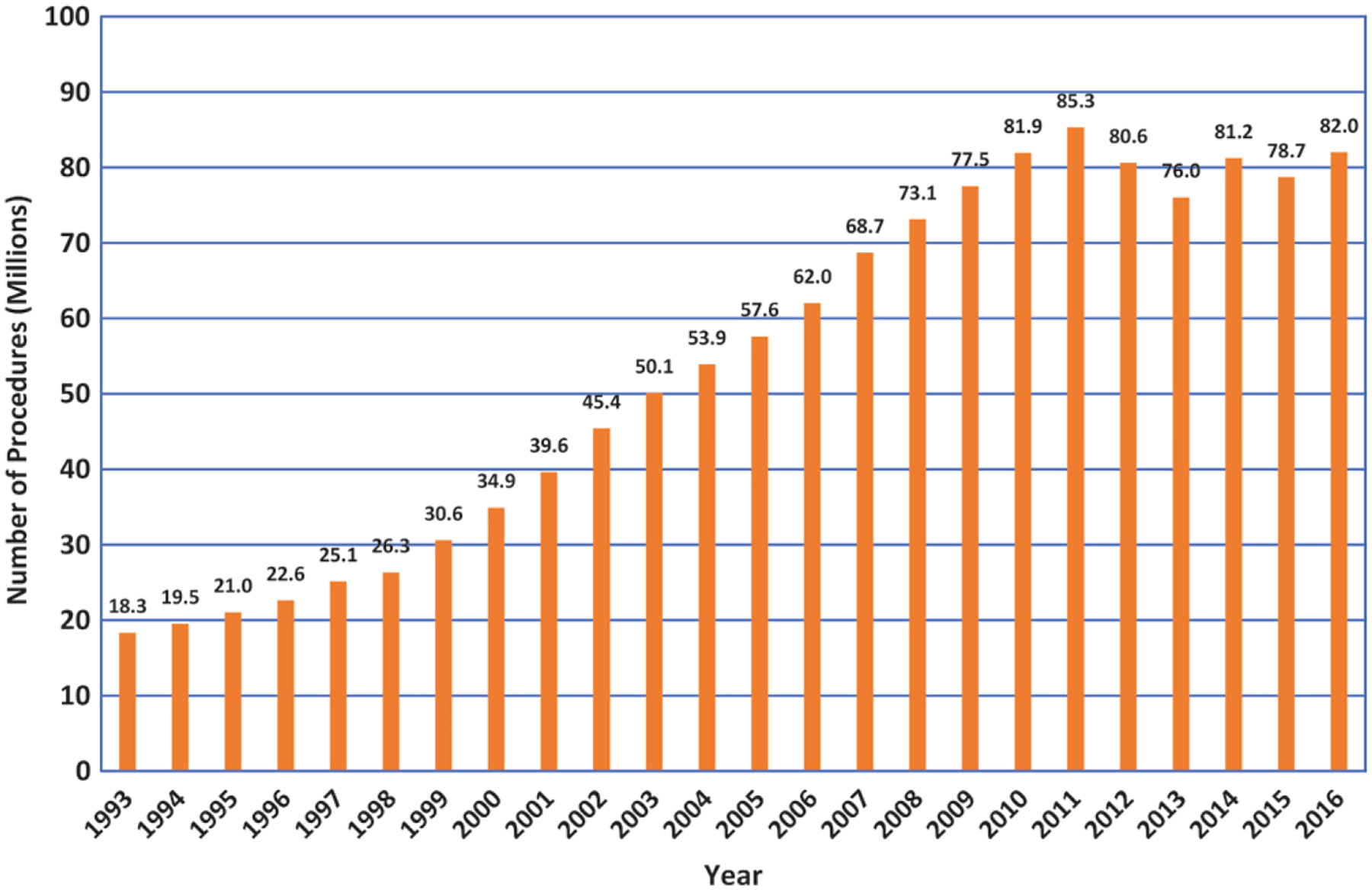
Bar graph shows total CT procedure volume trend data reported by IMV Medical Information Division in the United States from 1993 to 2016 (IMV 2017 CT Benchmark Survey). This provides general temporal trend information. The sudden drop from 2011 to 2012 is partially due to the Centers for Medicare and Medicaid Services’ bundling of certain procedures, including abdomen and pelvis. (Note: These values are slightly different from those estimated in National Council on Radiation Protection and Measurements [NCRP] Report 184 as these IMV values do not include PET/CT, SPECT/CT, or multiple scans within a single procedure or adjustments made for possible overestimation of certain types of procedures.) Adapted with permission of the NCRP.
Table 2:
Number and Type of CT Procedures and Scans for 2016
| Type of CT Scan | No. of CT Procedures | No. of CT Scans after Accounting for Multiple Scans in Certain Examinations | Effective Dose Per Scan Based on ICRP 60 (E60) (mSv) | Ratio of E103 and E60 | Effective Dose Per Scan Based on ICRP 103 (E103) (mSv) | Collective Effective Dose Based on ICRP 60 (S60) (Person-Sv) | Collective Effective Dose Based on ICRP 103 (S103) (Person-Sv) |
|---|---|---|---|---|---|---|---|
| Brain | 15 300 000 | 15 891 371 | 1.9 | 0.84 | 1.6 | 30 193 | 25 426 |
| Head and neck | 7 200 000 | 7 700 481 | 1.4 | 0.87 | 1.2 | 10 780 | 9240 |
| Chest | 12 700 000 | 13 250 657 | 5.4 | 1.14 | 6.2 | 71 553 | 82 154 |
| Calcium scoring* | 57 492 | 57 492 | 1.5 | 1.14 | 1.7 | 86 | 97 |
| Cardiac* | 281 920 | 281 920 | 7.6 | 1.14 | 8.7 | 2142 | 2452 |
| Abdomen and pelvis | 20 100 000 | 22 137 153 | 8.7 | 0.88 | 7.7 | 192 593 | 170 456 |
| CT colonography | 200 000 | 200 000 | 7.5 | 0.88 | 6.6 | 1500 | 1320 |
| Spine | 6 400 000 | 6 457 522 | 9.2 | 0.96 | 8.8 | 59 409 | 56 826 |
| CT angiography (noncardiac) | 6 600 000 | 13 027 708 | 5.4 | 0.94 | 5.1 | 70 349 | 66 441 |
| Interventional* | 863 280 | 863 280 | 5.2 | 0.96 | 5.0 | 4489 | 4316 |
| Upper extremity* | 471 100 | 479 228 | 2.0 | 0.87 | 1.7 | 958 | 814 |
| Lower extremity* | 1 203 716 | 1 223 064 | 3.2† | 1 | 3.2 | 3913 | 3913 |
| Miscellaneous. | 300 000 | 300 000 | 5.2 | 0.96 | 5.0 | 1560 | 1500 |
| Subtotal | 71 677 508 | 81 869 876 | … | … | … | 449 530 | 424 959 |
| PET/CT | 1 821 610 | 1 821 610 | 10.0 | 1 | 10.0 | 18 216 | 18 216 |
| SPECT/CT | 314 206 | 314 206 | 3.0 | 1 | 3.0 | 942 | 942 |
| Total (including PET/CT and SPECT/CT) | 73 813 324 | 84 005 692 | … | … | … | 468 688 | 444 118 |
Note.—The effect of using International Commission on Radiological Protection (ICRP) Publication 60 or ICRP Publication 103 tissue-weighting factors on the effective dose per scan and on the collective effective dose is demonstrated in columns 4–8. PET/CT is a new category assuming all procedures are for localization of whole-body PET/CT procedures. Miscellaneous includes whole-body screening, bone densitometry, follow-up and others. The EUS without PET/CT and SPECT/CT was 1.39 mSv with ICRP 60 and 1.32 mSv with ICRP 103. The EUS with PET/CT and SPECT/CT was 1.45 mSv with ICRP 60 and 1.37 mSv with ICRP 103. E60 = effective dose based on ICRP Publication 60 (8), E103 = effective dose based on ICRP Publication 103 (9), EUS = average individual effective dose in the United States from radiography and nuclear medicine (whether the person was exposed or not), S60 = collective effective dose based on ICRP Publication 60 (8), S103 = collective effective dose based on ICRP Publication 103 (9). Adapted with permission of the National Council on Radiation Protection and Measurements.
Cardiac CT, calcium scoring, interventional, and extremity (upper and lower) scans are scaled Medicare counts by a factor of four to obtain the numbers because IMV Medical Information Division numbers did not correlate with Medicare or Department of Veterans Affairs data.
Value is for CT of hip. Lower values can be applied for CT scans of knees and ankles.
Nuclear Medicine
Up until 2006, nuclear medicine experienced rapid growth, but after that, the number of procedures stabilized and then decreased substantially (Fig 4). From 2006 to 2016, the annual number of procedures decreased by more than 20% from approximately 17 million to 13.5 million. PET/CT for tumor imaging increased from 1.3 million examinations in 2006 to approximately 1.9 million examinations in 2016. With use of ICRP 1990 tissue-weighting factors for both 2006 and 2016 data, the value of S60 from nuclear medicine decreased by 40%, and the average individual effective dose from nuclear medicine decreased by 44%. Details are shown in Table 3.
Figure 4:
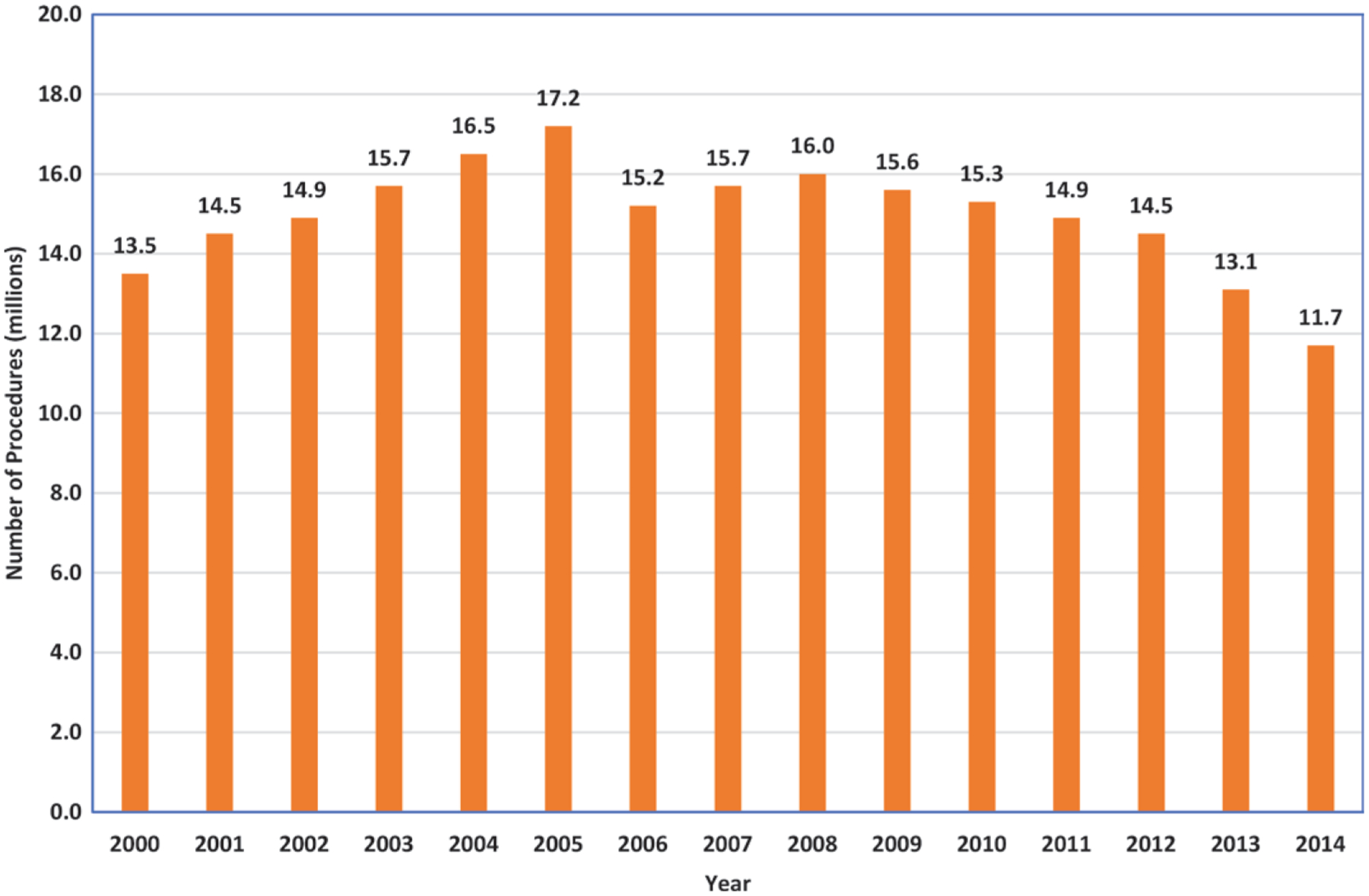
Bar graph shows IMV Medical Information Division estimates of trend data for total nuclear medicine procedures (non-PET) according to year (IMV 2015 [15]). Estimates for 2016 were a total of 13.5 million procedures, of which about 1.9 million were clinical PET procedures. Adapted with permission of the National Council on Radiation Protection and Measurements.
Table 3:
Estimated Effective and Collective Dose with ICRP Publication 60 versus ICRP Publication 103 Using the ICRP Phantom for Various Nuclear Medicine Procedures
| Study Type | No. of Procedures* | E60 Per Procedure (mSv) | ICRP 60 Collective Dose (Person-Sv)* | E103 Per Procedure (mSv) | ICRP 103 and ICRU Collective Dose (Person-Sv)† | Ratio of ICRP 103 and ICRP 60 Values |
|---|---|---|---|---|---|---|
| Cardiac (including PET) | 6 440 000 (47.6) | 12.9 | 82 918 (62.2) | 9.7 | 62 159 (58.7) | 0.75 |
| Tumor (PET) | 1 970 000 (14.6) | 14.1 | 27 698 (20.8) | 12.7 | 24 928 (23.5) | 0.90 |
| Bone and marrow | 1 670 000 (12.3) | 5.3 | 8934 (6.7) | 4.0 | 6659 (6.3) | 0.745 |
| Gastrointestinal | 930 000 (6.9) | 3.2 | 2941 (2.2) | 2.9 | 2684 (2.5) | 0.913 |
| Thyroid | 590 000 (4.4) | 4.7 | 2784 (2.1) | 4.5 | 2648 (2.5) | 0.951 |
| Tumor (non-PET) | 400 000 (3.0) | 6.4 | 2564 (1.9) | 4.1 | 1622 (1.5) | 0.633 |
| Inflammation | 280 000 (2.1) | 6.1 | 1719 (1.3) | 5.9 | 1641 (1.5) | 0.955 |
| Neurology (including PET of brain) | 200 000 (1.5) | 7.7 | 1532 (1.2) | 6.6 | 1316 (1.2) | 0.859 |
| Lung | 690 000 (5.1) | 1.8 | 1231 (0.9) | 2.5 | 1740 (1.6) | 1.414 |
| Genitourinary | 360 000 (2.7) | 2.5 | 901 (0.7) | 1.4 | 517 (0.5) | 0.574 |
| Total | 13 530 000 (100) | … | 133 222 (100) | … | 105 914 (100) | 0.795 |
Note.—Data are for procedures in the United States in 2016. The effective dose per category depends on assumptions made in distribution of specific procedures, distribution of radiopharmaceuticals used, administered activity, and dose conversion coefficients for radiopharmaceuticals in that category. E60 = effective dose based on International Commission on Radiological Protection (ICRP) Publication 60 (8), E103 = effective dose based on ICRP Publication 103 (9), ICRU = International Commission on Radiological Units and Measurements. Adapted with permission of the National Council on Radiation Protection and Measurements.
Numbers in parentheses are the percentage of the total.
Numbers in parentheses are percentages.
Radiography and Fluoroscopy
Since 2006, fundamental changes have taken place in the type of image receptors used, with essentially complete replacement of screen-film units with digital detectors. Despite this, the effective dose per procedure appears to have changed little. Radiographic intravenous urography has been almost completely replaced by CT and MRI urography, and fluoroscopic examinations of the gastrointestinal tract have declined substantially. In 2006, an estimated 281 million radiographic and diagnostic fluoroscopic procedures were performed. This decreased to approximately 275 million in 2016 even though the population increased from approximately 300 million to approximately 323 million. An interval decrease occurred in chest, abdomen and pelvis, and urologic radiographic examinations, and an increase occurred in hip and extremity radiographic and mammographic examinations. The decrease in procedures (particularly abdomen and pelvis) has resulted in a reduction in collective effective dose (S60) from radiography and diagnostic fluoroscopy. Details are shown in Table 4.
Table 4:
Estimated Number of Procedures, Effective Dose per Procedure, and Collective Effective Dose from Radiography, Mammography, and Diagnostic Fluoroscopy for Specific Examinations in 2016
| Examination | No. of Procedures (Thousands) | Effective Dose Per Procedure (E103) (mSv) | Collective Effective Dose (S103) (Person-Sv) |
|---|---|---|---|
| Chest | 110 388 | 0.1 | 11 039 |
| Mammography | 39 252 | 0.36 | 14 131 |
| Skull | 229 | 0.14 | 32 |
| Cervical spine | 4884 | 0.36 | 977 |
| Thoracic spine | 2509 | 1.0 | 2509 |
| Lumbar spine | 11 255 | 1.4 | 15 757 |
| Esophagus | 2105 | 0.7 | 1474 |
| Upper gastrointestinal | 938 | 6.0 | 5628 |
| Abdomen | 12 228 | 0.6 | 7337 |
| Barium enema | 192 | 6.0 | 1152 |
| Urography | 647 | 3.0 | 1941 |
| Pelvis | 5411 | 0.4 | 2164 |
| Hip | 14 995 | 0.4 | 5996 |
| Hands and feet | 31 194 | 0 | 0 |
| Knees | 25 757 | 0.003 | 77 |
| Shoulder | 11 951 | 0.006 | 72 |
| Other head and neck | 1121 | 0.22 | 247 |
| Total | 275 556 | … | 70 532 |
| Average individual effective dose (EUS 103) (mSv) | … | … | 0.22 |
Note.—Effective dose used in National Council on Radiation Protection and Measurements (NCRP) Report 160 was modified according to E103/E60 ratios from Wall et al 2011 (36) and rounded. Results for 2016 were computed by using 2007 International Commission on Radiological Protection (ICRP) Publication 103 tissue-weighting factors. E60 = effective dose calculated according to ICRP Publication 60 (8), E103 = effective dose calculated according to ICRP Publication 103 (9), EUS 103 = effective dose per person in the United States using tissue-weighting factors from ICRP Publication 103 (9), S103 = collective effective dose using tissue-weighting factors from ICRP Publication 103 (9). Adapted with permission from the NCRP.
Cardiac Interventional Fluoroscopy
As of 2016, the estimated total number of cardiac procedures performed in catheterization or angiography laboratories has remained at approximately 4.1 million cases annually. More coronary diagnostic and percutaneous interventions are now combined in a single procedure. Greater estimated numbers of electrophysiologic procedures and a much smaller (but rapidly increasing) number of structural heart procedures exist. No substantial impact of the change exists in ICRP tissue-weighting factors. Details are shown in Table 5.
Table 5:
Estimated Number of Procedures, Effective Dose per Procedure, and Collective Effective Dose for Fluoroscopically Guided Interventional Cardiologic Procedures Performed in the United States during 2006 and 2016
| Procedure | No. of Procedures in 2006 | Effective Dose in 2006 (mSv) | Collective Effective Dose in 2006 (Person-Sv)* | No. of Procedures in 2016 | Effective Dose in 2016 (mSv) | Collective Effective Dose in 2016 (Person-Sv) |
|---|---|---|---|---|---|---|
| Diagnostic arteriography | 2 137 050 | 7 | 14 959 | 2 500 000 | 7 | 17 500 |
| Percutaneous intervention | 498 250 | 23 | 11 457 | 850 000 | 23 | 19 550 |
| Combined diagnostic and intervention† | 1 323 000 | 30 | 39 690 | … | … | … |
| Electrophysiology nonpacemaker | 264 600 | 3.2 | 847 | 350 000 | 3.2 | 1120 |
| Pacemaker | 361 800 | 1 | 362 | 360 000 | 1 | 360 |
| Other | 60 750 | 15 | 911 | … | … | … |
| Structural | … | … | … | 70 000 | 50 | 3500 |
| Total | 4 645 450 | … | 68 226 | 4 130 000 | … | 42 030 |
Note.—Adapted with permission of the National Council on Radiation Protection and Measurements.
Effective dose conversion International Commission on Radiological Protection Reports 60 and 103 taken to be 1.0.
Data for this category were not reported by the IMV Medical Information Division for 2016 and are now included in the other categories.
Noncardiac Interventional Fluoroscopy
Many procedures (eg, tissue biopsy, aspiration, arthrography, and central venous catheter insertions) for which fluoroscopy previously was the main imaging method now only use minimal or no fluoroscopic guidance, and diagnostic imaging is often performed with CT, US, or MRI. This has resulted in a substantial reduction (from 12 million to 4 million procedures) in the number of what were classified in NCRP Report 160 as noncardiac interventional fluoroscopy procedures. However, neither the IMV nor the Medicare billing data allow for analysis of the frequency of specific interventional procedures to assess effective dose. For example, billing codes may specify “embolization” or “percutaneous biopsy” but not anatomic location (which is necessary to estimate effective dose). IMV trend data on the total number and broad categories of cases and procedures performed in angiographic or catheterization laboratories and the total number of such examinations show relative stability from 2006 to 2016. With these limited data, it is possible to estimate broadly the total collective effective dose. The value for collective effective dose (S103) of 40 000 person-sievert used in NCRP Report 184 for 2016 is the same as was used in NCRP Report 160 (NCRP 2009) for those procedures that were likely performed in interventional angiography or cardiac catheterization laboratories. Substantial uncertainty surrounds these values.
Dental Radiography
The collective effective doses for dental radiography presented in NCRP Report 160 (NCRP 2009) were based on estimates of images acquired. In NCRP Report 184, the data were derived from a large workload survey of facilities, and the data on number and types of procedures, which may include more than one image, were considered to be much more accurate and the numbers higher than those estimated for 2006. This, combined with changes in ICRP tissue-weighting factors for salivary glands and upper airway, has substantially increased the estimated collective effective dose. Details are shown in Table 6.
Table 6:
Estimated Number and Effective Doses for Dental Radiography in the United States in 2016
| Examination | No. of Procedures (Millions) | Effective Dose Per Procedure (E103) (μSv) | Collective Effective Dose (S103) (Person-Sv) | Average Annual Individual Effective Dose (EUS 103 Dental) (mSv) |
|---|---|---|---|---|
| Intraoral | 296 | 43.4 | 12 800 | … |
| Panoramic | 21 | 26 | 550 | … |
| Cone beam CT | 5.2 | 176 | 620 | … |
| Cephalometric | <1% | ~5–10 | NA* | … |
| Total | 320 (rounded) | … | 14 000 (rounded) | 0.04 |
Note.—Adapted with permission from the National Council on Radiation Protection and Measurements. EUS 103 Dental = average individual effective dose in the United States (whether the person was exposed or not) from dental sources calculated according to International Commission on Radiological Protection (ICRP) Publication 103 (9), E103 = effective dose based on ICRP Publication 103 (9), S103 = collective effective dose based on ICRP Publication 103 (9).
NA = not available, insufficient data. Procedure is rarely performed, and the absorbed dose is low.
Radiation Oncology
It is estimated that in 2016, a little more than 1 million courses of radiation therapy were administered to about 800 000 patients. The high doses and high dose rates delivered to radiation therapy targets and nearby normal tissues can exceed several grays, which is well above levels that are typically applicable to effective dose. As such, they were not considered in this report. However, patients undergoing radiation therapy also experience radiation exposure from imaging performed as part of simulation, portal evaluation, and before or during each treatment. Tissue doses associated with the many types of radiation therapy imaging are described in NCRP Report 184. These imaging procedures have not been included in assessment of procedure numbers or effective dose in the modality sections such as CT. The tissue doses from these imaging procedures can be a few percentages of the treatment dose. Doses to distant normal tissues may be an important issue for future assessments as the number of cancer survivors treated with radiation therapy increases.
Pediatric Exposure
Pediatric exposures were not addressed separately in NCRP Report 160 (NCRP 2009). Since effective dose is based on risks to an age-averaged population, its application to children is complex. Assessment of the collective effective dose as a result of medical imaging to the pediatric population is also difficult due to lack of recent and comprehensive data on the numbers of specific examinations in the pediatric population. The major contributor to pediatric collective effective dose from medical exposure is CT scanning (7). A reduction in pediatric exposure has likely occurred due to implementation of size-specific protocols, iterative reconstruction, and awareness of radiation dose by both parents and physicians, although the exact magnitude of these efforts is difficult to assess. For 2016, the collective effective dose from medical exposure of pediatric patients is estimated to be approximately 21 500 person-sievert (3% of the approximately 750 000 person-sievert total collective dose to the U.S. population from patient medical exposures).
Discussion
The current analysis of frequency and effective doses demonstrates that the previous rapid increase in patient radiation exposure through the year 2006 has slowed and, in some cases, declined. The exception to this trend is the continuing increase in frequency of CT from an estimated 62 million procedures to 74 million procedures in 2016. Overall, however, the total collective effective dose to the U.S. population has decreased since 2006 from 885 000 person-sievert to 717 000 person-sievert. The reasons for this decline were not analyzed but are likely multifactorial, including awareness of radiation dose, education, attempts to optimize doses, newer technology, changes in practices, and reduction in reimbursement.
A major strength of this 2016 analysis is that the methodology and major data sources were largely the same as were used in the 2006 NCRP analysis, allowing for accurate comparison and clear identification of trends. In addition, new data sources, particularly dosimetry-related data for the largest sources (CT and nuclear medicine), have been included based on millions of CT data procedures from the American College of Radiology Dose Index Registry and accreditation data of thousands of nuclear medicine practices. This should help increase accuracy. Our report also includes new sections on the impact of changes in ICRP tissue-weighting factors and dosimetric computational phantoms representing the patients, analysis of pediatric exposure, and tissue doses during image-guided radiation therapy.
The estimated effective doses per procedure and to the U.S. population should be used to compare with other population radiation sources. The values of dose per procedure are averages, and they do not apply to a specific individual. In addition, estimation of radiation detriment should be based on organ dose. The estimated effective dose per person in the United States is an average and does not represent a specific individual. The range of doses that might be incurred by a patient can vary substantially from the average. One should always be careful to place any such estimates in the context of the typically much greater medical benefits of the procedure.
Evaluation of nationwide doses is difficult for many reasons. One is reconciling diverse data sources that have been collected for disparate reasons. For example, use of billing data for frequency of procedures is affected by changes in coding, which must be identified and taken into account. An extremely wide range of reported doses also exists for a single specific procedure (26,27), and the change in ICRP tissue-weighting factors in 2007 requires care in analysis of doses to be sure that estimated effective doses are meaningful (30). This is especially important for mammographic examinations and procedures in the head and neck.
Limitations of this analysis include the need for various assumptions and judgments to make a coherent picture from divergent data sources and literature. Another limitation is the timeliness of the data and analysis. It would be interesting to have more current data than 2016; however, 2016 data were not available until the end of 2017, and subsequent analysis and compilation took an additional 18 months. Also, few current data exist on distribution and frequency of pediatric procedures. Little effort was devoted to collecting data from either very rare or very low-dose procedures (eg, bone densitometry with effective dose of about 0.001 mSv).
Evaluation of uncertainties is an important issue. Much of the literature and data sources do not contain sufficient information for a precise mathematic analysis. As a result, subjective uncertainty intervals are presented in this report. The subject experts arrived at these intervals after analyzing the available data and coherence for each modality. Intervals ranged from low to high depending on the modality. For the modalities that account for more than 90% of collective effective dose (CT, nuclear medicine, radiography, mammography, and coronary arteriography) the uncertainty was judged to be low (<30%). It was moderate (30%–90%) for diagnostic fluoroscopy and dental and high for noncardiac interventional and rarely performed procedures.
The production of this report resulted in several recommendations for future similar efforts. If Medicare data remain an important data source, it would be helpful to have a better analysis of the differences in diseases and frequency of different procedures in the Medicare population versus the general U.S. population. Additional dose metrics could be incorporated into patients’ medical records, and Digital Imaging and Communications in Medicine standardization for dose reporting and collection would help allow automated collection of absorbed organ doses, which may provide a better indicator of risk in the future. Expansion of automated dose reporting and dose registries by professional societies would be helpful, as would be additional Nationwide Evaluation of X-ray Trends surveys by the Food and Drug Administration in cooperation with the states. Also likely are additional dose databases and registries from professional societies (eg, American College of Cardiology and American College of Radiology). These can be used to help define types of procedures, analogous to the naming conventions used in the CT Dose Index Registry, and to improve dose estimation. More accurate and complete national data are likely only with a single-payer health care system or universal electronic medical records (eg, Norway and the United Kingdom), which could be anonymized and accessed.
In conclusion, we found that at the average annual individual effective dose in the United States from diagnostic and interventional patient radiation exposure decreased between 2006 and 2016. We have identified issues that may have affected the doses and associated number of procedures over time, but the magnitude and importance of factors such as payment models, impact of guidelines, and technologic advances could be better assessed. This could reduce the uncertainties of the estimates of doses and number of procedures in future analyses of this kind.
Key Result.
U.S. annual individual (per capita) effective dose from diagnostic and interventional medical procedures was estimated as 2.9 mSv in 2006 and 2.3 mSv in 2016, a decrease of approximately 20% over 10 years.
Acknowledgments
This work was performed by Scientific Committee 4–9 of the National Council on Radiation Protection and Measurements under a grant from the U.S. Centers for Disease Control and Prevention (grant 5NUE1EH001315).
The findings, conclusions, or statements in this manuscript are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Abbreviations
- ICRP
International Commission on Radiological Protection
- NCRP
National Council on Radiation Protection and Measurements
Footnotes
Disclosures of Conflicts of Interest: F.A.M. disclosed no relevant relationships. M.M. disclosed no relevant relationships. M.B.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received reimbursement from the American College of Radiology for travel/accommodations/meeting expenses. Other relationships: disclosed no relevant relationships. C.E.C. disclosed no relevant relationships. J.G.E. disclosed no relevant relationships. D.P.F. disclosed no relevant relationships. D.L.M. disclosed no relevant relationships. H.D.R. disclosed no relevant relationships. M.T.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author’s institution has received royalties from Wolters Kluwer; has received an honorarium from Galera Therapeutics. Other relationships: disclosed no relevant relationships. D.C.S. disclosed no relevant relationships. A.J.A. disclosed no relevant relationships. W.E.B. disclosed no relevant relationships. G.M.G. disclosed no relevant relationships. R.H.S. disclosed no relevant relationships. J.M.S. disclosed no relevant relationships. R.J.V. disclosed no relevant relationships.
References
- 1.Population exposure to x-rays, United States, 1964, Public Health Service Publication No. 1519. Washington DC: DHEW, Public Health Service, 1966. [Google Scholar]
- 2.Population exposure to x-rays, United States 1970, Department of Health Education and Welfare publication (FDA) No. 73-8047. Washington, DC: DHEW, Food and Drug Administration, Bureau of Radiological Health, 1973. [Google Scholar]
- 3.Radiation Experience Data: Documentation and results of the 1980 survey of U.S. Hospitals. Department of Health and Human Services publication (FDA) No. 86-8253. Washington, DC: DHHS, Food and Drug Administration, Center for Devices and Radiological Health, 1985. [Google Scholar]
- 4.Mettler FA Jr. Diagnostic radiology: Usage and trends in the United States, 1964–1980. Radiology 1987;162(1 Pt 1):263–266. [DOI] [PubMed] [Google Scholar]
- 5.Mettler FA Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: Frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology 2009;253(2):520–531. [DOI] [PubMed] [Google Scholar]
- 6.National Council on Radiation Protection and Measurements. Ionizing radiation exposure of the population of the United States: 2006. NCRP report No 160, Bethesda, Md: National Council on Radiation Protection and Measurements, 2009. [Google Scholar]
- 7.National Council on Radiation Protection and Measurements. Medical Radiation Exposure of Patients in the United States, NCRP Report 184, Bethesda, Md: National Council on Radiation Protection and Measurements, 2019. [Google Scholar]
- 8.1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 1991;21(1–3):1–201. [PubMed] [Google Scholar]
- 9.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37(2–4):1–332. [DOI] [PubMed] [Google Scholar]
- 10.IMV. Benchmark Report, Radiographic Fluoroscopy. Des Plaines, Ill: IMV Medical Information Division, 2012. [Google Scholar]
- 11.IMV. Benchmark Report, Interventional Angiography. Des Plaines, Ill: IMV Medical Information Division, 2013. [Google Scholar]
- 12.IMV. Benchmark Report, Interventional Angiography. Des Plaines, Ill: IMV Medical Information Division, 2014. [Google Scholar]
- 13.IMV. Benchmark Report, Cardiac Catheterization. Des Plaines, Ill: IMV Medical Information Division, 2014. [Google Scholar]
- 14.IMV. Benchmark Report, Nuclear Medicine. Des Plaines, Ill: IMV Medical Information Division, 2015. [Google Scholar]
- 15.IMV. Benchmark Report, PET Imaging. Des Plaines, Ill: IMV Medical Information Division, 2015. [Google Scholar]
- 16.IMV. Benchmark Report, X-ray CR/DR. Des Plaines, Ill: IMV Medical Information Division, 2015. [Google Scholar]
- 17.IMV. Benchmark Report, CT. Des Plaines, Ill: IMV Medical Information Division, 2016. [Google Scholar]
- 18.IMV. Benchmark Report, CT. Des Plaines, Ill: IMV Medical Information Division, 2017. [Google Scholar]
- 19.IMV. Benchmark Report, X-ray CR/DR. Des Plaines, Ill: IMV Medical Information Division, 2017. [Google Scholar]
- 20.IMV. Benchmark Report, Radiation Therapy. Des Plaines, Ill: IMV Medical Information Division, 2017. [Google Scholar]
- 21.IMV. Benchmark Report, PET Imaging. Des Plaines, Ill: IMV Medical Information Division, 2018. [Google Scholar]
- 22.American College of Radiology. National Radiology Data Registry - Feedback Report. Dose Index Registry. https://nrdrsupport.acr.org/support/solutions/articles/11000044558-dir-available-reports. Published 2018. Accessed June 2018.
- 23.McCollough C, Branham T, Herlihy V, et al. Diagnostic reference levels from the ACR CT Accreditation Program. J Am Coll Radiol 2011;8(11):795–803. [DOI] [PubMed] [Google Scholar]
- 24.Hendrick RE, Pisano ED, Averbukh A, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic imaging screening trial. AJR Am J Roentgenol 2010;194(2):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008;248(1):254–263. [DOI] [PubMed] [Google Scholar]
- 26.Andersson M Erratum to: Effective dose to adult patients from 338 radiopharmaceuticals estimated using ICRP biokinetic data, ICRP/ICRU computational reference phantoms and ICRP 2007 tissue-weighting factors. EJNMMI Phys 2015;2(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker MD, Butler PF, Bhargavan-Chatfield M, et al. Adult gamma camera myocardial perfusion imaging: Diagnostic reference levels and achievable administered activities derived from ACR accreditation data. J Am Coll Radiol 2016;13(6):688–695. [DOI] [PubMed] [Google Scholar]
- 28.European Commission. Medical Radiation Exposure of the European Population, Part 1 of 2. Radiation Protection No. 180. Luxembourg, European Commission, 2014. [Google Scholar]
- 29.European Commission. Radiation Protection, Report 180 Part 2, Diagnostic reference levels in 36 European countries. Luxembourg, European Commission, 2014. [Google Scholar]
- 30.Hirshfeld JW Jr, Ferrari VA, Bengel FM, et al. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71(24):e283–e351. [DOI] [PubMed] [Google Scholar]
- 31.Georges JL, Belle L, Etard C, et al. Radiation Doses to Patients in Interventional Coronary Procedures-Estimation of Updated National Reference Levels by Dose Audit. Radiat Prot Dosimetry 2017;175(1):17–25. [DOI] [PubMed] [Google Scholar]
- 32.Hart D, Wall B, Hillier M, Shrimpton P. Frequency and collective dose for medical and dental x-ray examinations in the UK, 2008. HPA-CRCE-012. Health Protection Agency, Center for Radiation, Chemical, and Environmental Hazards, Chilton, Didcot, Oxfordshire, United Kingdom: Health Protection Agency, 2012. [Google Scholar]
- 33.Ludlow JB, Davies-Ludlow LE, White SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. J Am Dent Assoc 2008;139(9):1237–1243. [DOI] [PubMed] [Google Scholar]
- 34.Miller DL, Hilohi CM, Spelic DC. Patient radiation doses in interventional cardiology in the U.S.: advisory data sets and possible initial values for U.S. reference levels. Med Phys 2012;39(10):6276–6286. [DOI] [PubMed] [Google Scholar]
- 35.Strauss KJ, Somasundaram E, Sengupta D, Marin JR, Brady SL. Radiation dose for pediatric CT: Comparison of pediatric versus adult imaging facilities. Radiology 2019;291(1):158–167. [DOI] [PubMed] [Google Scholar]
- 36.Wall BF, Haylock R, Hillier MC, et al. Radiation risks from medical x-ray examinations as a function of age and sex of the patient. Oxfordshire, England: Health Protection Agency, Center for Radiation, Chemical and Environmental Hazards, 2011. [Google Scholar]


